Fabrication and Surface Interactions of Super-Hydrophobic Silicon Carbide for Membrane Distillation
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemicals and Materials
2.2. Surface Modification
2.3. Material Characterization
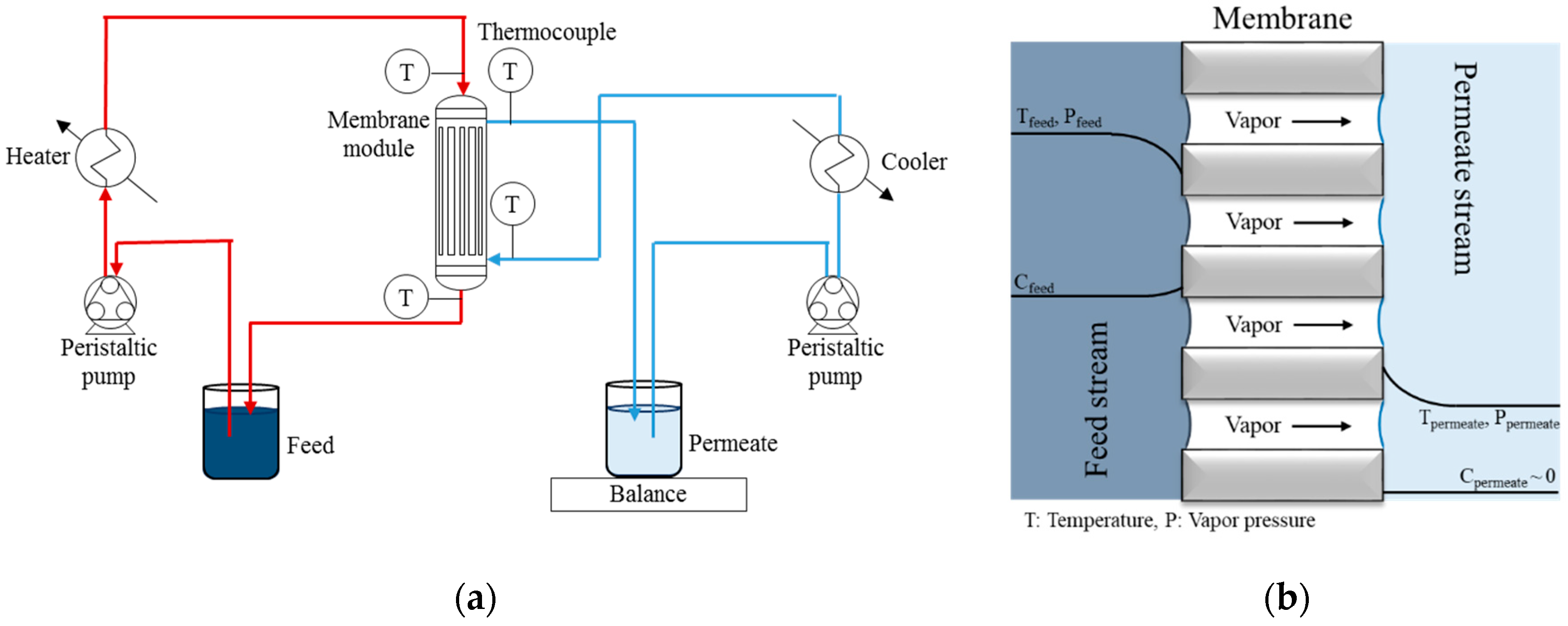
2.4. DCMD Experiments
3. Results and Discussion
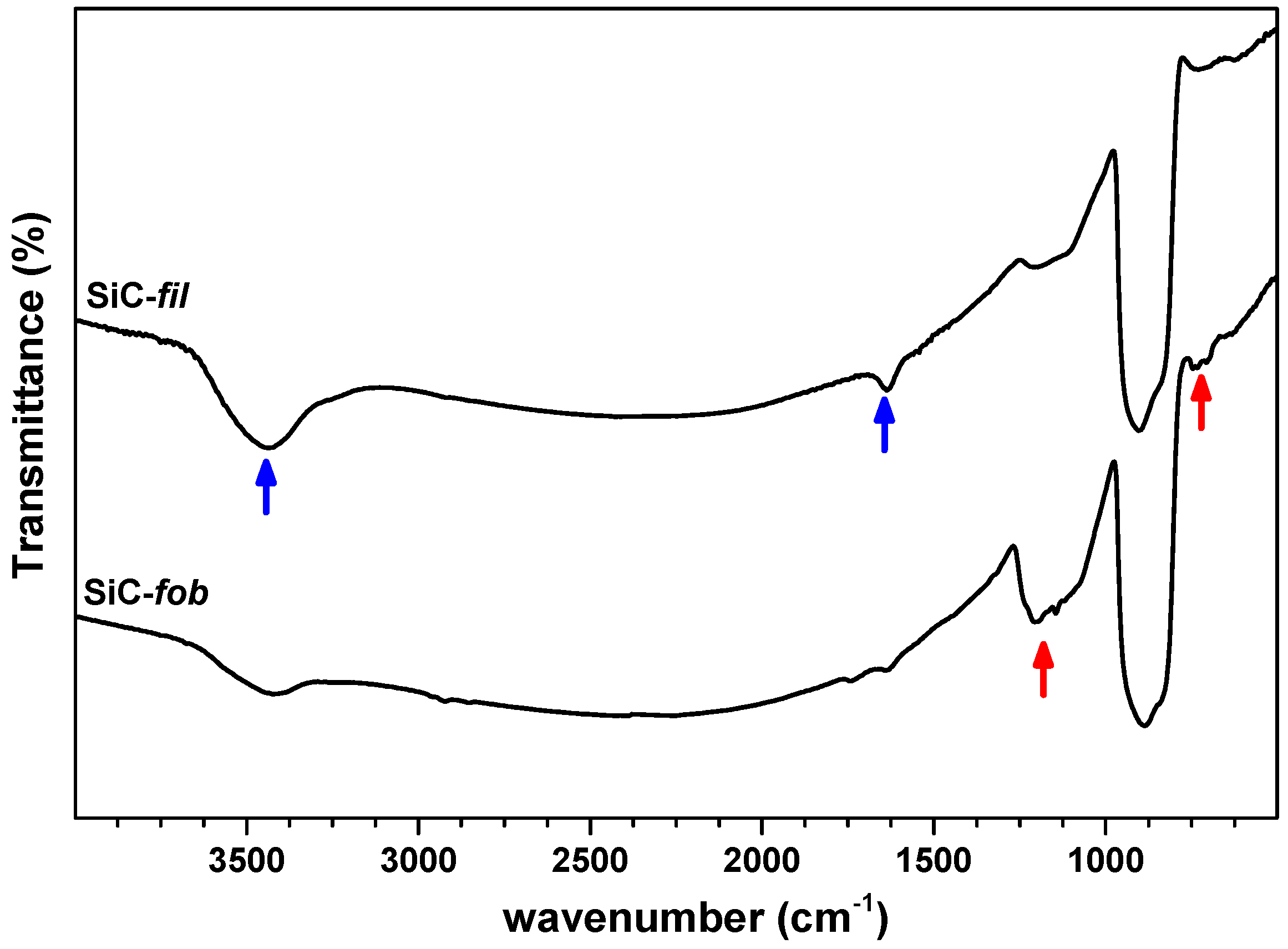
3.1. Surface Modification
3.2. Adsorption of Vapours
3.3. DCMD Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deka, B.J.; Lee, E.J.; Guo, J.; Kharraz, J.; An, A.K. Electrospun nanofiber membranes incorporating PDMS-aerogel superhydrophobic coating with enhanced flux and improved anti-wettability in membrane distillation. Environ. Sci. Technol. 2019, 53, 4948–4958. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Li, S.; Francis, L.; Zhenyu, L.; Linares Valladares, R.; Alsaadi, A.S.; Ghdaib, M.; Son, A.H.; Amy, G.; Ghaffour, N. Osmotically and Thermally Isolated Forward Osmosis–Membrane Distillation (FO–MD) Integrated Module. Environ. Sci. Technol. 2019, 53, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Macedonio, F.; Drioli, E. Pressure-driven membrane operations and membrane distillation technology integration for water purification. Desalination 2008, 223, 396–409. [Google Scholar] [CrossRef]
- Di Profio, G.; Curcio, E.; Drioli, E. Controlling protein crystallization kinetics in membrane crystallizers: Effects on morphology and structure. Desalination 2006, 200, 598–600. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Integrated membrane desalination systems with membrane crystallization units for resource recovery: A new approach for mining from the sea. Crystals 2016, 6, 36. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Sørensen, J.M.; Svenstrup, M.A.; Scarpa, L.; Carlsen, T.S.; Jensen, H.C.; Wybrandt, L.; Christensen, M.L. Membrane crystallization for phosphorus recovery and ammonia stripping from reject water from sludge dewatering process. Desalination 2018, 440, 156–160. [Google Scholar] [CrossRef]
- Hsu, S.T.; Cheng, K.T.; Chiou, J.S. Seawater desalination by direct contact membrane distillation. Desalination 2002, 143, 279–287. [Google Scholar] [CrossRef]
- Khayet, M. Treatment of radioactive wastewater solutions by direct contact membrane distillation using surface modified membranes. Desalination 2013, 321, 60–66. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Ashoor, B.B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S.W. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Koter, S.; Kujawski, W. Highly efficient hydrophobic titania ceramic membranes for water desalination. ACS Appl. Mater. Interfaces 2014, 6, 14223–14230. [Google Scholar] [CrossRef]
- Ren, C.; Fang, H.; Gu, J.; Winnubst, L.; Chen, C. Preparation and characterization of hydrophobic alumina planar membranes for water desalination. J. Eur. Ceram. Soc. 2015, 35, 723–730. [Google Scholar] [CrossRef]
- Larbot, A.; Gazagnes, L.; Krajewski, S.; Bukowska, M.; Kujawski, W. Water desalination using ceramic membrane distillation. Desalination 2004, 168, 367–372. [Google Scholar] [CrossRef]
- Zhang, J.W.; Fang, H.; Wang, J.W.; Hao, L.Y.; Xu, X.; Chen, C.S. Preparation and characterization of silicon nitride hollow fiber membranes for seawater desalination. J. Membr. Sci. 2014, 450, 197–206. [Google Scholar] [CrossRef]
- Wang, J.W.; Li, L.; Zhang, J.W.; Xu, X.; Chen, C.S. β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. Soc. 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Cerneaux, S.; Strużyńska, I.; Kujawski, W.M.; Persin, M.; Larbot, A. Comparison of various membrane distillation methods for desalination using hydrophobic ceramic membranes. J. Membr. Sci. 2009, 337, 55–60. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, S.; Zhao, H.; Liu, Y. Distillation membrane constructed by TiO2 nanofiber followed by fluorination for excellent water desalination performance. Desalination 2017, 405, 51–58. [Google Scholar] [CrossRef]
- Garofalo, A.; Donato, L.; Drioli, E.; Criscuoli, A.; Carnevale, M.C.; Alharbi, O.; Aljlil, S.A.; Algieri, C. Supported MFI zeolite membranes by cross flow filtration for water treatment. Sep. Purif. Technol. 2014, 137, 28–35. [Google Scholar] [CrossRef]
- Zhang, J.W.; Fang, H.; Hao, L.Y.; Xu, X.; Chen, C.S. Preparation of silicon nitride hollow fibre membrane for desalination. Mater. Lett. 2012, 68, 457–459. [Google Scholar] [CrossRef]
- Ko, C.C.; Chen, C.H.; Chen, Y.R.; Wu, Y.H.; Lu, S.C.; Hu, F.C.; Li, C.L.; Tung, K.L. Increasing the performance of vacuum membrane distillation using micro-structured hydrophobic aluminum hollow fiber membranes. Appl. Sci. 2017, 7, 357. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P. Chemically modified ceramic membranes. Microporous Mesoporous Mater. 1998, 22, 321–332. [Google Scholar] [CrossRef]
- Dafinov, A.; Garcia-Valls, R.; Font, J. Modification of ceramic membranes by alcohol adsorption. J. Memb. Sci. 2002, 196, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Krajewski, S.R.; Kujawski, W.; Dijoux, F.; Picard, C.; Larbot, A. Larbot, Grafting of ZrO2 powder and ZrO2 membrane by fluoroalkylsilanes. Colloids Surf. A Physicochem. Eng. Asp. 2004, 243, 43–47. [Google Scholar] [CrossRef]
- García-Fernández, L.; Wang, B.; García-Payo, M.C.; Li, K.; Khayet, M. Morphological design of alumina hollow fiber membranes for desalination by air gap membrane distillation. Desalination 2017, 420, 226–240. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W.; Knozowska, K. Hydrophobic ceramic membranes for water desalination. Appl. Sci. 2017, 7, 402. [Google Scholar] [CrossRef]
- Ko, C.C.; Ali, A.; Drioli, E.; Tung, K.L.; Chen, C.H.; Chen, Y.R.; Macedonio, F. Performance of ceramic membrane in vacuum membrane distillation and in vacuum membrane crystallization. Desalination 2018, 440, 48–58. [Google Scholar] [CrossRef]
- Chau, J.; Singh, D.; Sirkar, K.K. 110th Anniversary: Liquid separation membranes based on nanowire substrates for organic solvent nanofiltration and membrane distillation. Ind. Eng. Chem. Res. 2019, 58, 31. [Google Scholar] [CrossRef]
- Mabry, J.M.; Vij, A.; Iacono, S.T.; Viers, B.D. Fluorinated polyhedral oligomeric silsesquioxanes (F-POSS). Angew. Chem. Int. Ed. 2008, 47, 4137–4140. [Google Scholar] [CrossRef]
- Bazzar, M.; Mousa, G.; Alizadeh, R. Novel fluorescent light-emitting polymer composites bearing 1,2,4-triazole and quinoxaline moieties: Reinforcement and thermal stabilization with silicon carbide nanoparticles by epoxide functionalization. Polym. Degr. Stab. 2012, 97, 1690–1703. [Google Scholar] [CrossRef]
- Da Silva, C.R.S.; Justo, J.F.; Pereyra, I. Crystalline silicon oxycarbide: Is there a native oxide for silicon carbide? Appl. Phys. Lett. 2004, 84, 4845–4847. [Google Scholar] [CrossRef]
- Winter, R.; Nixon, P.G.; Gard, G.L.; Graham, D.J.; Castner, D.G.; Holcomb, N.R.; Grainger, D.W. Self-assembled organic monolayers terminated in perfluoroalkyl pentafluoro-λ6-sulfanyl (-SF5) chemistry on gold. Langmuir 2004, 20, 5776–5781. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009; p. 284. [Google Scholar]
- Law, K.G. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef]
- Majer, V.; Svoboda, V. Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation; Blackwell Scientific Publications: Oxford, UK, 1985; p. 300. [Google Scholar]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]







| Atom% | Water Contact Angle | ||||||
|---|---|---|---|---|---|---|---|
| Si | C | O | F | wet sample | |||
 | |||||||
| SiC-fil | 36.6 | 57.2 | 6.2 | 0.0 | 31.5° ± 3.7° | ||
| after modification | 1 day in H2O—100 °C | 3 days in H2O—100 °C | |||||
 |  |  | |||||
| SiC-fob | 36.6 | 57.0 | 5.6 | 1.5 | 143.2° ± 0.5° | 146.8° ± 0.7° | 148.9° ± 1.4° |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boffa, V.; Lunghi, C.; Quist-Jensen, C.A.; Magnacca, G.; Calza, P. Fabrication and Surface Interactions of Super-Hydrophobic Silicon Carbide for Membrane Distillation. Nanomaterials 2019, 9, 1159. https://doi.org/10.3390/nano9081159
Boffa V, Lunghi C, Quist-Jensen CA, Magnacca G, Calza P. Fabrication and Surface Interactions of Super-Hydrophobic Silicon Carbide for Membrane Distillation. Nanomaterials. 2019; 9(8):1159. https://doi.org/10.3390/nano9081159
Chicago/Turabian StyleBoffa, Vittorio, Cristian Lunghi, Cejna A. Quist-Jensen, Giuliana Magnacca, and Paola Calza. 2019. "Fabrication and Surface Interactions of Super-Hydrophobic Silicon Carbide for Membrane Distillation" Nanomaterials 9, no. 8: 1159. https://doi.org/10.3390/nano9081159
APA StyleBoffa, V., Lunghi, C., Quist-Jensen, C. A., Magnacca, G., & Calza, P. (2019). Fabrication and Surface Interactions of Super-Hydrophobic Silicon Carbide for Membrane Distillation. Nanomaterials, 9(8), 1159. https://doi.org/10.3390/nano9081159








