Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer
Abstract
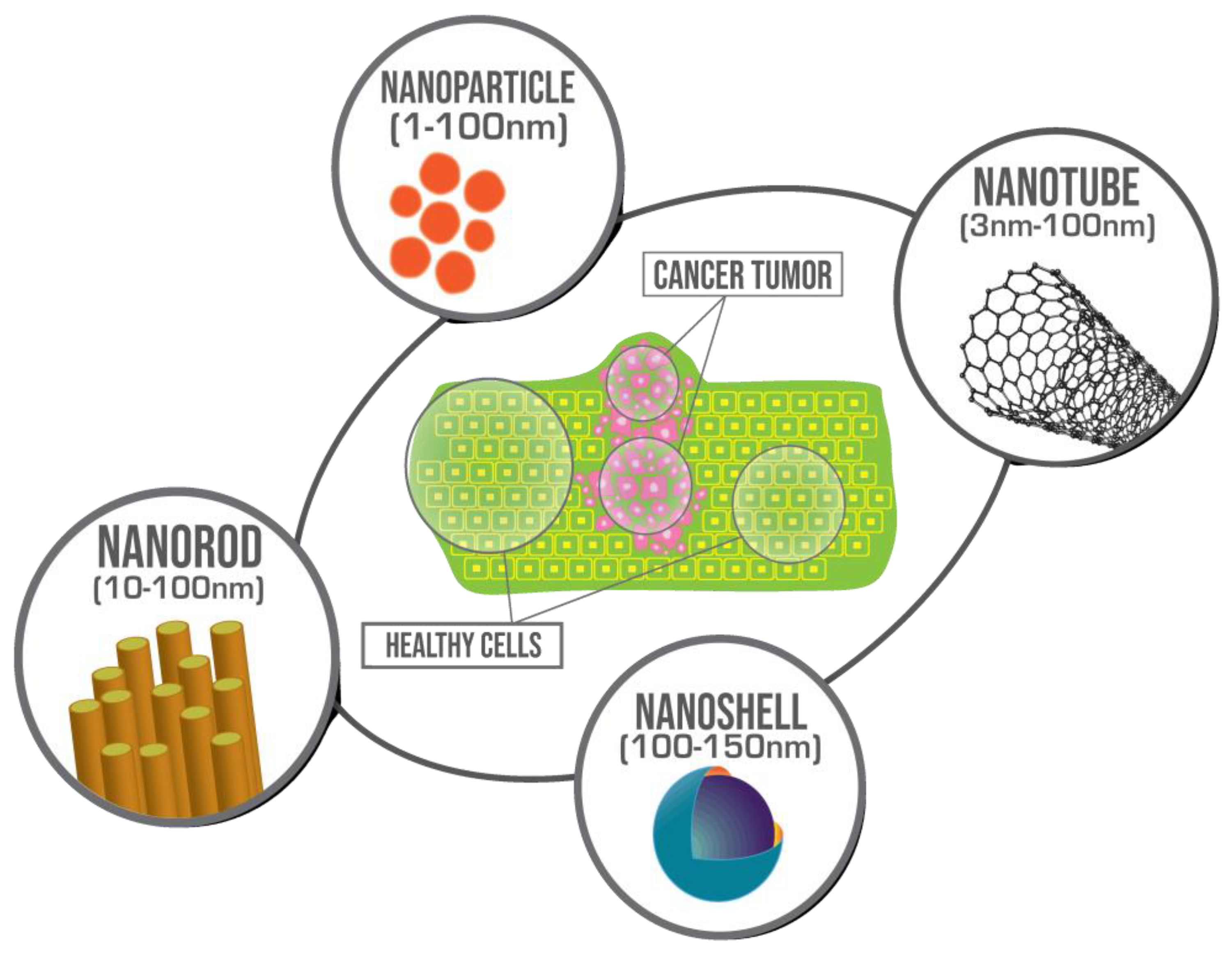
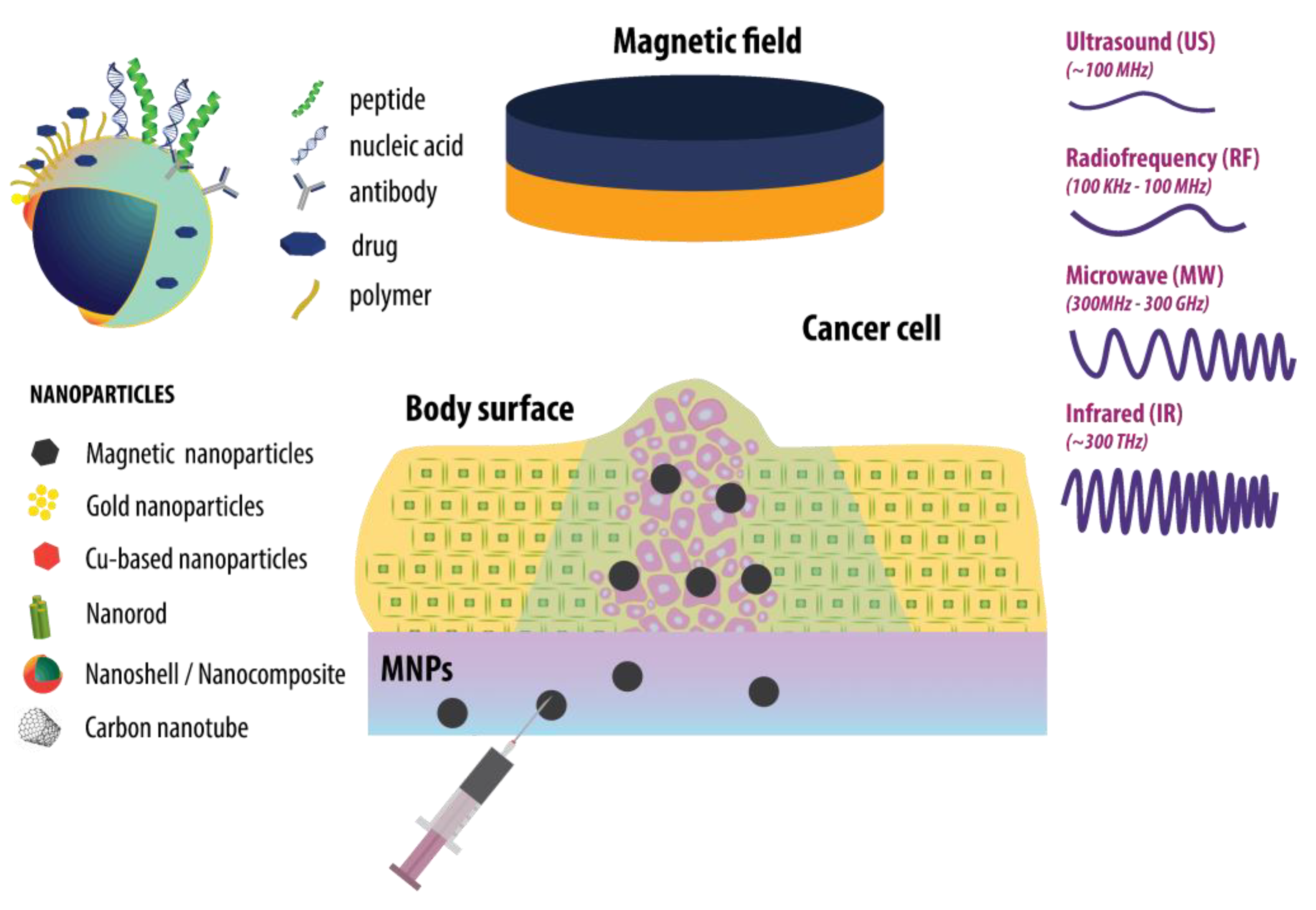
:1. Introduction
2. Magnetic Nanoparticles (MNP)
3. Gold Nanoparticles (AuNP)
4. CuS Nanoparticles
5. Nanorods
6. Carbon Nanotubes (CNTs)
7. Nanoshells/Nanocomposites
7.1. Nanoshells
7.2. Nanocomposites
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research in Cancer. Latest Global Cancer Data, 2018; Press Release #263; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Ren, Y.; Qi, H.; Chen, Q.; Ruan, L. Thermal dosage investigation for optimal temperature distribution in gold nanoparticle enhanced photothermal therapy. Int. J. Heat Mass Transf. 2017, 106, 212–221. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP–dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Brace, C.L.; Lee, F.T.; Goldberg, S.N. Principles of and Advances in Percutaneous Ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Saccomandi, P.; Fong, Y. Laser Ablation for Cancer: Past, Present and Future. J. Funct. Biomater. 2017, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Carrafiello, G.; Laganà, D.; Mangini, M.; Fontana, F.; Dionigi, G.; Boni, L.; Rovera, F.; Cuffari, S.; Fugazzola, C. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int. J. Surg. 2008, 6 (Suppl. 1), 65–69. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for Thermal Cancer Therapy. J. Biomech. Eng. 2009, 131, 074001. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, G.A.; Mohazzabi, P.; McCormack, P.; Jabbari, S. Nanotechnology in cancer prevention, detection and treatment: Bright future lies ahead. World Rev. Sci. Technol. Sustain. Dev. 2007, 4, 226. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.Q.; Zhu, Y.J.; Ao, H.Y.; Qi, C.; Chen, F. Synthesis and characterization of magnetic iron oxide/calcium silicate mesoporous nanocomposites as a promising vehicle for drug delivery. ACS Appl. Mater. Interfaces 2012, 4, 6969–6974. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Naghdi, N.; Nokhbatolfoghahaei, H.; Bagheri, H. The combination of laser therapy and metal nanoparticles in cancer treatment originated from epithelial tissues: A literature review. J. Lasers Med. Sci. 2016, 7, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Manthe, R.L.; Foy, S.P.; Krishnamurthy, N.; Sharma, B.; Labhasetwar, V. Tumor ablation and nanotechnology. Mol. Pharm. 2010, 7, 1880–1898. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Wust, P.; Fähling, H.; John, W.; Hinz, A.; Felix, R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int. J. Hyperth. 2009, 25, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Letfullin, R.R.; Iversen, C.B.; George, T.F. Modeling nanophotothermal therapy: Kinetics of thermal ablation of healthy and cancerous cell organelles and gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Irudayaraj, J. Multifunctional magnetic-optical nanoparticle probes for simultaneous detection, separation, and thermal ablation of multiple pathogens. Small 2010, 6, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Burford, C.D.; Bhattacharyya, K.D.; Boriraksantikul, N.; Whiteside, P.J.D.; Robertson, B.P.; Peth, S.M.; Islam, N.E.; Viator, J.A. Nanoparticle mediated thermal ablation of breast cancer cells using a nanosecond pulsed electric field. IEEE Trans. Nanobiosci. 2013, 12, 112–118. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Hilger, I.; Hiergeist, R.; Hergt, R.; Winnefeld, K.; Schubert, H.; Kaiser, W.A. Thermal ablation of tumors using magnetic nanoparticles: An in vivo feasibility study. Investig. Radiol. 2002, 37, 580–586. [Google Scholar] [CrossRef]
- Attar, M.M.; Haghpanahi, M.; Amanpour, S.; Mohaqeq, M. Analysis of bioheat transfer equation for hyperthermia cancer treatment. J. Mech. Sci. Technol. 2014, 28, 763–771. [Google Scholar] [CrossRef]
- Toy, R. The Effect of Particle Size and Shape on the In Vivo Journey of Nanoparticles. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2014. [Google Scholar]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Gui, C.; Cui, D.-X. Functionalized gold nanorods for tumor imaging and targeted therapy. Cancer Biol. Med. 2012, 9, 221–233. [Google Scholar] [PubMed]
- Gajbhiye, K.R.; Gajbhiye, J.M. EPR effect based nanocarriers targeting for treatment of cancer. Int. J. Drug Deliv. 2017, 8, 117–124. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- García-Jimeno, S.; Ortega-Palacios, R.; Cepeda-Rubio, M.; Vera, A.; Leija, L.; Estelrich, J. Improved Thermal Ablation Efficacy Using Magnetic Nanoparticles: A Study in Tumor Phantoms. Prog. Electromagn. Res. 2012, 128, 229–248. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Original Contribution Thermal dose Determination in Cancer Therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Haemmerich, D.; Wood, B.J. Hepatic radiofrequency ablation at low frequencies preferentially heats tumour tissue. Int. J. Hyperth. 2006, 22, 563–574. [Google Scholar] [CrossRef]
- Gassino, R.; Liu, Y.; Konstantaki, M.; Vallan, A.; Pissadakis, S.; Perrone, G. A fiber optic probe for tumor laser ablation with integrated temperature measurement capability. J. Light. Technol. 2017, 35, 3447–3454. [Google Scholar] [CrossRef]
- Jacques, S.L. Erratum: Optical properties of biological tissues: A review (Physics in Medicine and Biology (2013) 58). Phys. Med. Biol. 2013, 58, 5007–5008. [Google Scholar] [CrossRef]
- Mathew, M.; Mikhail, A.S.; Wood, B.J.; Partanen, A.; Graham, C.; Negussie, A.H. Evaluation of a tissue-mimicking thermochromic phantom for radiofrequency ablation. Med. Phys. 2016, 43, 4304–4311. [Google Scholar]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, H. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Vedernikova, I.A. Magnetic nanoparticles: Advantages of using, methods for preparation, characterization, application in pharmacy. Rev. J. Chem. 2015, 5, 256–280. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef] [PubMed]
- Abenojar, E.C.; Wickramasinghe, S.; Bas-Concepcion, J.; Samia, A.C.S. Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles. Prog. Nat. Sci. Mater. Int. 2016, 26, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Nedelcu, G. Magnetic nanoparticles impact on tumoral cells in the treatment by magnetic fluid hyperthermia. Dig. J. Nanomater. Biostruct. 2008, 3, 103–107. [Google Scholar]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Particles, F.; Hilger, I.; Hergt, R.; Andr, W.; Ambly, C.G.; Hilger, I. Physical Limits of Hyperthermia Using Magnetite Physical Limits of Hyperthermia Using Magnetite Fine Particles. IEEE Trans. Magn. 1998, 34, 3745–3754. [Google Scholar]
- Salloum, M.; Ma, R.H.; Weeks, D.; Zhu, L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int. J. Hyperth. 2008, 24, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hergt, R.; Dutz, S.; Zeisberger, M. Validity limits of the Néel relaxation model of magnetic nanoparticles for hyperthermia. Nanotechnology 2010, 21, 015706. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.D.S.R.K. Effects of Electromagetic Heating on Internal Viscera: A Preliminary to the Treatment of Human Tumors. Ann. Surg. 1965, 161, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Dürr, S.; Janko, C.; Lyer, S.; Tripal, P.; Schwarz, M.; Zaloga, J.; Tietze, R.; Alexiou, C. Magnetic nanoparticles for cancer therapy. Nanotechnol. Rev. 2013, 2, 395–409. [Google Scholar] [Green Version]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment (Review). Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Kettering, M.; Winter, J.; Zeisberger, M.; Bremer-Streck, S.; Oehring, H.; Bergemann, C.; Alexiou, C.; Hergt, R.; Halbhuber, K.J.; Kaiser, W.A.; et al. Magnetic nanoparticles as bimodal tools in magnetically induced labelling and magnetic heating of tumour cells: An in vitro study. Nanotechnology 2007, 18, 175101. [Google Scholar] [CrossRef]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-Dependent Heating of Magnetic Iron Oxide Nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef]
- Hilger, I.; Andrä, W.; Hergt, R.; Hiergeist, R.; Schubert, H.; Kaiser, W.A. Electromagnetic Heating of Breast Tumors in Interventional Radiology: In Vitro and in Vivo Studies in Human Cadavers and Mice. Radiology 2001, 218, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Hergt, R.; Kaiser, W.A. Towards breast cancer treatment by magnetic heating. J. Magn. Magn. Mater. 2005, 293, 314–319. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Maier-Hauf, K.; Landeghem, F.K.H.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neurooncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldofner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of Prostate Cancer Using Magnetic Nanoparticles: Feasibility, Imaging, and Three-Dimensional Temperature Distribution. Eur. Urol. 2007, 52, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Thiesen, B.; Jordan, A.; Taymoorian, K.; Gneveckow, U.; Waldofner, N.; Scholz, R.; Koch, M.; Lein, M.; Jung, K.; et al. Magnetic fluid hyperthermia (MFH) reduces prostate cancer growth in the orthotopic Dunning R3327 rat model. Prostate 2005, 64, 283–292. [Google Scholar] [CrossRef]
- Rich, J.N.; Robinson, J.W.; Rowitch, D.H.; Sampson, J.H.; Taylor, M.D.; Workman, P.; Gilbertson, R.J. Statement brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar]
- Bredlau, A.L.; McCrackin, M.A.; Motamarry, A.; Helke, K.; Chen, C.; Broomee, A.-M.; Haemmerich, D. Thermal Therapy Approaches for Treatment of Brain Tumors in Animals and Humans. Crit Rev Biomed Eng. 2016, 44, 443–457. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Bruners, P.; Braunschweig, T.; Hodenius, M.; Pietsch, H.; Penzkofer, T.; Baumann, M.; Gunter, R.W.; Schmitz-Rode, T.; Mahnken, A.H. Thermoablation of malignant kidney tumors using magnetic nanoparticles: An in vivo feasibility study in a rabbit model. Cardiovasc. Intervent. Radiol. 2010, 33, 127–134. [Google Scholar] [CrossRef]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Taymoorian, K.; Thiesen, B.; Waldöfner, N.; Scholz, R.; Jung, K.; Jordan, A.; Wust, P.; Loening, S.A. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int. J. Hyperth. 2007, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: A mouse study. BMC Cancer 2010, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Wiekhorst, F.; Steinhoff, U.; Trahmz, L. Localization and quantification of magnetic nanoparticles by multichannel magnetorelaxometry for in vivo hyperthermia studies in carcinoma models. IFMBE Proc. 2009, 25, 302–305. [Google Scholar]
- Andrä, W.; D’Ambly, C.G.; Hergt, R.; Hilger, I.; Kaiser, W.A. Temperature distribution as function of time around a small spherical heat source of local magnetic hyperthermia. J. Magn. Magn. Mater. 1999, 194, 197–203. [Google Scholar] [CrossRef]
- Versiani, A.F.; Andrade, L.M.; Martins, E.M.N.; Scalzo, S.; Geraldo, J.M.; Chaves, C.R.; Ferreira, D.C.; Ladeira, M.; Guatimosim, S.; Ladeira, L.O.; et al. Gold nanoparticles and their applications in biomedicine. Futur Med. Virol. 2016, 11, 293–309. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold nanoparticle based photothermal therapy: Development and application for effective cancer treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Cardinal, J.; Klune, J.R.; Chory, E.; Jeyabalan, G.; Kanzius, J.S.; Nalesnik, M.; Geller, D.A. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery 2008, 144, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, C.J.; Patra, C.R.; Bhattacharya, R.; Mukherjee, P.; Curley, S.A. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J. Nanobiotechnol. 2008, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.S.; Zhu, C.; Massey, K.L.; Thompson, C.S.; Kaluarachchi, W.D.; Hamir, A.N.; Curley, S.A. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin. Cancer Res. 2010, 16, 5712–5721. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.S.; Curley, S.A. Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles. Cancer 2010, 116, 3285–3293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, E.; de Palma, R.; Orditura, M.; de Vita, F.; Ciardiello, F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin. Exp. Immunol. 2009, 158, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Determination of the Minimum Temperature Required for Selective Photothermal Destruction of Cancer Cells with the Use of Immunotargeted Gold Nanoparticles. Photochem. Photobiol. 2006, 82, 412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Panderi, I.; Yan, D.D.; Szulak, K.; Li, Y.; Chen, Y.; Ma, H.; Niesen, D.B.; Seeram, N.; Ahmed, A.; et al. A comparative study of hollow copper sulfide nanoparticles and hollow gold nanospheres on degradability and toxicity. ACS Nano 2013, 7, 8780–8793. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, Y.; Shi, S.; Hao, S.; Wei, J.; Chen, X. Copper sulfide nanoparticles with phospholipid-PEG coating for in vivo near-infrared photothermal cancer therapy. Chem. Asian J. 2015, 10, 370–376. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Y.; Adachi, M.; Wen, X.; Erwin, B.; Mawlawi, O.; Lai, S.Y.; Li, C. Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer. Biomaterials 2015, 57, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: A potential cancer diagnostic marker. Nano Lett. 2007, 7, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Zhou, L.; Liu, Y.; Meng, L.; Zhang, K.; Wu, X.; Zhang, L.; Li, B.; Chen, C. Characterization of gold nanorods in vivo by integrated analytical techniques: Their uptake, retention, and chemical forms. Anal. Bioanal. Chem. 2010, 396, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Huff, T.B.; Tong, L.; Zhao, Y.; Hansen, M.N.; Cheng, J.X.; Wei, A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine 2007, 2, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Liu, Y.; Wang, L.; Xu, L.; Bai, R.; Ji, Y.; Wu, X.; Zhao, Y.; Li, Y.; Chen, C. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 2010, 31, 7606–7619. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, Y.; Tian, Q.; Yang, S. Small gold nanorods: Recent advances in synthesis, biological imaging, and cancer therapy. Materials 2017, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Fang, C.; Zhu, X.M.; Ruan, Q.; Wang, Y.-X.J.; Wang, J. Synthesis of Absorption-Dominant Small Gold Nanorods and Their Plasmonic Properties. Langmuir 2015, 31, 7418–7426. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 1–16. [Google Scholar] [CrossRef]
- von Maltzahn, G.; Park, J.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef]
- Huang, H.C.; Rege, K.; Heys, J.J. Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano 2010, 4, 2892–2900. [Google Scholar] [CrossRef]
- Soni, S.; Tyagi, H.; Taylor, R.A.; Kumar, A. Investigation on nanoparticle distribution for thermal ablation of a tumour subjected to nanoparticle assisted thermal therapy. J. Therm. Biol. 2014, 43, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Emoto, K.; Su, L.J.; Yang, X.; Flaig, T.W.; Park, W. Functionalized gold nanorods for thermal ablation treatment of bladder cancer. J. Biomed. Nanotechnol. 2014, 10, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.; Schena, E.; Zhumkhawala, A.; Aboody, K.S.; Berlin, J.M. Internal temperature increase during photothermal tumour ablation in mice using gold nanorods. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 2563–2566. [Google Scholar]
- Mooney, R.; Schena, E.; Saccomandi, P.; Zhumkhawala, A.; Aboody, K.; Berlin, J.M. Gold nanorod-mediated near-infrared laser ablation: In vivo experiments on mice and theoretical analysis at different settings. Int. J. Hyperth. 2017, 33, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.; El-Sayed, I.H.; Chu, H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, B.; Kim, Y.S.; Choi, Y. Effects of gold Nanorod concentration on the depth-related temperature increase during hyperthermic ablation. Small 2011, 7, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Mceuen, P.L.; Fuhrer, M.S.; Park, H. Single-walled carbon nanotube electronics. IEEE Trans. Nanotechnol. 2002, 1, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Elhissi, A.; Ahmed, W.; Dhanak, V.R.; Subramani, K. Carbon Nanotubes in Cancer Therapy and Drug Delivery, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.G.; Lu, G.Q.; Yan, Z.F.; Wilson, M.A. Recent Advances in the Preparation and Utilization of Carbon Nanotubes for Hydrogen Storage. J. Nanosci. Nanotechnol. 2001, 1, 7–29. [Google Scholar] [CrossRef]
- Zhou, F.; Xing, D.; Ou, Z.; Wu, B.; Resasco, D.E.; Chen, W.R. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J. Biomed. Opt. 2009, 14, 021009. [Google Scholar] [CrossRef]
- Iancu, C.; Mocan, L. Advances in cancer therapy through the use of carbon nanotube-mediated targeted hyperthermia. Int. J. Nanomed. 2011, 6, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Nakatsuji, H.; Inada, M.; Matoba, Y.; Umeyama, T.; Tsujimoto, M.; Isoda, S.; Hashida, M.; Imahori, H. Photodynamic and photothermal effects of semiconducting and metallic-enriched single-walled carbon nanotubes. J. Am. Chem. Soc. 2012, 134, 17862–17865. [Google Scholar] [CrossRef] [PubMed]
- Kam, N.W.S.; O’Connell, M.; Wisdom, J.A.; Dai, H. Selective Cancer Cell Destruction Linked references are available on JSTOR for this article: Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Pnas 2005, 102, 1600–11605. [Google Scholar]
- Madani, S.Y.; Naderi, N.; Dissanayake, O.; Tan, A.; Seifalian, A.M. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int. J. Nanomed. 2011, 6, 2963–2979. [Google Scholar] [Green Version]
- Ji, S.R.; Liu, C.; Zhang, B.; Yang, F.; Xu, J.; Long, J.; Jin, C.; Fu, D.; Ni, Q.; Yu, X. Carbon nanotubes in cancer diagnosis and therapy. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.; Botelho, E.C.; Costa, M.L.; Bandeira, C.F. Carbon nanotube buckypaper reinforced polymer composites: A review. Polímeros 2017, 27, 247–255. [Google Scholar] [CrossRef]
- Mayr, E. American Association for the Advancement of Science Handbook. Science 1998, 266, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Munge, B.; Patel, V.; Jensen, G.; Bhirde, A.; Gong, J.D.; Kim, S.N.; Gillespie, J.; Gutkind, J.S.; Papadimitrakopoulos, F.; et al. Carbon nanotube amplification strategies for highly sensitive immunodetection of cancer biomarkers. J. Am. Chem. Soc. 2006, 128, 11199–11205. [Google Scholar] [CrossRef]
- Park, D.-W.; Kim, Y.-H.; Kim, B.S.; So, H.-M.; Won, K.; Lee, J.-O.; Kong, K.-J.; Chang, H. Detection of Tumor Markers Using Single-Walled Carbon Nanotube Field Effect Transistors. J. Nanosci. Nanotechnol. 2006, 6, 3499–3502. [Google Scholar] [CrossRef]
- Liu, X.; Tao, H.; Yang, K.; Zhang, S.; Lee, S.T.; Liu, Z. Optimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials 2011, 32, 144–151. [Google Scholar] [CrossRef]
- Gannon, C.J.; Cherukuri, P.; Yakobson, B.I.; Cognet, L.; Kanzius, J.S.; Kittrell, C.; Weisman, R.B.; Pasquali, M.; Schmidt, H.K.; Smalley, R.E.; et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer 2007, 110, 2654–2665. [Google Scholar] [CrossRef]
- Marches, R.; Mikoryak, C.; Wang, R.H.; Pantano, P.; Draper, R.K.; Vitetta, E.S. The importance of cellular internalization of antibody-targeted carbon nanotubes in the photothermal ablation of breast cancer cells. Nanotechnology 2011, 22, 095101. [Google Scholar] [CrossRef]
- Hashida, Y.; Tanaka, H.; Zhou, S.; Kawakami, S.; Yamashita, F.; Murakami, T.; Umeyama, T.; Imahori, H.; Hashida, M. Photothermal ablation of tumor cells using a single-walled carbon nanotube-peptide composite. J. Control. Release 2014, 173, 58–66. [Google Scholar] [CrossRef]
- Fisher, J.W.; Sarkar, S.; Buchanan, C.F.; Szot, C.S.; Whitney, J.; Hatcher, H.C.; Torti, S.V.; Rylander, C.G.; Rylander, M.N. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res. 2010, 70, 9855–9864. [Google Scholar] [CrossRef]
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Bele, C.; Orza, A.I.; Lucan, C.; Stiufiuc, R.; Manaila, I.; Iulia, F.; Dana, I.; et al. Selective ex-vivo photothermal ablation of human pancreatic cancer with albumin functionalized multiwalled carbon nanotubes. Int. J. Nanomed. 2011, 6, 915–928. [Google Scholar] [Green Version]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [Green Version]
- Lal, S.; Clare, S.E.; Halas, N.J. Photothermal Therapy: Impending Clinical Impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef]
- Liu, A.; Wang, G.; Wang, F.; Zhang, Y. Gold nanostructures with near-infrared plasmonic resonance: Synthesis and surface functionalization. Coord. Chem. Rev. 2017, 336, 28–42. [Google Scholar] [CrossRef]
- Ke, H.; Wang, J.; Tong, S.; Jin, Y.; Wang, S.; Qu, E.; Bao, G.; Dai, Z. Gold nanoshelled liquid perfluorocarbon magnetic nanocapsules: A nanotheranostic platform for bimodal ultrasound/magnetic resonance imaging guided photothermal tumor ablation. Theranostics 2014, 4, 12–23. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Bernardi, R.J.; Lowery, A.R.; Thompson, P.A.; Blaney, S.M.; West, J.L. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: An in vitro evaluation using human cell lines. J. Neurooncol. 2008, 86, 165–172. [Google Scholar] [CrossRef]
- Stern, J.M.; Stanfield, J.; Kabbani, W.; Hsieh, J.T.; Cadeddu, J.A. Selective prostate cancer thermal ablation with laser activated gold nanoshells. J. Urol. 2008, 179, 748–753. [Google Scholar] [CrossRef]
- Frémeaux, S.; Noël, C. Une analyse philosophique du management de la RSE: De la difficile conciliation entre l’ordre économique, l’ordre juridique et l’ordre moral. Manag. Avenir 2014, 73, 107. [Google Scholar] [CrossRef]
- Schwartz, J.A.; Price, R.E.; Gill-Sharp, K.L.; Sang, K.L.; Khorchani, J.; Goodwin, B.S.; Payne, J.D. Selective nanoparticle-directed ablation of the canine prostate. Lasers Surg. Med. 2011, 43, 213–220. [Google Scholar] [CrossRef]
- Xu, Y.; Mahmood, M.; Li, Z.; Dervishi, E.; Trigwell, S.; Zharov, V.P.; Ali, N.; Saini, V.; Biris, A.R.; Lupu, D.; et al. Cobalt nanoparticles coated with graphitic shells as localized radio frequency absorbers for cancer therapy. Nanotechnology 2008, 19, 435102. [Google Scholar] [CrossRef]
- Gyergyek, S.; Huskić, M.; Makovec, D.; Drofenik, M. Superparamagnetic nanocomposites of iron oxide in a polymethyl methacrylate matrix synthesized by in situ polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 49–55. [Google Scholar] [CrossRef]
- Xu, Y.; Karmakar, A.; Wang, D.; Mahmood, M.W.; Watanabe, F.; Zhang, Y.; Fejleh, A.; Fejleh, P.; Li, Z.; Kannarpady, G.; et al. Multifunctional Fe3O4 cored magnetic-quantum dot fluorescent nanocomposites for RF nanohyperthermia of cancer cells. J. Phys. Chem. C 2010, 114, 5020–5026. [Google Scholar] [CrossRef]
- Shi, X.; Gong, H.; Li, Y.; Wang, C.; Cheng, L.; Liu, Z. Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials 2013, 34, 4786–4793. [Google Scholar] [CrossRef]











| Type of MNP/MNP with a Surface Coating | Nanoparticle Size | Injection Dose/Nanoparticle Concentration | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Temperature, °C | Cell Death | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Fe3O4 | 20–100 nm | 5 mg of Fe2O3 per gram of tissue | intratumoral | 200 to 240 oersteds, 3 min | RF ablation | dog’s lymph nodes | max 50 °C | N/A | [45] |
| Fe3O4 | 50–100 nm | 5 mg of Fe2O3 per gram of tissue | intratumoral | 55,000 cycles/second, 500 oesterds, 30 min | RF ablation | lymph node metastases by | max 50 °C | N/A | [46] |
| Fe3O4 | 10–20 nm | 21 mg ± 9 of magnetite per 299 mm3 of tumor tissue | intratumoral | 1.2–6.5 kA/m; 400 kHz, 242 s | AMF | human breast tissue | max 79 °C | N/A | [53] |
| Fe3O4 | 10 nm | 5 ± 0.3 mg magnetite per 100 mg of tumor tissue | intratumoral | 400 kHz, 6.5 kA/m, 4 min | AMF | human breast adenocarcinoma cells | max 73 °C at tumor center and 12 °C at tumor periphery | N/A | [20] |
| Fe3O4—dextran coated | 10–20 nm | 107 pg/cells | intratumoral | 410 kHz, 10 kA/m, 242 s | AMF | human breast adenocarcinoma cells | max 71 °C at tumor center | N/A | [54] |
| Fe3O4—starch coated | 11.4 nm ± 0.38 | 0.32 mg Fe mL−1 culture medium | intratumoral | 400 kHz, 24.6 kA m−1 | AMF | breast carcinoma cell line BT474 | to 28.2 ± 0.4 °C | N/A | [51] |
| Fe3O4—amino-silane coated | 15 nm | N/A | intratumoral | 100 kHz, 0–18 kA/m | AMF | RG-2 glioma cells | max 43–47 °C | N/A | [55] |
| Fe3O4 | 20 nm | 11.4 mL per 100 mg of tumor tissue | interstitial | 100 kHz, 2.5–15 kA/m | AMF | prostate cancer | max 55 °C | N/A | [57] |
| Fe3O4—amino-silane coated | 15 nm | 112 mg/mL | transperineally | 100 kHz, 2.5–18.0 kA/m, 60 min | AMF | prostate cancer | max 50 °C | N/A | [56] |
| Fe3O4—amino-silane coated | 15 nm | 200–400 µl of MNP per 0.5 mL/cm3 tumor volume and 120 mg/mL | intratumoral | 100 kHz, 18.0 kA/m | AMF | prostate cancer | max 54.88 °C centrally and 41.28 °C—peripherally | N/A | [58] |
| Type of NP/NP with a Surface Coating | Nanoparticle Size | Injection Dose/Nanoparticle Concentration | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Temperature, °C | Cell Death | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AuNP—anti-EGFR antibody coated | 40 nm | N/A | intratumoral | 57 W/cm2, 514 nm, 4 min | Laser photothermal therapy | Epithelial carcinoma HaCaT cells | N/A | 100% | [72] |
| AuNP—citrate coated | 13 nm | N/A | intratumoral | 10–100 W, 7 min | Radiowave ablation | HepG2 cancerous cells | >50 °C | 80% | [73] |
| AuNP | 5 nm | 67 µM/L | intratumoral | 13.56 MHz, 5 min | RF ablation | Hepatocellular (Hep3B) and Pancreatic cancerous cells (Panc-1) | N/A | 99.8 ± 3.1 Hep3B 96.5 ± 8.4 Panc-1 | [74] |
| AuNP—anti-EGFR coated | N/A | N/A | intratumoral | 200 nW | Laser photothermal therapy | Cancerous cell | 70–80 °C | N/A | [69] |
| AuNP—anti-EGFR coated | 20 nm | N/A | intratumoral | 13.56 MHz, 200 S, 10−15 kV/m | RF ablation | Pancreatic cancerous cell line | N/A | Panc-1 61% | [76] |
| AuNP—PAM4 hemi-antibody coated and AuNP—C225 antibody-coated | 36.9 ± 1.5 nm 32.6 ± 0.7 nm | 100 µg/mL | invivo | 600 W, 10 min | RF ablation | Panc-1 and Capan-1 pancreatic carcinoma cell lines | N/A | N/A | [75] |
| Type of Nanoparticle/Nanoparticle with a Surface Coating | Nanoparticle Size | Injection Dose/Nanoparticle Concentration | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Cell Death | Temperature, °C | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Thioglycolic acid-stabilized CuS NPs | 3 nm | 4.6 g/cm3, 770 µM | intratumoral | 24 W/cm2 for 5 min | photothermal ablation | HeLa cells | 55.6 ± 5.8% | Increased to 12.7 °C | [79] |
| Chitosan-coated HCuSNPs | 10 × 12 nm | intratumoral | photothermal ablation | [81] | |||||
| Phospholipid-PEG coated– CuS NPs | 3.8 nm | 400 mg/mL−1 | intratumoral | 1.0 W cm2, 8 min | Photothermal ablation | HeLa cells | >80% | Max 59.2 °C | [82] |
| PEG coated—CuS NP | N/A | 400 mg/mL, 0.1 mm | intratumoral | 2.5 W/cm2 | Photothermal ablation | Anaplastic thyroid carcinoma | N/A | Max 98 °C | [83] |
| Type of Nanorods/Nanorods with a Surface Coating | Nanorod Size/Concentration | Injection Dose/Nanorods Concentration | Injection Rote | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Cell Death | Temperature, °C | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AuNP | 5 nm | 0.001% volume fraction | intratumoral | 1.25 W/cm2, 300 s | photothermal therapy | Skin tumor | Total cell death | 75 °C at tumor surface, 43–48 °C at tumor depth | [93] |
| PEGylated gold nanorods– C225 antibody | 3 mm | 4 mg/mL | intratumoral | 20 W/cm2 to 90 W/cm2, from 30 s to 3 min | photothermal laser ablation | HTB-9 cells | N/A | N/A | [94] |
| PEGylated gold nanorods | dimensions 12 nm in width and 50 nm in length | N/A | intravenous | 3 min | photothermal therapy | HSC-3 human squamous carcinoma cells | >90% | N/A | [97] |
| PEG-coated gold nanorods | N/A | N/A | intratumoral | 2 W cm–2, 5 min. | photothermal ablation | Cancer tumor | N/A | 50–52 °C | [98] |
| gold nanorods | N/A | N/A | intratumoral | 30 J/cm2, 30 s | photothermal laser ablation | human KB cells | N/A | Increased by 5 °C | [86] |
| PEG-coated gold nanorods | N/A | 20 mg Au/kg in PBS | intravenous | 2 W cm–2, 5 min | photothermal ablation | MDA-MB-435 human cancerouscells | Within 10 days all the irradiated, PEG-NR-targeted tumors completely disappeared | over 70 °C | [91] |
| gold nanorods | N/A | N/A | intratumoral | 20 W/cm2, 4 to 20 min | photothermal ablation | prostate cancerouscells | N/A | N/A | [92] |
| gold nanorods | N/A | N/A | intratumoral | 1.4 and 2 W/cm2, and 0.5, 1, 2, and 5 min | photothermal ablation | MDA-MB-231 human breast cancerous cells | N/A | About 55 °C | [95,96] |
| Type of Carbon Nanotubes/Carbon Nanotubes with a Surface Coating | Carbon Nanotube Size | Injection Dose/Carbon Nanotube Concentration | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Cell Death | Temperature, °C | Reference |
|---|---|---|---|---|---|---|---|---|---|
| SWNT–polymer coated | N/A | 50 mg/mL | intratumoral | 600 W, 13.56 MHz | RF ablation | human cancer cell lines (HepG2, Hep3B and Panc-1 | 100% | Increase by 1.6 °C per second | [114] |
| Carbon nanotube—anti-Her2+ antibody coated | N/A | N/A | intratumoral | 9.5 W cm−2, 4 min | laser ablation | Her2+ human breast carcinoma cancer cells | 90% | N/A | [115] |
| Carbon nanotube—(KFKA)7 –peptide coated | N/A | 0.75 μg/mL for colon26 cells 2.5 μg/mL for HepG2 cells | intratumoral | 30 s | photothermal therapy | Colon and HepG2 cells | N/A | 43 °C | [116] |
| Multi-walled carbon nanotube | 900 nm | N/A | intratumoral | 15.3 W/cm2, 5 min | laser ablation | prostate cancer cell line (PC3) and murine renal cancer cell line (RENCA) | N/A | 43 °C | [117] |
| Carbon nanotube— human albumin protein coated | N/A | N/A | Ex vivo | 5 W/cm2, 20 min | laser-mediated ablation | pancreatic cancer Panc-1 cells | N/A | 29.3 °C at tumor centre | [118] |
| Type of Nanoshells/Nanocomposites (Core/Shell) | Size and/or Core/Shell Thickness | Injection Dose/Concertation | Injection Route | Exposure Conditions | Thermal Ablation Type | Type of Tumor | Temperature, °C | Cell Death | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Gold nanoshells (silica/gold) | 110 nm/10 nm | N/A | intratumoral | 35 W/cm2, 7 min | photothermal therapy | breast cancer SK-BR-3 cells | >37.4 °C | N/A | [119] |
| Gold nanoshells (gold/PEG) | 110 nm/8–10 nm | 100 µL of 2.4 × 1011 nanoshells/mL solution | intratumoral | 10 days | laser ablation | CT26.WT murine colon carcinoma tumor cells | 50 °C | 100% | [121] |
| Gold nanoshells (silica/gold) | 110 ± 11 nm/10 nm | 8.5 µL/gm body weight | intratumoral | 21 days | NIR laser ablation | prostate cancer tumor | 65.4 °C | 93% | [126] |
| anti-HER2—silica core nanoshell anti-IL13Ra2 antibody—silica core nanoshell (silica/gold) | 100 nm and 10 nm | 32.61 and 48.52 mg/g | intratumoral | N/A | photothermal ablation | medulloblastoma and glioma cell lines | N/A | 100% | [126] |
| Gold nanoshells (silica/gold) | 150 nm | N/A | intratumoral | 3.5 W for 3 min | laser ablation | canine prostate cancer | N/A | 100% | [128] |
| Graphitic carbon coated C–Co-NPs | 7 nm | 20 μg mL−1 | intratumoral | 350 kHz, 5 kW, 10 min | RF ablation | HeLa cells | N/A | 98% | [129] |
| Fe3O4 nanoparticles– silica shell (silica/Fe3O4) | N/A | 1.66 µg/mL | intratumoral | 350 kHz, 10 min | RF ablation | Panc-1 cell line | N/A | 98.7– 99.2% | [131] |
| GO-IONP-Au-PEG | N/A | 50 mg/mL | intratumoral | 0.75 W/cm2, 5 min | Laser ablation | 4T1 tumor cells | Max 55 °C | N/A | [132] |
| Type of Nanoparticles/Source of Ablation | RF | MW | Laser Ablation | Photothermal Ablation |
|---|---|---|---|---|
| Magnetic nanoparticle | ||||
| Gold nanoparticle | ||||
| Cu-based nanoparticle | ||||
| Nanorod | ||||
| Carbon nanotubes | ||||
| Nanoshell/Nanocomposite |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashikbayeva, Z.; Tosi, D.; Balmassov, D.; Schena, E.; Saccomandi, P.; Inglezakis, V. Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials 2019, 9, 1195. https://doi.org/10.3390/nano9091195
Ashikbayeva Z, Tosi D, Balmassov D, Schena E, Saccomandi P, Inglezakis V. Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials. 2019; 9(9):1195. https://doi.org/10.3390/nano9091195
Chicago/Turabian StyleAshikbayeva, Zhannat, Daniele Tosi, Damir Balmassov, Emiliano Schena, Paola Saccomandi, and Vassilis Inglezakis. 2019. "Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer" Nanomaterials 9, no. 9: 1195. https://doi.org/10.3390/nano9091195
APA StyleAshikbayeva, Z., Tosi, D., Balmassov, D., Schena, E., Saccomandi, P., & Inglezakis, V. (2019). Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials, 9(9), 1195. https://doi.org/10.3390/nano9091195









