Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Synthesis of Carbon Nanotube Quicklime Nanocomposites
2.3. Material Characterizations
2.4. Modification of Screen-Printed Carbon Electrodes and Electrochemical Measurement
2.4.1. Modification of Screen-Printed Electrodes Using Nanomaterials
2.4.2. Cyclic Voltammetry Studies of the Modified SPCE
3. Results and Discussions
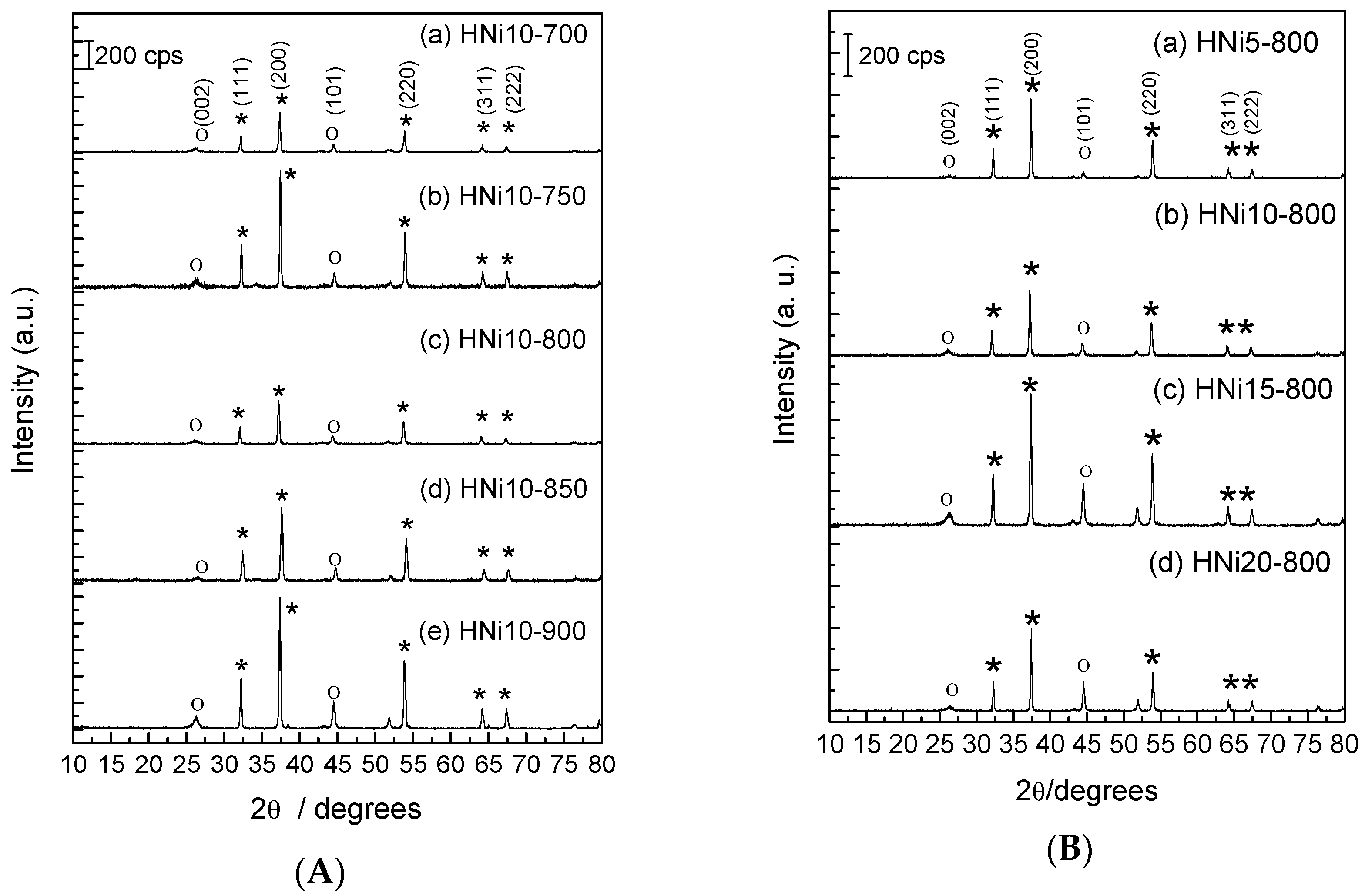
3.1. X-Ray Diffraction Results
3.2. Raman Spectroscopy
3.3. BET Surface Area and Porosity of Ni-Catalyzed CQNs
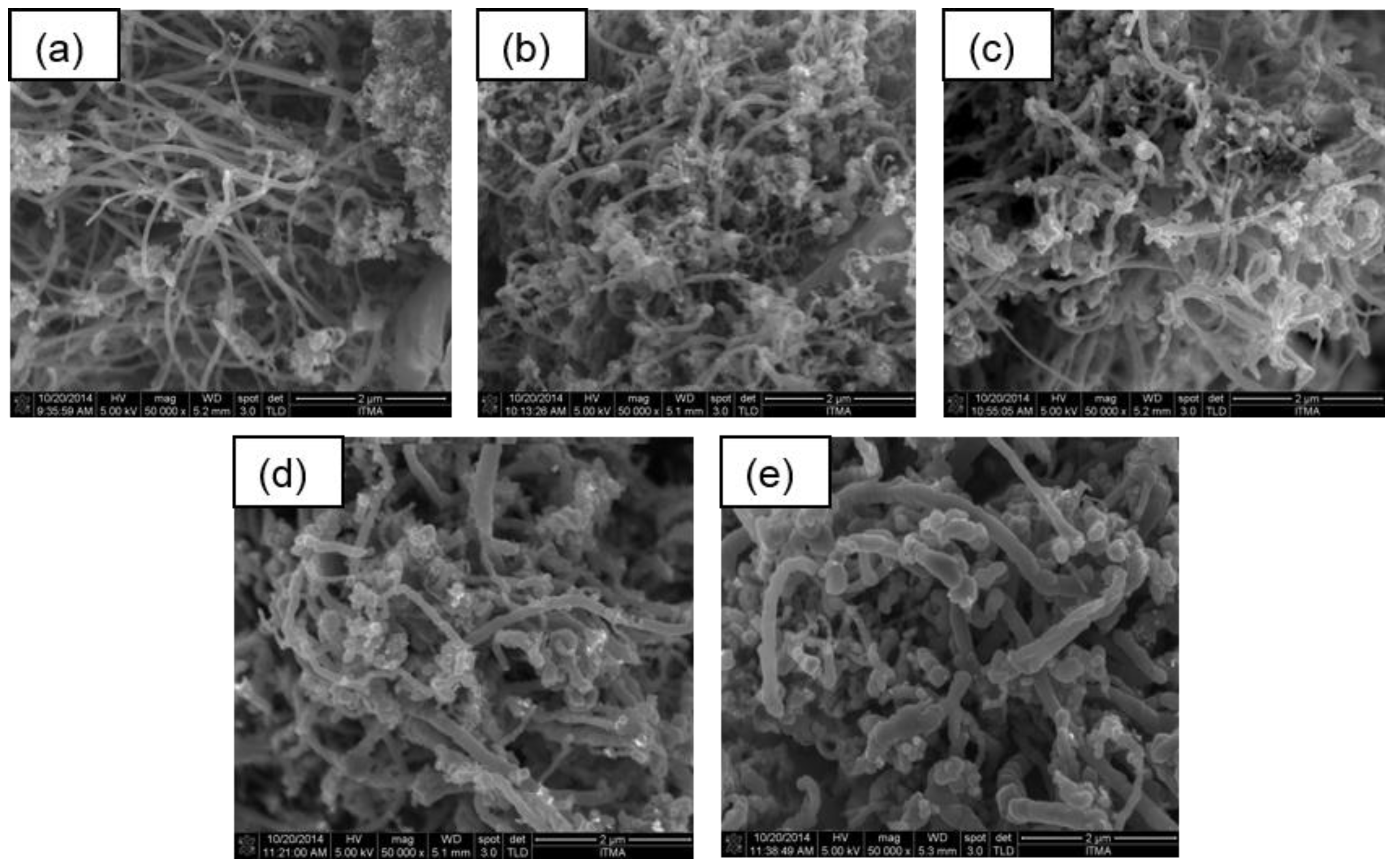
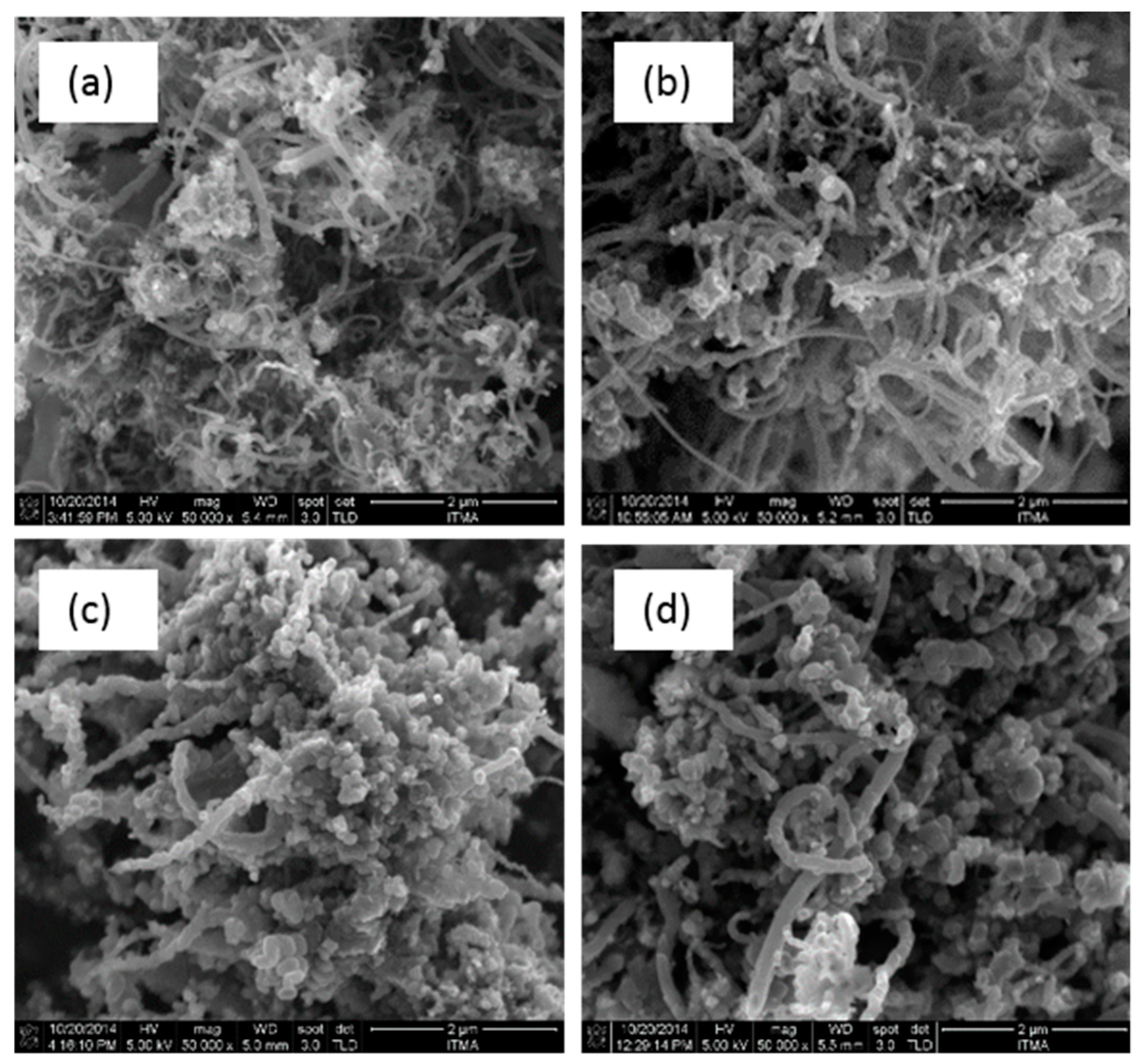
3.4. Field Emission Scanning Electron Microscopy
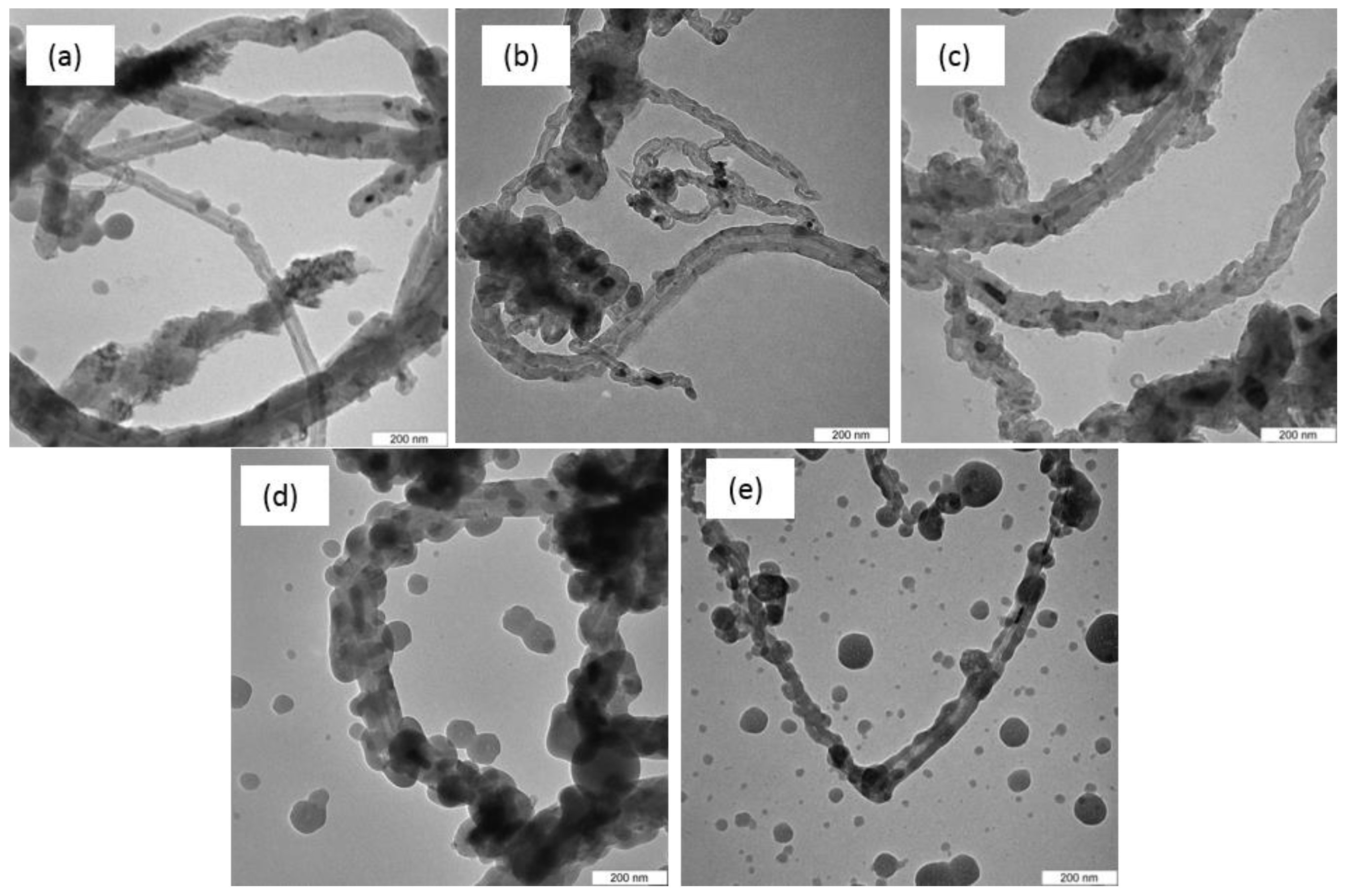
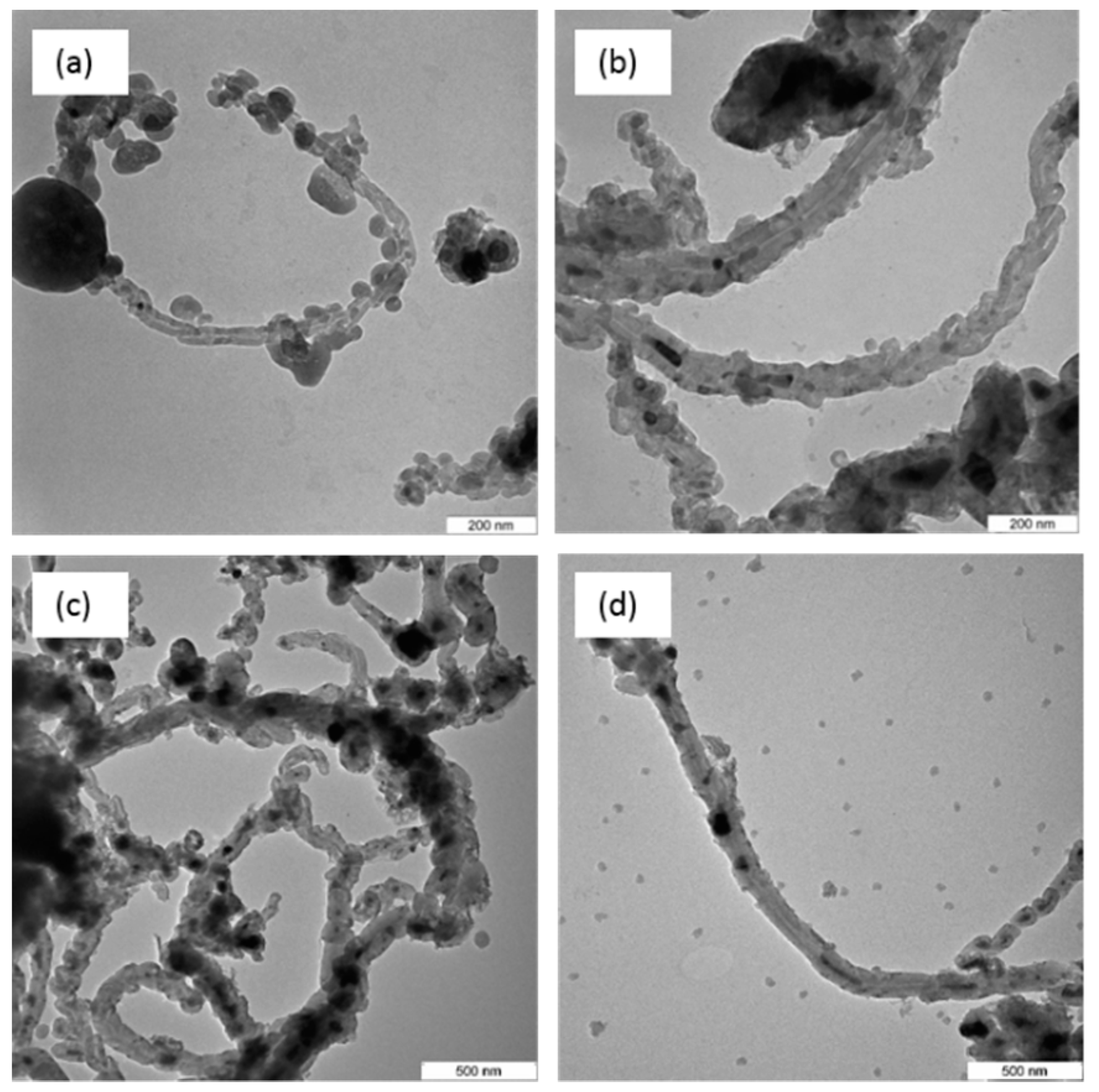
3.5. Transmission Electron Microscopy
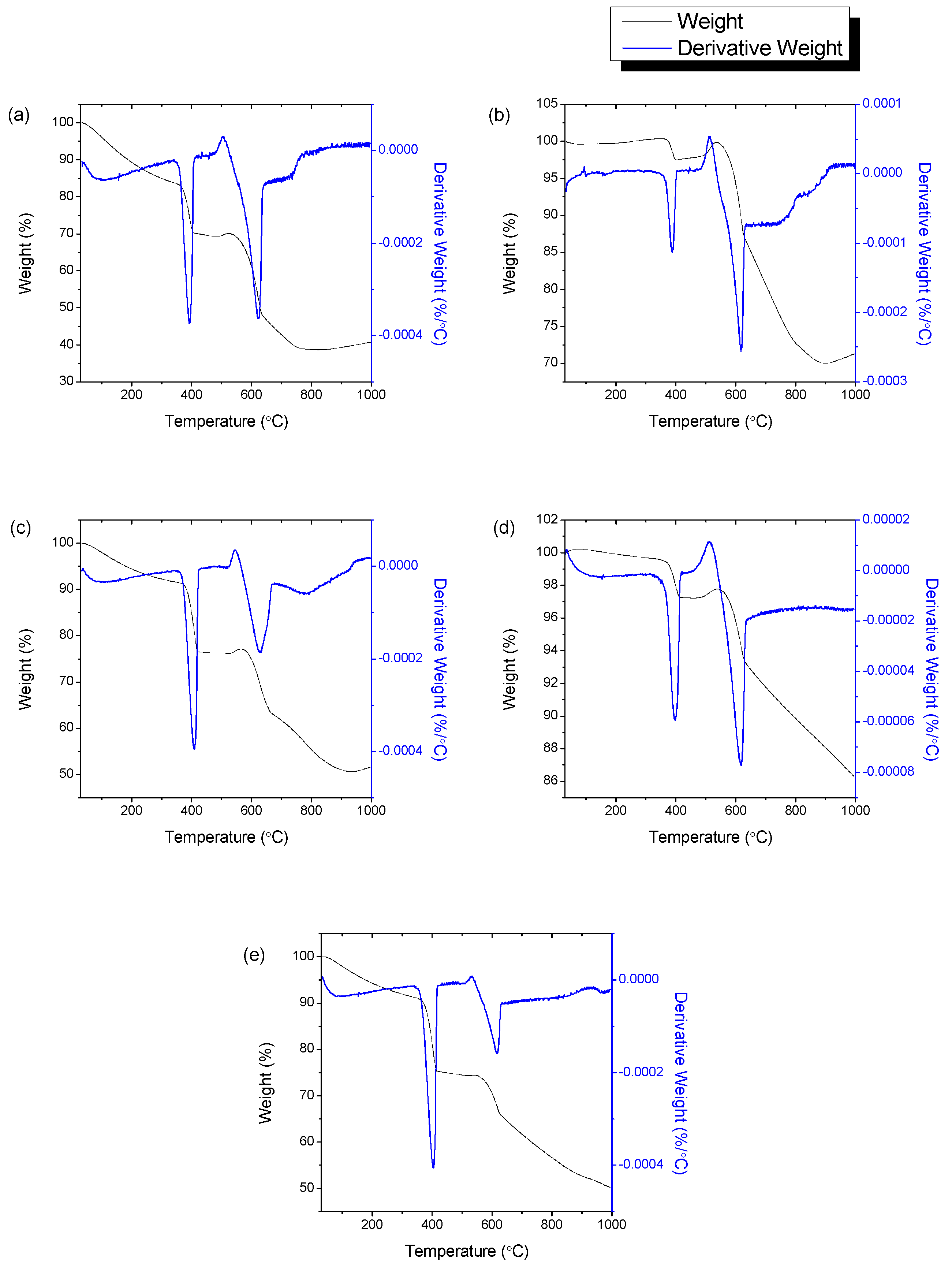
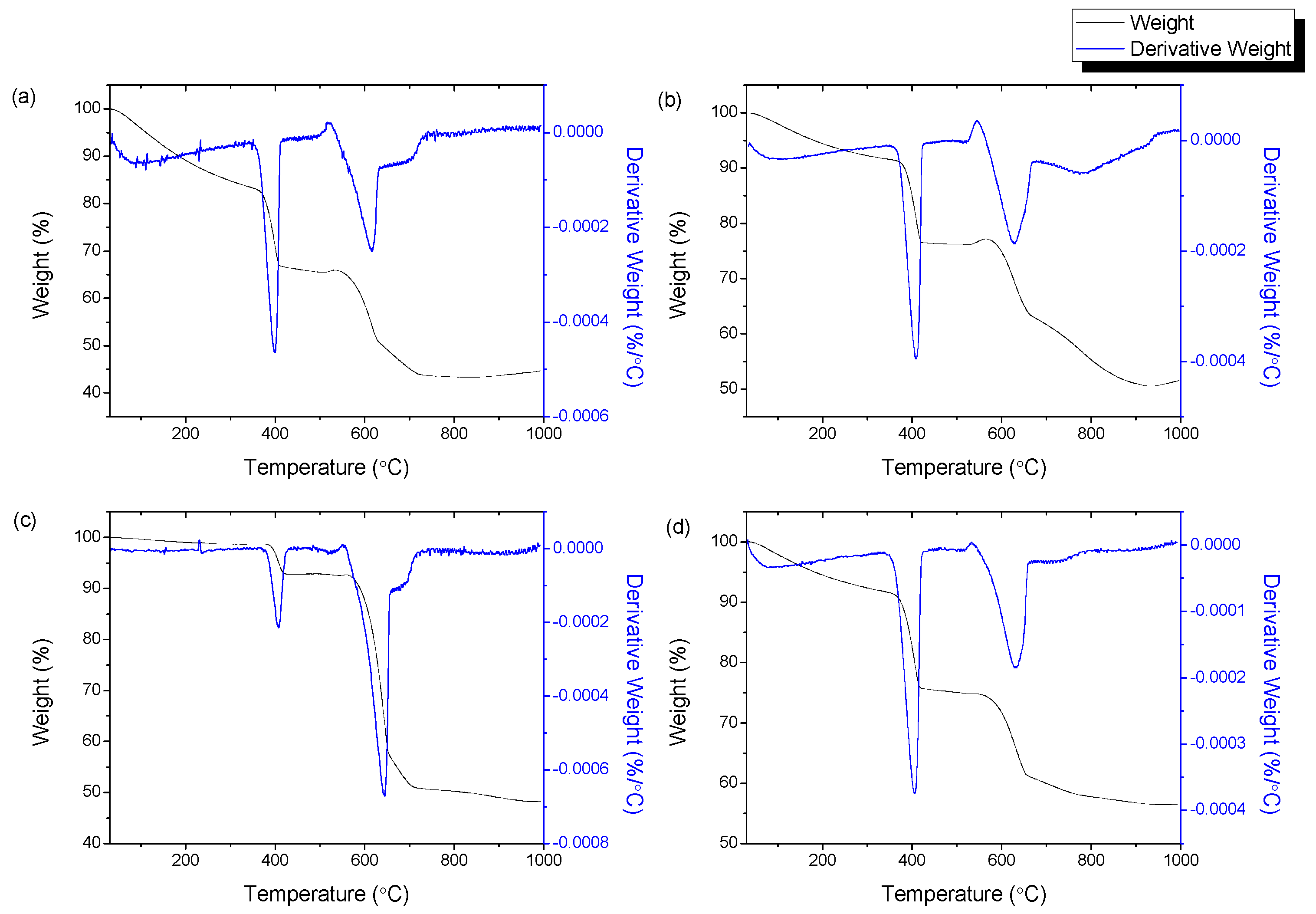
3.6. Thermogravimetric Analysis
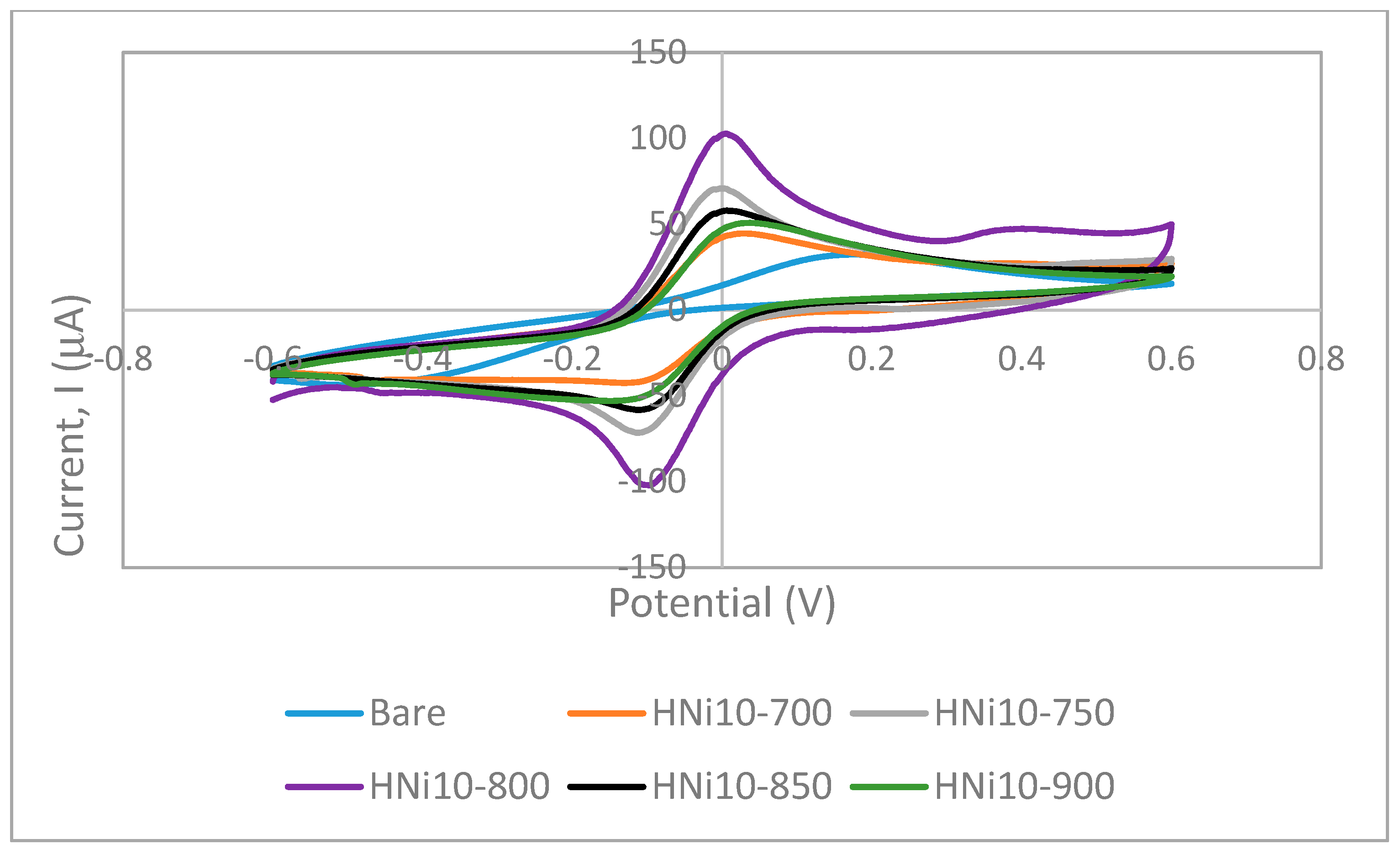
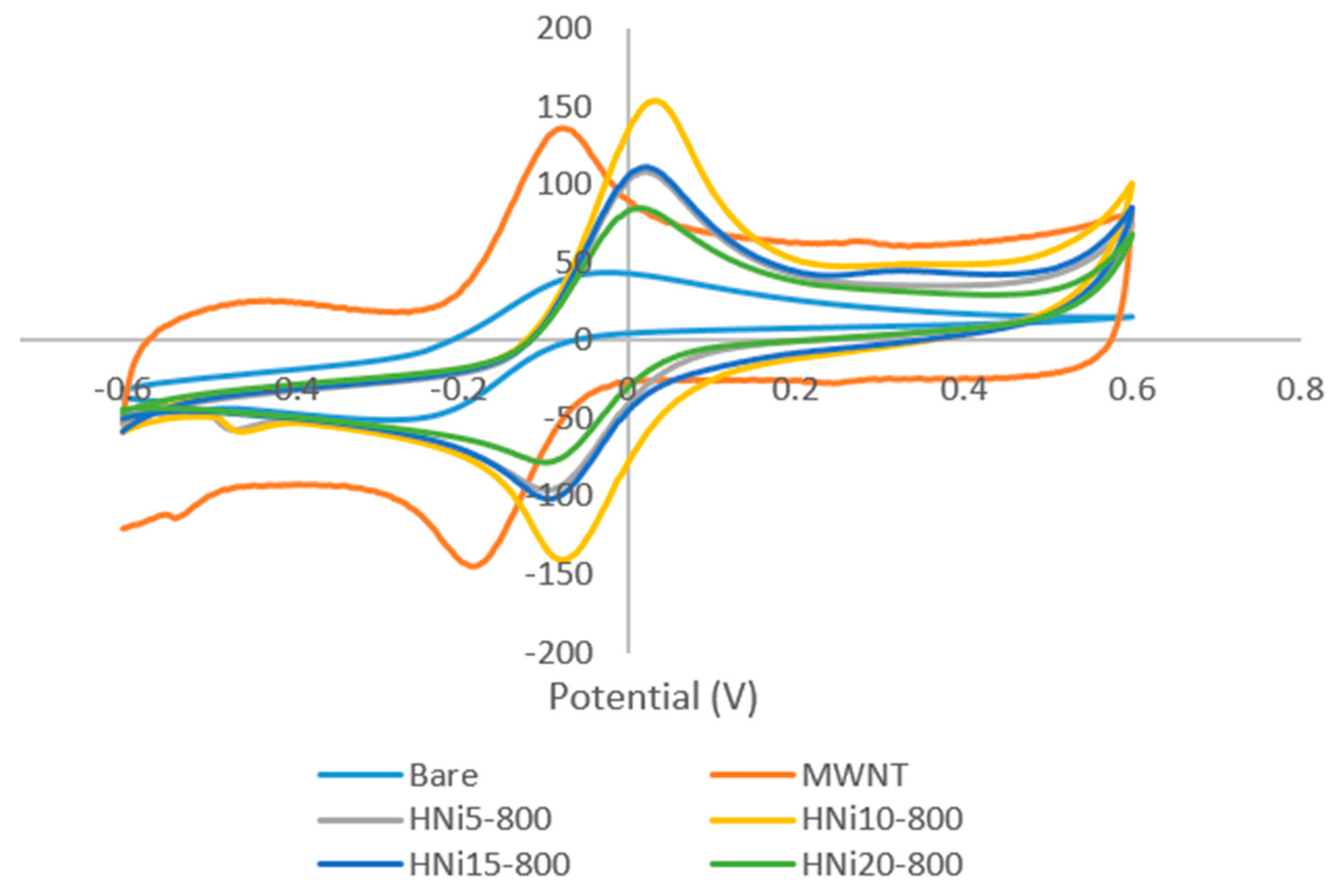
3.7. Cyclic Voltammetry of Nanocomposite-Modified Screen-Printed Electrodes
3.7.1. Electroactive Surface Area of HNi Nanocomposite-Modified Screen-Printed Electrode
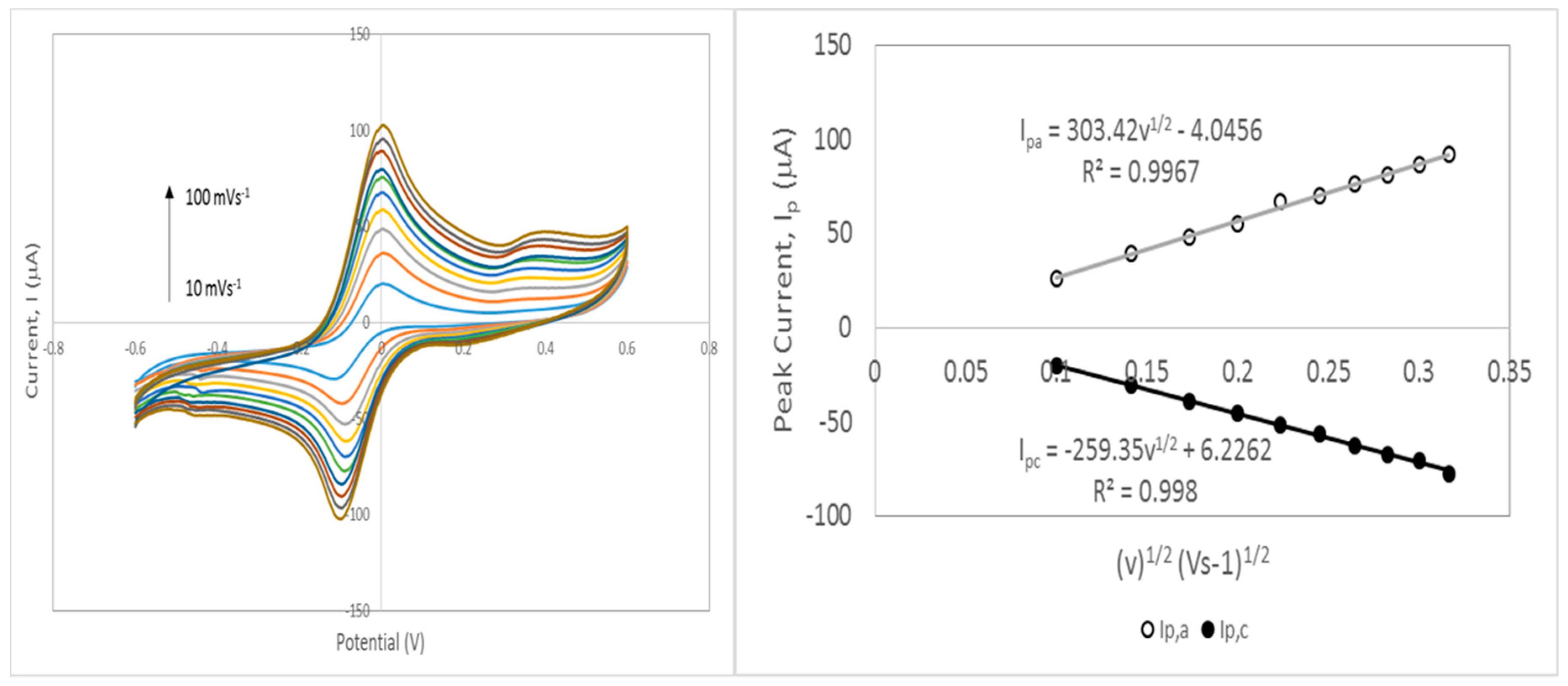
3.7.2. Estimation of Kinetic Parameter: Heterogeneous Electron Transfer Rate Constant (k0)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Liu, J.H.; Huang, X.J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, A.M.S.; Dresselhaus, G.; Hofmann, M.; Fundamentals, C.E.; Jan, D.A.; Hofmann, M. Raman Spectroscopy as a Probe of Graphene and Carbon Nanotubes. Philos. Trans. Math. Phys. Eng. Sci. 2013, 366, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tsoufis, T.; Xidas, P.; Jankovic, L.; Gournis, D.; Saranti, A.; Bakas, T.; Karakassides, M.A. Catalytic production of carbon nanotubes over Fe-Ni bimetallic catalysts supported on MgO. Diam. Relat. Mater. 2007, 16, 155–160. [Google Scholar] [CrossRef]
- Maruyama, S.; Kojima, R.; Miyauchi, Y.; Chiashi, S.; Kohno, M. Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol. Chem. Phys. Lett. 2002, 360, 229–234. [Google Scholar] [CrossRef]
- Mendoza, D.; Santiago, P.; Mendozaa, D.; P’erez, E.R. Carbon nanotubes produced from hexane and ethanol. Rev. Mex. F´IsicaCarta. 2006, 52, 1–5. [Google Scholar]
- Li, Z.; Dervishi, E.; Xu, Y.; Saini, V.; Mahmood, M.; Oshin, O.D.; Biris, A.R.; Biris, A.S. Carbon nanotube growth on calcium carbonate supported molybdenum-transition bimetal catalysts. Catal. Letters 2009, 131, 356–363. [Google Scholar] [CrossRef]
- Buasri, A.; Rochanakit, K.; Wongvitvichot, W. The Application of Calcium Oxide and Magnesium Oxide from Natural Dolomitic Rock for Biodiesel Synthesis; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Radfarnia, H.R.; Iliuta, M.C. Developmentof Zirconium-Stabilized Calcium Oxide Absorbent for Cyclic High-Temperature CO2 Capture. Ind. Eng. Chem. Res. 2012, 51, 10390–10398. [Google Scholar] [CrossRef]
- Sultana, K.S.; Tran, D.T.; Walmsley, J.C.; Ronning, M.; Chen, D. CaO Nanoparticles Coated by ZrO2 Layers for Enhanced CO2 Capture Stability. Ind. Eng. Chem. Res. 2015, 54, 8929–8939. [Google Scholar] [CrossRef]
- Zhuiykov, S. Electrochemistry of Zirconia Gas Sensors; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Wang, Z.L. Nanowires and Nanobelts:Materials, Properties and Devices: Volume 2: Nanowires and Nanobelts of Functional Materials; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Kobun, R.; Siddiquee, S.; Shaarani, S.M. Sensitive Determination of Tartrazine (E 102) Based on Chitosan/Nanoparticles/MWCNTs Modified Gold Electrode in Food and Beverage Products. Trans. Sci. Technol. 2016, 3, 176–180. [Google Scholar]
- Kathyayini, H.; Nagaraju, B.; Fonseca, A.; Nagy, J.B. Catalytic activity of Fe, Co and Fe/Co supported on Ca and Mg oxides, hydroxides and carbonates in the synthesis of carbon nanotubes. Mol. Catal. 2004, 223, 129–136. [Google Scholar] [CrossRef]
- Couteau, E.; Hernadi, K.; Seo, J.W.; Thiên-Nga, L.; Mikó, C.; Gaál, R.; Forró, L. CVD synthesis of high-purity multiwalled carbon nanotubes using CaCO3 catalyst support for large-scale production. Chem. Phys. Lett. 2003, 378, 9–17. [Google Scholar] [CrossRef]
- See, C.H.; Harris, A.T. CaCO3 Supported Co-Fe Catalysts for Carbon Nanotube Synthesis in Fluidized Bed Reactors. AIChE J. 2008, 54, 657–664. [Google Scholar] [CrossRef]
- Dikio, E.D.; Shooto, N.D. Raman and TGA Study of Carbon Nanotubes Synthesized Over Mo / Fe Catalyst on Aluminium Oxide, Calcium Carbonate and Magnesium Oxide Support. Chem. Sci. Trans. 2011, 2, 1160–1173. [Google Scholar]
- Wang, B.; Han, Y.; Liu, S. Effect of highly dispersed carbon nanotubes on the flexural toughness of cement-based composites. Constr. Build. Mater. 2013, 46, 8–12. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsayed, S.H.; Aqel, M. Hybrid effect of carbon nanotube and nano-clay on physico-mechanical properties of cement mortar. Constr. Build. Mater. 2011, 25, 145–149. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Highly dispersed carbon nanotube reinforced cement based materials. Cem. Concr. Res. 2010, 40, 1052–1059. [Google Scholar] [CrossRef]
- Nien, Y.; Huang, C. The mechanical study of acrylic bone cement reinforced with carbon nanotube. Mater. Sci. Eng. B 2010, 169, 134–137. [Google Scholar] [CrossRef]
- Sobolkina, A.; Mechtcherine, V.; Khavrus, V.; Maier, D.; Mende, M.; Ritschel, M.; Leonhardt, A. Dispersion of carbon nanotubes and its influence on the mechanical properties of the cement matrix. Cem. Concr. Compos. 2012, 34, 1104–1113. [Google Scholar] [CrossRef]
- Boey, P.L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef]
- Marinkovic, D.M.; Stankovic, M.V.; Velickovic, A.V.; Avramovic, J.M.; Miladinovic, M.R.; Stamenkovic, O.O.; Veljkovic, V.B.; Jovanovic, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Chen, Y.; Shi, Y.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. Sulfonated multi-walled carbon nanotubes for biodiesel production through triglycerides transesterification. RSC Adv. 2017, 7, 7250–7258. [Google Scholar] [CrossRef]
- Zu, Y.; Tang, J.; Zhu, W.; Zhang, M.; Liu, G.; Liu, Y.; Zhang, W.; Jia, M. Graphite oxide-supported CaO catalysts for transesterification of soybean oil with methanol. Bioresour. Technol. 2011, 102, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, X.; Pan, L.; Li, H.; Sun, Z.; Sun, C.; Tay, K. Carbon nanotube – zinc oxide electrode and gel polymer electrolyte for electrochemical supercapacitors. J. Alloys Compd. 2009, 480, 17–19. [Google Scholar] [CrossRef]
- Sakellariou, K.G.; Karagiannakis, G.; Criado, Y.A.; Konstandopoulos, A.G. Calcium oxide based materials for thermochemical heat storage in concentrated solar power plants. Sol. Energy. 2015, 122, 215–230. [Google Scholar]
- Yen, M.; Hsiao, M.; Liao, S.; Liu, P.; Tsai, H. Preparation of graphene/multi-walled carbon nanotube hybrid and its use as photoanodes of dye-sensitized solar cells. Carbon 2011, 49, 3597–3606. [Google Scholar] [CrossRef]
- Ansaldo, A.; Haluška, M.; Čech, J.; Meyer, J.C.; Ricci, D.; Gatti, F.; di Zitti, E.; Cincotti, S.; Roth, S. A study of the effect of different catalysts for the efficient CVD growth of carbon nanotubes on silicon substrates. Phys. E Low-dimensional Syst. Nanostructures 2007, 37, 6–10. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Kang, J.; Li, J.; Zhao, N.; Du, X.; Shi, C.; Nash, P. The effect of catalyst evolution at various temperatures on carbon nanostructures formed by chemical vapor deposition. J. Mater. Sci. 2009, 44, 2471–2476. [Google Scholar] [CrossRef]
- Kamalakar, G.; Hwang, D.W.; Hwang, L.P. Synthesis and characterization of multiwalled carbon nanotubes produced using zeolite Co-beta. J. Mater. Chem. 2002, 12, 1819–1823. [Google Scholar] [CrossRef]
- Andrews, R.; Jaqques, D.; Qian, D.; Rantell, T. Multiwall carbon nanotubes: Synthesis and application. J. Acc. Chem. Res. 2002, 35, 1008–1017. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-palen, E.; Bachmatiuk, A.; Rümmeli, M.H.; Gemming, T.; Kalenczuk, R.J. Iron filled carbon nanostructures from different precursors. Energy Convers. Manag. 2008, 49, 2483–2486. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, D.; Su, Y.; Li, J.; Jiang, Z.; Jiang, Y.; Yuan, W. Covalent functionalization of multi-walled carbon nanotubes by lipase. J. Nanoparticle Res. 2007, 9, 1205–1210. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Ren, Z.; Wang, G.; Bai, J. Co-production of hydrogen and multi-wall carbon nanotubes from ethanol decomposition over Fe/Al2O3 catalysts. Appl. Catal. B Environ. 2008, 84, 433–439. [Google Scholar] [CrossRef]
- Birch, M.E.; Ruda-eberenz, T.A.; Chai, M.; Andrews, R.; Hatfield, R.L. Properties that Influence the Specific Surface Areas of Carbon Nanotubes and Nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar]
- Alexiadis, V.I.; Boukos, N.; Verykios, X.E. Influence of the composition of Fe2O3/Al2O3 catalysts on the rate of production and quality of carbon nanotubes. Mater. Chem. Phys. 2011, 128, 96–108. [Google Scholar] [CrossRef]
- Alves, J.O.; Zhuo, C.; Levendis, Y.A.; Tenório, J.A.S. Catalytic conversion of wastes from the bioethanol production into carbon nanomaterials. Appl. Catal. B Environ. 2011, 106, 433–444. [Google Scholar] [CrossRef]
- Hoyos-Palacio, L.M.; García, A.G.; Pérez-Robles, J.F.; González, J.; Martínez-Tejada, H.V. Catalytic effect of Fe, Ni, Co and Mo on the CNTs production. IOP Conf. Ser. Mater. Sci. Eng. 2014, 59, 1–8. [Google Scholar] [CrossRef]
- Mizoguti, E.; Nihey, F.; Yudasaka, M.; Iijima, S.; Ichihashi, T.; Nakamura, K. Purification of single-wall carbon nanotubes by using ultrafine gold particles. Chem. Phys. Lett. 2000, 321, 297–301. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Chng, E.L.K. Impurities in graphenes and carbon nanotubes and their influence on the redox properties. Chem. Sci. 2012, 3, 3347. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Chen, C.H.; Chen, L.Y.; Chen, C.C.; Luo, J.D.; Chiou, C.C.; Tian, Y.C.; Lin, C.Y.; Yang, C.M.; Lai, H.C.; et al. Speckled ZnO Nanograss Electrochemical Sensor for Staphylococcus epidermidis Detection. J. Electrochem. Soc. 2017, 164, B205–B211. [Google Scholar] [CrossRef]
- Silva, T.A.; Zanin, H.; Saito, E.; Medeiros, R.A.; Vincentini, F.C.; Corat, E.J.; Fatibello-filho, O. Electrochemical behaviour of vertically aligned carbon nanotubes and graphene oxide nanocomposite as electrode material. Electrochim. Acta 2014, 119, 114–119. [Google Scholar] [CrossRef]
- Bollo, S.; Finger, S.; Sturm, J.C.; Squella, J.A. Cyclic voltammetry and scanning electrochemical microscopy studies of the heterogeneous electron transfer reaction of some nitrosoaromatic compounds. Electrochim. Acta 2007, 52, 4892–4898. [Google Scholar] [CrossRef]
- Mohammad, M.; Aslam, M. Heterogeneous Electron Transfer Studies on the Reduction of Some Pyridinium Cations: Substituents and the Inner-Reorganization Energy. J. Chem. Soc. Pakistan 2011, 33, 12–20. [Google Scholar]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]


| No. | Sample | Catalyst Ni (wt%) | BET * Surface Area (m2/g) | Total Pore Volume (cm3/g) | BJH * Average Pore Diameter(Å) |
|---|---|---|---|---|---|
| 1 | HNi10-700 | 10 | 13 | 0.084 | 260 |
| 2 | HNi10-750 | 10 | 12 | 0.078 | 250 |
| 3 | HNi10-800 | 10 | 10 | 0.069 | 290 |
| 4 | HNi10-850 | 10 | 6 | 0.051 | 340 |
| 5 | HNi10-900 | 10 | 7 | 0.063 | 360 |
| 6 | HNi5-800 | 5 | 8 | 0.067 | 330 |
| 7 | HNi15-800 | 15 | 11 | 0.069 | 240 |
| 8 | HNi20-800 | 20 | 8 | 0.070 | 370 |
| Sample | Weight Loss 1 | Weight Loss 2 | ||
|---|---|---|---|---|
| T (°C) | wt% | T (°C) | wt% | |
| HNi10-700 | 350–373 | 13.11 | 537–576 | 14.06 |
| HNi10-750 | 330–423 | 2.73 | 537–655 | 15.26 |
| HNi10-800 | 200–477 | 18.35 | 573–708 | 15.78 |
| HNi10-850 | 300–452 | 2.98 | 540–600 | 11.47 |
| HNi10-900 | 357–381 | 13.90 | 549–609 | 26.77 |
| HNi5-800 | 345–369 | 17.24 | 537–579 | 8.68 |
| HNi15-800 | 349–376 | 8.43 | 540–581 | 5.76 |
| HNi20-800 | 342–367 | 13.46 | 523–568 | 8.83 |
| Electrode | Geometrical Area (cm2) | Electroactive Area (cm2) | Roughness Factor |
|---|---|---|---|
| Bare | 0.0707 | 0.098 ± 0.010 | 1.39 |
| HNi10-700 | 0.0707 | 0.168 ± 0.031 | 2.37 |
| HNi10-750 | 0.0707 | 0.334 ± 0.053 | 4.73 |
| HNi10-800 | 0.0707 | 0.380 ± 0.051 | 5.37 |
| HNi10-850 | 0.0707 | 0.277 ± 0.050 | 3.93 |
| HNi10-900 | 0.0707 | 0.188 ± 0.026 | 2.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, R.; Hussein, M.Z.; Yusof, N.A.; Abu Bakar, F. Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones. Nanomaterials 2019, 9, 1239. https://doi.org/10.3390/nano9091239
Ibrahim R, Hussein MZ, Yusof NA, Abu Bakar F. Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones. Nanomaterials. 2019; 9(9):1239. https://doi.org/10.3390/nano9091239
Chicago/Turabian StyleIbrahim, Ruzanna, Mohd Zobir Hussein, Nor Azah Yusof, and Fatimah Abu Bakar. 2019. "Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones" Nanomaterials 9, no. 9: 1239. https://doi.org/10.3390/nano9091239
APA StyleIbrahim, R., Hussein, M. Z., Yusof, N. A., & Abu Bakar, F. (2019). Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones. Nanomaterials, 9(9), 1239. https://doi.org/10.3390/nano9091239





