Flower-Like MoSe2/MoO2 Composite with High Capacity and Long-Term Stability for Lithium-Ion Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization
2.3. Electrochemical Measurements
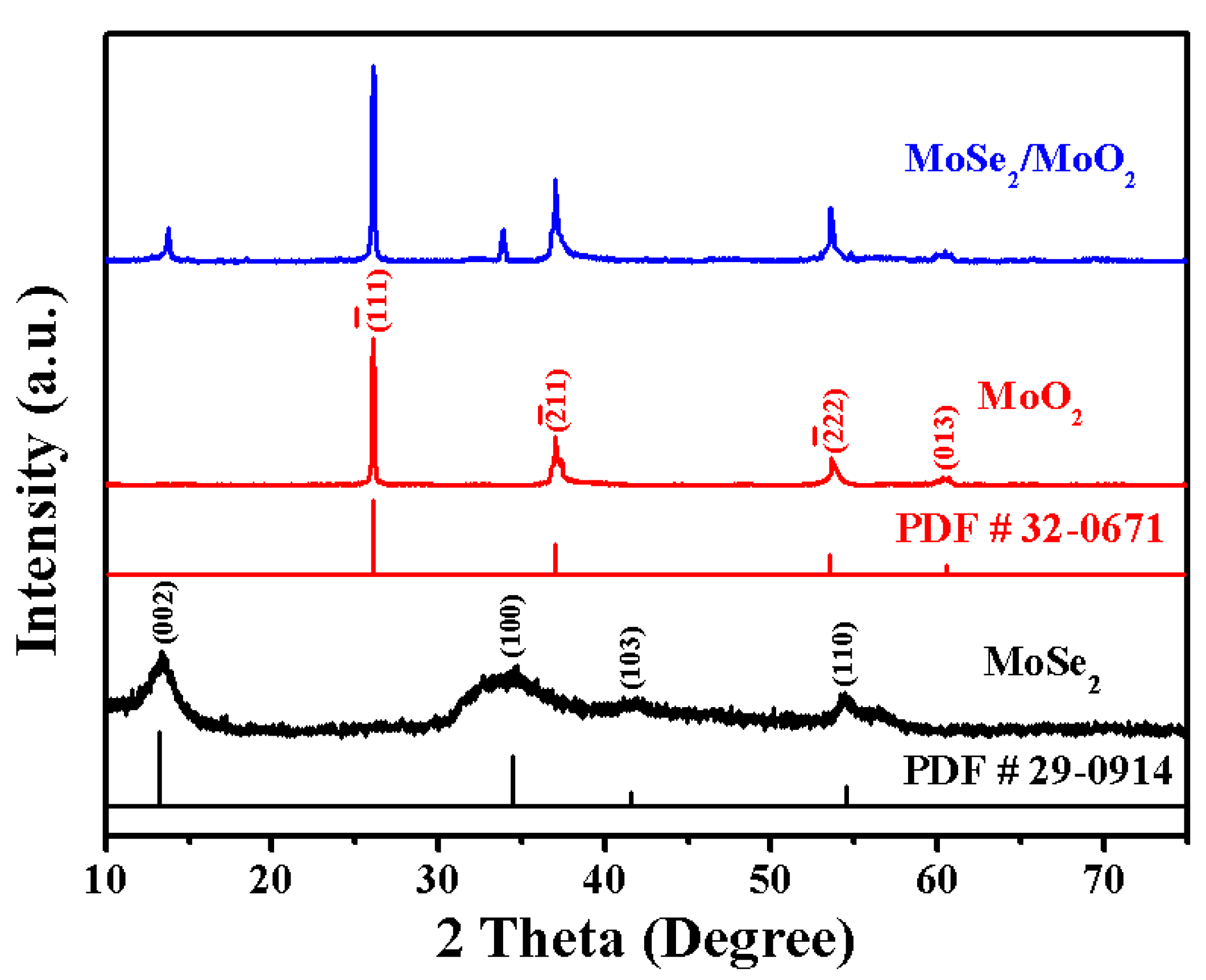
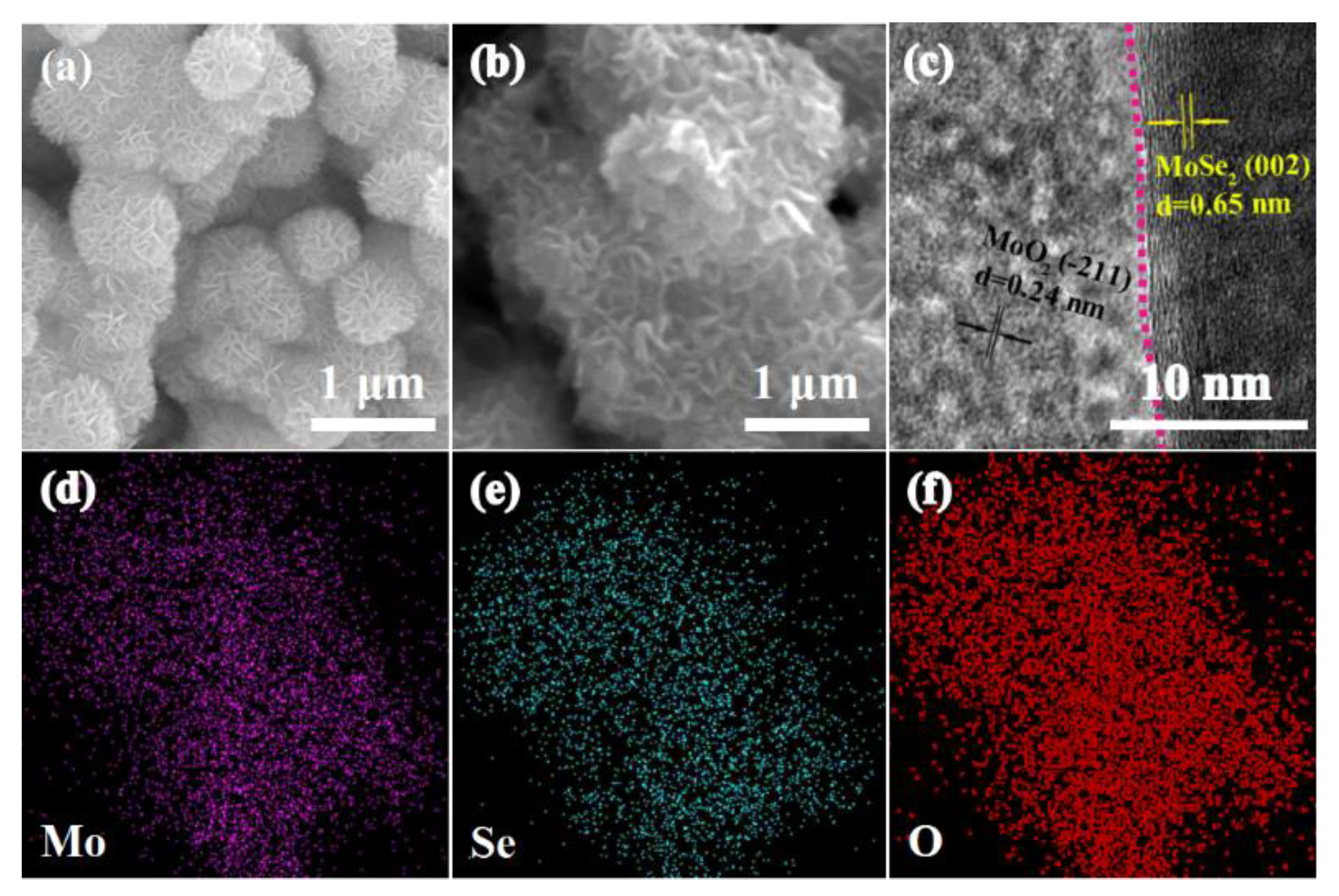
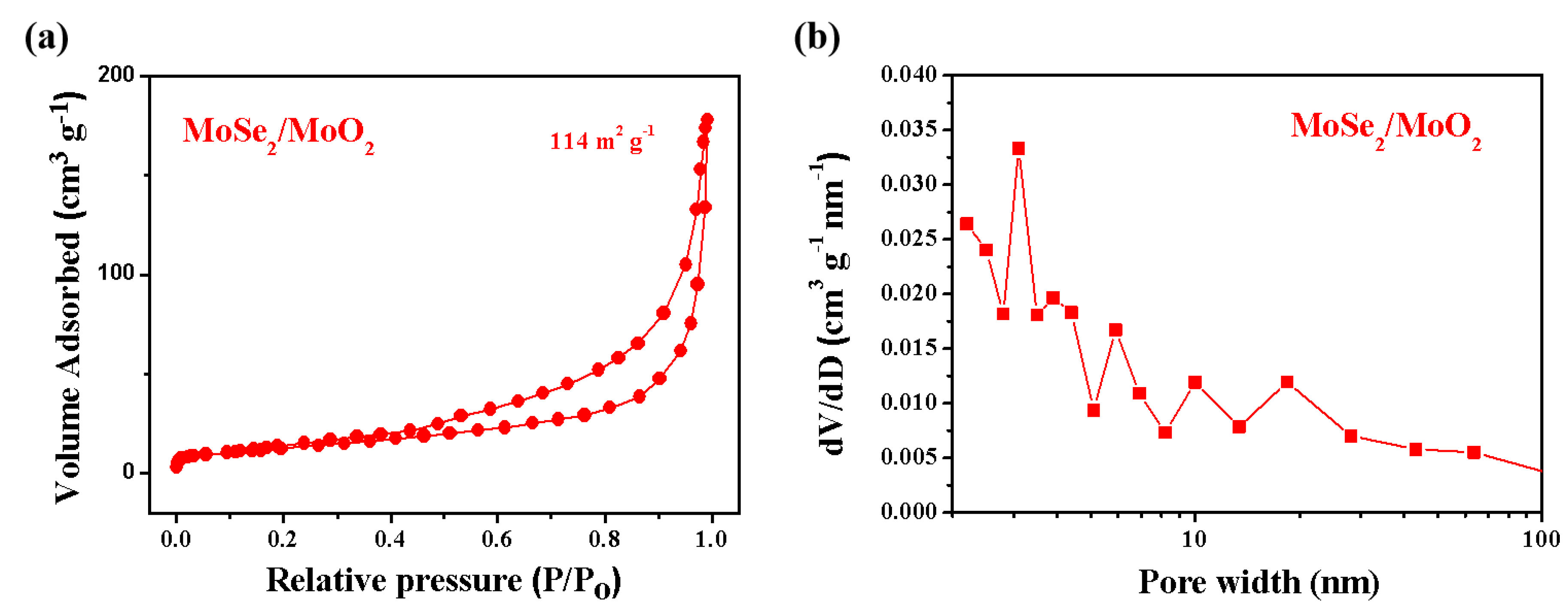
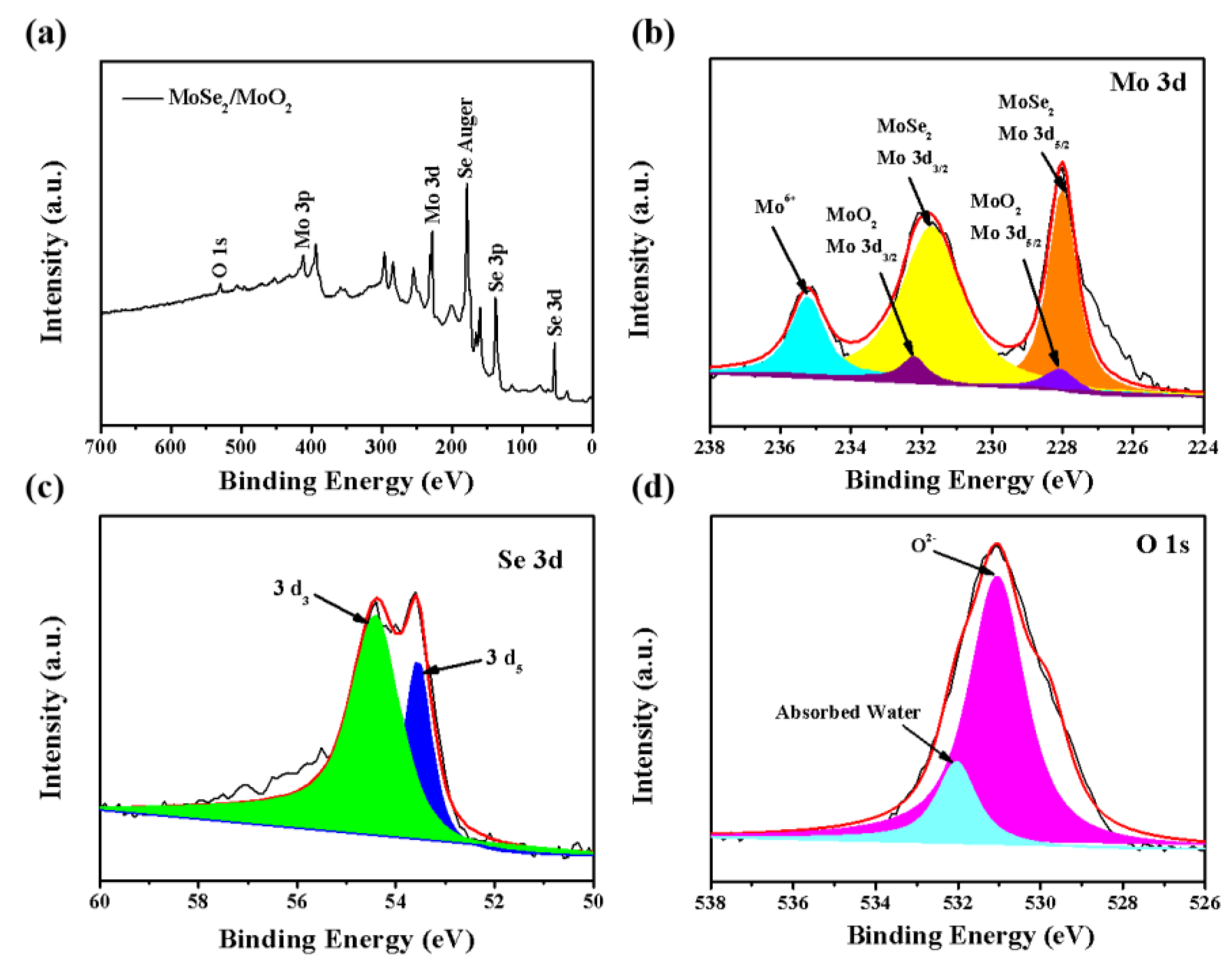
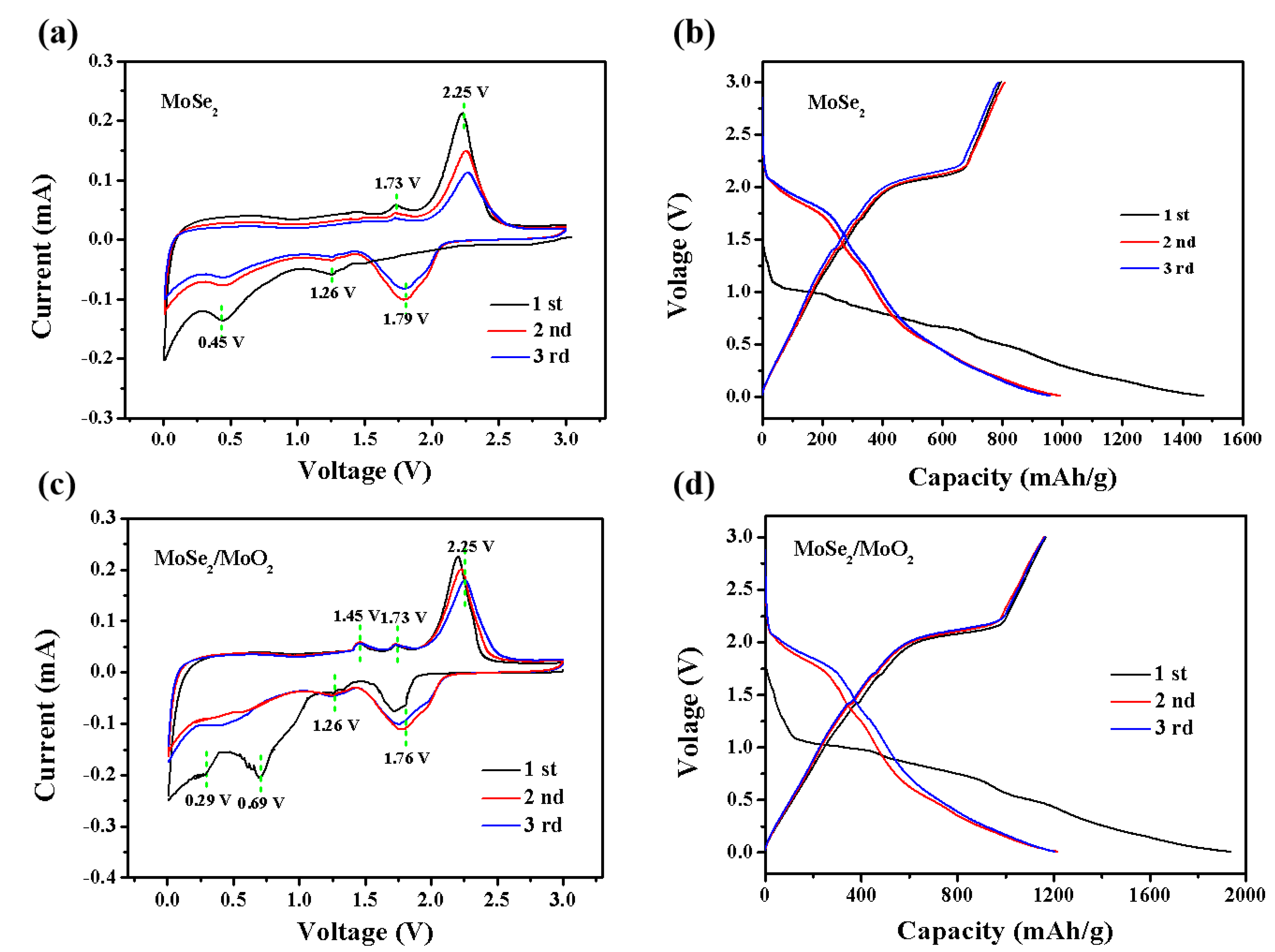
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Yan, L.; Luo, H.; Guo, S. Nanoscale engineering of heterostructured anode materials for boosting lithium-ion storage. Adv. Mater. 2016, 28, 7580–7602. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, B.; Lu, Z.; Liu, N.; Jia, Z. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Yu, S.H.; Feng, X.; Zhang, N.; Seok, J.; Abruña, H.D. Understanding conversion-type electrodes for lithium rechargeable batteries. Acc. Chem. Res. 2018, 51, 273–281. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A review on design strategies for carbon based metal oxides and sulfides nanocomposites for high performance Li and Na ion battery anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar]
- Wang, S.; Guan, B.Y.; Yu, L.; Lou, X.W. Rational design of three-layered TiO2@Carbon@MoS2 hierarchical nanotubes for enhanced lithium storage. Adv. Mater. 2017, 29, 1702724. [Google Scholar] [CrossRef]
- Zhou, X.; Le, Y.; Xiong, W.D.L. Formation of uniform N-doped carbon-coated SnO2 submicroboxes with enhanced lithium storage properties. Adv. Energy Mater. 2016, 6, 1600451. [Google Scholar] [CrossRef]
- Gang, H.; Feifei, Z.; Xinchuan, D.; Yuling, Q.; Dongming, Y.; Limin, W. Metal organic frameworks route to in situ insertion of multiwalled carbon nanotubes in Co3O4 polyhedra as anode materials for lithium-ion batteries. ACS Nano 2015, 9, 1592–1599. [Google Scholar]
- Xue, H.; Jie, W.; Wang, S.S.; Muhammad, S.; Yun, Z. Core-shell MoS2@graphene composite microspheres as stable anode for Li-ion batteries. New J. Chem. 2018, 42, 15340–15345. [Google Scholar] [CrossRef]
- Le, S.; Zhao, T. Recent advances in inorganic 2D materials and their applications in lithium and sodium batteries. J. Mater. Chem. A 2017, 5, 3735–3758. [Google Scholar]
- Ge, P.; Hou, H.; Banks, C.E.; Foster, C.W.; Li, S.; Zhang, Y.; He, J.; Zhang, C.; Ji, X. Binding MoSe2 with carbon constrained in carbonous nanosphere towards high-capacity and ultrafast Li/Na-ion storage. Energy Storage Mater. 2018, 12, 310–323. [Google Scholar] [CrossRef]
- Zhang, P.; Qin, F.; Zou, L.; Wang, M.; Zhang, K.; Lai, Y.; Li, J. Few-layered MoS2/C with expanding d-spacing as a high-performance anode for sodium-ion batteries. Nanoscale 2017, 9, 12189–12195. [Google Scholar] [CrossRef]
- Zhu, M.; Luo, Z.; Pan, A.; Yang, H.; Zhu, T.; Liang, S.; Cao, G. N-doped one-dimensional carbonaceous backbones supported MoSe2 nanosheets as superior electrodes for energy storage and conversion. Chem. Eng. J. 2018, 334, 2190–2200. [Google Scholar] [CrossRef]
- Tang, W.; Xie, D.; Shen, T.; Wang, X.; Wang, D.; Zhang, X.; Xia, X.; Wu, J.; Tu, J. Smart construction of nitrogen-doped carbon coated MoSe2 microspheres with enhanced performance for lithium storage. Chem. Eur. J 2017, 23, 12924–12929. [Google Scholar] [CrossRef]
- Zhao, X.; Sui, J.; Li, F.; Fang, H.; Wang, H.; Li, J.; Cai, W.; Cao, G. Lamellar MoSe2 nanosheets embedded with MoO2 nanoparticles: novel hybrid nanostructures promoted excellent performances for lithium ion batteries. Nanoscale 2016, 8, 17902. [Google Scholar] [CrossRef]
- Jin, R.; Cui, Y.; Wang, Q.; Li, G. Facile fabrication of CNTs@C@MoSe2@Se hybrids with amorphous structure for high performance anode in lithium-ion batteries. J. Colloid Interface Sci. 2017, 508, 435–442. [Google Scholar] [CrossRef]
- Kang, J.; Su, Q.; Feng, H.; Huang, P.; Du, G.; Xu, B. MoSe2 nanosheets-wrapped flexible carbon cloth as binder-free anodes for high-rate lithium and sodium ion storages. Electrochim. Acta 2019, 301, 29–38. [Google Scholar] [CrossRef]
- Qin, F.; Hu, H.; Jiang, Y.; Kai, Z.; Zhao, F.; Lai, Y.; Jie, L. Mesoporous MoSe2/C composite as anode material for sodium/lithium ion batteries. J. Electroanal. Chem. 2018, 823, 67–72. [Google Scholar] [CrossRef]
- Eftekhari, A. Molybdenum diselenide (MoSe2) for energy storage, catalysis, and optoelectronics. Appl. Mater. Today 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Xiang, T.; Tao, S.; Xu, W.; Fang, Q.; Wu, C.; Liu, D.; Zhou, Y.; Khalil, A.; Muhammad, Z.; Chu, W. Stable 1T-MoSe2 and carbon nanotubes hybridized flexible film: Binder-free and high-performance Li-ion anode. ACS Nano 2017, 11, 6483–6491. [Google Scholar] [CrossRef]
- Xie, D.; Xia, X.H.; Tang, W.J.; Zhong, Y.; Wang, Y.D.; Wang, D.H.; Wang, X.L.; Tu, J.P. Novel carbon channels from loofah sponge for construction of metal sulfide/carbon composites with robust electrochemical energy storage. J. Mater. Chem. A 2017, 5, 7578–7585. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, H.; Guan, C.; Cheng, C. Ultrathin MoS2 nanosheets@ metal organic framework-derived n-doped carbon nanowall arrays as sodium ion battery anode with superior cycling life and rate capability. Adv. Funct. Mater. 2017, 27, 1702116. [Google Scholar] [CrossRef]
- Cong, L.; Xie, H.; Li, J. Hierarchical structures based on two-dimensional nanomaterials for rechargeable lithium batteries. Adv. Energy Mater. 2017, 7, 1601906. [Google Scholar] [CrossRef]
- Deng, M.; Qi, J.; Xiang, L.; Xiao, Y.; Yang, L.; Xiang, Y.; Hui, W.; Yuan, B.; Gao, Q. MoC/C nanowires as high-rate and long cyclic life anode for lithium ion batteries. Electrochim. Acta 2018, 277, 205–210. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Liu, H.; Zhang, X.; Wang, J.G. Constructing hierarchical MoO2/N-doped carbon hydrangea-like spheres with superior lithium storage properties. J. Alloys Compd. 2019, 787, 45–52. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Q.; Gao, Y.; Zhang, H.; Xu, H.; Zhang, Y. Hierarchical MoO2/C microspheres: Preparation and application as anode materials for lithium ion batteries. J. Alloys Compd. 2017, 723, 1113–1120. [Google Scholar] [CrossRef]
- Xu, G.; Ping, L.; Ren, Y.; Huang, X.; Wang, H. Three-dimensional MoO2 nanotextiles assembled from elongated nanowires as advanced anode for Li ion batteries. J. Power Sources 2017, 361, 1–8. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, B.; Zheng, S.; Yang, X.; Jin, W. 3D interconnected MoO2 nanocrystals on nickel foam as binder-free anode for Li-ion batteries. Mater. Sci. Ed. 2018, 33, 1315–1322. [Google Scholar] [CrossRef]
- Fu, H.; Xu, Z.; Wang, T.; Li, K.; Shen, X.; Li, J.; Huang, J. Rate behavior of MoO2/graphene oxide lithium-ion battery anodes from electrochemical contributions. J. Electrochem. Soc. 2018, 165, A439–CA447. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhi, G.; Xu, G.; Wang, Q.; Zhang, J. 2D layered mesoporous MoO2/rGO composites for high performance anode materials in lithium-ion battery. Microporous Mesoporous Mater. 2017, 246, 14–23. [Google Scholar] [CrossRef]
- Wu, S.; Yao, D.; Sun, S. Transition metal dichalcogenide based nanomaterials for rechargeable batteries. Chem. Eng. J. 2017, 307, 189–207. [Google Scholar] [CrossRef]
- Yu, Z.; Hui, X.; Wang, C.; He, Q.; Qin, L.; Muhammad, Z.; Haleem, Y.A.; Yuan, S.; Chen, S.; Li, S. Probing lithium storage mechanism of MoO2 nanoflowers with rich oxygen-vacancy grown on graphene sheets. J. Phys. Chem. C 2017, 121, 15589–15596. [Google Scholar]
- Yun, Y.; Shi, Z.; Shao, J.; Qu, Q.; Gao, Y.; Chen, Z.; Chen, Y.; Zheng, H. Strongly surface-bonded MoO2@Carbon nanocomposites by nitrogen-doping with outstanding capability for fast and stable Li storage. Chem. Nanostruct. Mater. 2018, 4, 1247–1253. [Google Scholar] [CrossRef]
- Li, Q.; Qi, Y.; Zhao, Y.; Wan, B. Carbon-based coating containing ultrafine MoO2 nanoparticles as an integrated anode for high-performance lithium-ion batteries. J. Nano Res. 2017, 19, 332. [Google Scholar] [CrossRef]
- Zhou, E.; Wang, C.; Shao, M.; Deng, X.; Xu, X. MoO2 nanoparticles grown on carbon fibers as anode materials for lithium-ion batteries. Ceram. Int. 2017, 43, 760–765. [Google Scholar] [CrossRef]
- Hui, W.; Wang, X.; Li, W.; Jin, W.; Jiang, D.; Li, G.; Yan, Z.; Zhong, H.; Yang, J. Phase transition mechanism and electrochemical properties of nanocrystalline MoSe2 as anode materials for the high performance lithium-ion battery. J. Phys. Chem. C 2015, 119, 10197–10205. [Google Scholar]
- Xing, Y.; Zhang, Z.; Shi, X. Rational design of coaxial-cable MoSe2/C: Towards high performance electrode materials for lithium-ion and sodium-ion batteries. J. Alloys Compd. 2016, 686, 413–420. [Google Scholar]
- Yao, J.; Liu, B.; Ozden, S.; Wu, J.; Yang, S.; Rodrigues, M.T.F.; Kalaga, K.; Pei, D.; Peng, X.; Zhang, Y. 3D nanostructured molybdenum diselenide/graphene foam as anodes for long-cycle life lithium-ion batteries. Electrochim. Acta 2015, 176, 103–111. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Chen, D. Sheet-like MoSe2/C composites with enhanced Li-ion storage properties. J. Mater. Chem. A 2015, 3, 11857–11862. [Google Scholar] [CrossRef]
- Ying, W.; Huang, Z.; Wang, Y. A new approach to synthesize MoO2@C for high-rate lithium ion batteries. J. Mater. Chem. A 2015, 3, 21314–21320. [Google Scholar]
- Huang, B.; Li, X.; Pei, Y.; Li, S.; Cao, X.; Massé, R.C.; Cao, G. Novel carbon-encapsulated porous SnO2 anode for lithium-ion batteries with much improved cyclic stability. Small 2016, 12, 1945–1955. [Google Scholar] [CrossRef]
- Devina, W.; Hwang, J.; Kim, J. Synthesis of MoO2/Mo2C/RGO composite in supercritical fluid and its enhanced cycling stability in Li-ion batteries. Chem. Eng. J. 2018, 345, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Rui, X.; Tang, Y.; Liu, Y.; Chen, X. Wet-chemical processing of phosphorus composite nanosheets for high-rate and high-capacity lithium-ion batteries. Adv. Energy Mater. 2016, 6, 1502409. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Q.; Cui, G.; Zhao, Y.; Bakenov, Z. Flower-Like MoSe2/MoO2 Composite with High Capacity and Long-Term Stability for Lithium-Ion Battery. Nanomaterials 2019, 9, 1256. https://doi.org/10.3390/nano9091256
Hao Q, Cui G, Zhao Y, Bakenov Z. Flower-Like MoSe2/MoO2 Composite with High Capacity and Long-Term Stability for Lithium-Ion Battery. Nanomaterials. 2019; 9(9):1256. https://doi.org/10.3390/nano9091256
Chicago/Turabian StyleHao, Qiuyan, Guoliang Cui, Yan Zhao, and Zhumabay Bakenov. 2019. "Flower-Like MoSe2/MoO2 Composite with High Capacity and Long-Term Stability for Lithium-Ion Battery" Nanomaterials 9, no. 9: 1256. https://doi.org/10.3390/nano9091256
APA StyleHao, Q., Cui, G., Zhao, Y., & Bakenov, Z. (2019). Flower-Like MoSe2/MoO2 Composite with High Capacity and Long-Term Stability for Lithium-Ion Battery. Nanomaterials, 9(9), 1256. https://doi.org/10.3390/nano9091256




