The Effect of the Kraft Pulping Process, Wood Species, and pH on Lignin Recovery from Black Liquor
Abstract
:1. Introduction
2. Experiment
2.1. Material
2.2. Methods
2.2.1. Kraft Pulping
2.2.2. Lignin Precipitation
2.2.3. Characterization of Wood, Pulp, and Precipitated Lignin
2.2.4. Material Balance
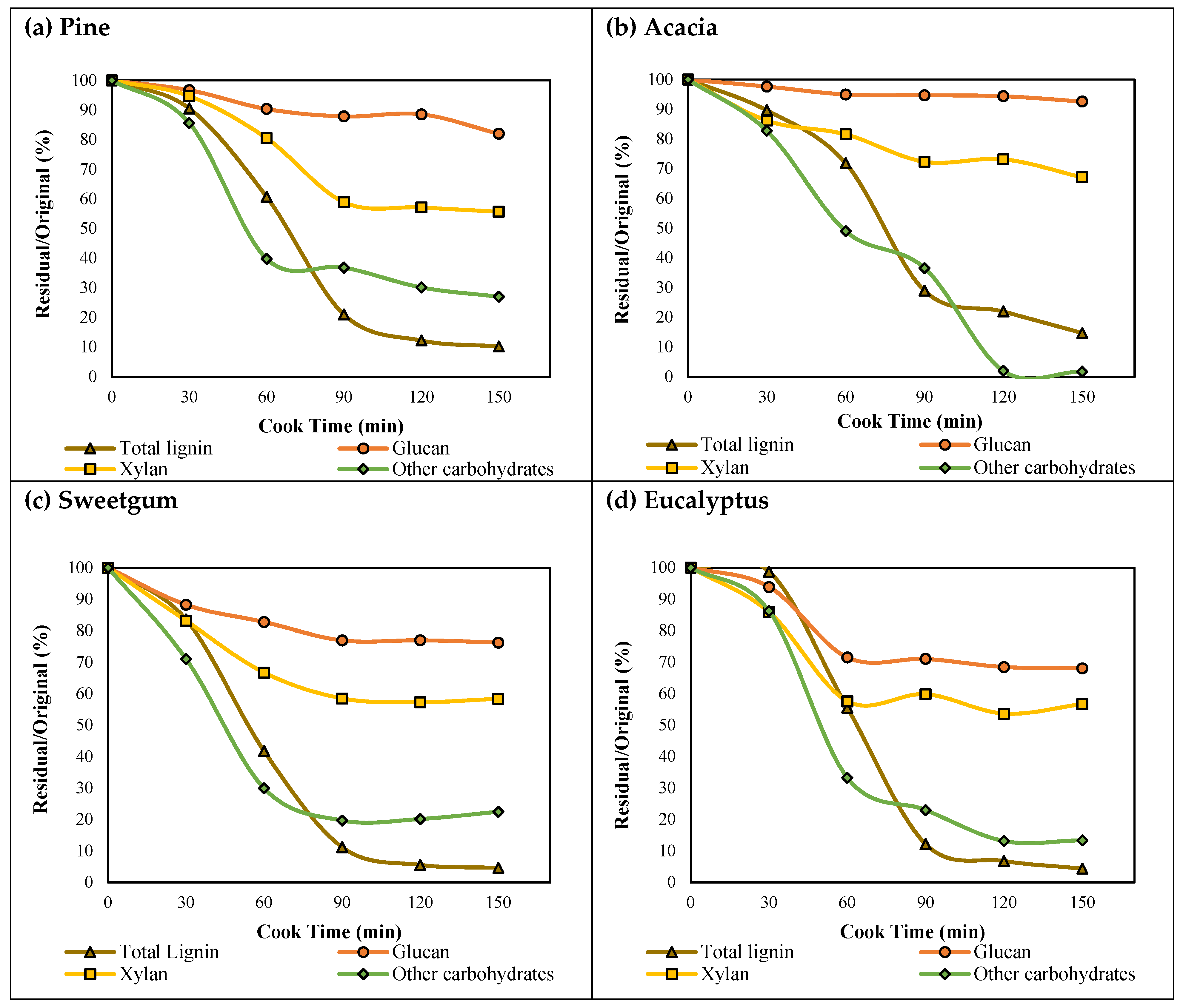
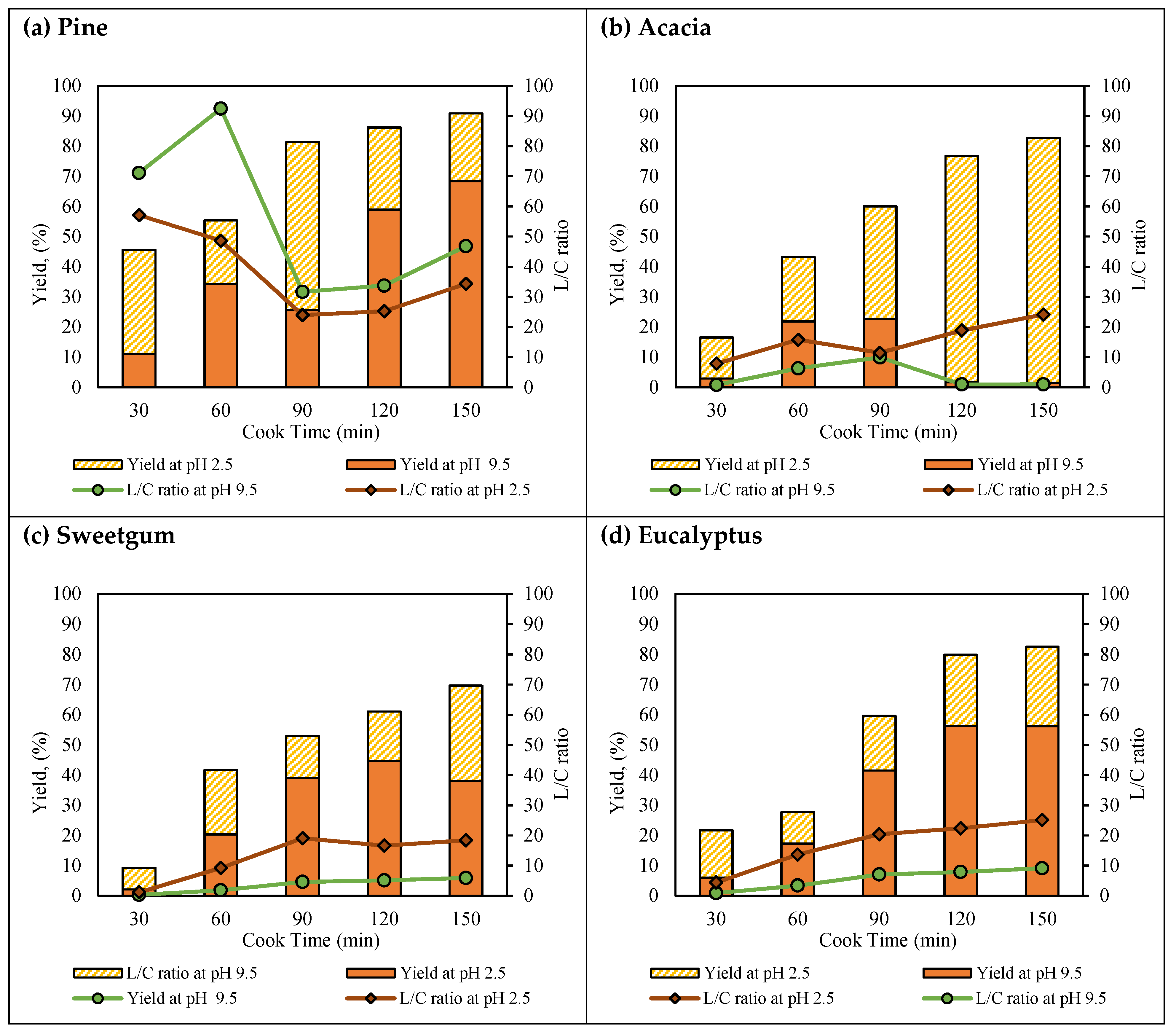
3. Results and Discussion
3.1. Kraft Pulping
3.2. Composition of Wood and Pulps Produced
4. Conclusions
- Lignin precipitation from pine, acacia, sweetgum, and eucalyptus black liquor samples were investigated, and it was shown that the lignin content in the black liquor and the subsequent recovery increased with pulping level. In the initial phase, the total lignin recovery ranged from ~9 to 55%, while in the later phase, the total lignin recovery varied from ~61 to 91%, depending on the species and pH. The increased lignin recovery was due to the increasing lignin concentration toward the end of pulping.
- Softwood showed higher lignin recovery and lower contamination with carbohydrates than the hardwoods, regardless of pH and pulping intensity.
- Hardwood lignin recovery was affected bythe S–G ratio, which resulted in higher yields at pH 9.5 with the increasing S–G ratio.
- Overall, more lignin was precipitated at pH 9.5 than at pH 2.5. However, lignin precipitation at pHs lower than 9.5 should be taken into consideration for samples that achieve low recovery yields. The precipitation of the lignin fraction at pH 2.5 contributed a ~7 to 81% recovery increase, depending on the pulping level, S–G ratio, and species.
- Acacia lignins, which have the lowest S–G ratio amongst the hardwoods, behaved very differently from the other species that were investigated, and the reasons for this are not yet fully understood. The further characterization of all lignins obtained herein will be performed using different spectroscopy and chromatography techniques, which will likely further amplify the lignin characteristics that account for such discrepancies.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hubbe, M.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin recovery from spent alkaline pulping liquors using acidification, membrane separation, and related processing steps: A review. Bioresources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Wallmo, H.; Richards, T.; Theliander, H. Lignin precipitation from kraft black liquors: Kinetics and carbon dioxide absorption. Pap. Ja Puu Pap. Timber 2007, 89, 436–442. [Google Scholar]
- Hu, Z.; Du, X.; Liu, J.; Chang, H.-M.; Jameel, H. Structural Characterization of Pine Kraft Lignin: BioChoice Lignin vs Indulin AT. J. Wood Chem. Technol. 2016, 36, 432–446. [Google Scholar] [CrossRef]
- Öhman, F.; Wallmo, H.; Theliander, H. A novel method for washing lignin precipitated from kraft black liquor–Laboratory trials. Nord. Pulp Pap. Res. J. 2007, 22, 9–16. [Google Scholar] [CrossRef]
- Ragnar, M.; Lindgren, C.T.; Nilvebrant, N.-O. pKa-Values of Guaiacyl and Syringyl Phenols Related to Lignin. J. Wood Chem. Technol. 2000, 20, 277–305. [Google Scholar] [CrossRef]
- Wallmo, H.; Littorin, A.; Karlsson, H.; Lindholm, K.; Stern, R.; Christiansen, G. The Evolution of LignoBoost Technology and the Lignin BioProducts Market, Part 2. In Proceedings of the 2016 International Bioenergy and Bioproducts Conference Proceedings, Jacksonville, FL, USA, 28–30 September 2016. [Google Scholar]
- Tomani, P. The lignoboost process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Fengel, D.; Gerd, W. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1984; ISBN 978-3-11-008481-8. [Google Scholar]
- Alén, R. Structure and chemical composition of wood. In Forest Products Chemistry; Stenius, P., Ed.; Fapet Oy: Helsinki, Finland, 2000; pp. 11–57. [Google Scholar]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Landmér, A.; Tomani, P.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Titirici, M.-M. From Waste to Wealth: From Kraft Lignin to Free-standing Supercapacitors. Carbon 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Akiyama, T.; Goto, H.; Nawawi, D.S.; Syafii, W.; Matsumoto, Y.; Meshitsuka, G. Erythro/threo ratio of β-O-4-5 structures as an important structural characteristic of lignin. Part 4: Variation in the erythro/threo ratio in softwood and hardwood lignins and its relation to syringyl/guaiacyl ratio. Holzforschung 2005, 59, 276–281. [Google Scholar] [CrossRef]
- Sarkanen, K.V.; Chang, H.M.; Allan, G.G. Species variation in lignins III. TAPPI J. 1967, 50, 587. [Google Scholar]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.-M.; Jameel, H. Lignin Structural Variation in Hardwood Species. J. Agric. Food Chem. 2012, 60, 4923–4930. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H.; Chang, H. A quantitative comparison of the precipitation behavior of lignin from sweetgum and pine kraft black liquors. Bioresources 2020, 15, 1–18. [Google Scholar] [CrossRef]
- Chen, C.L. Nitrobenzene and cupric oxide oxidations. In Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; pp. 301–321. [Google Scholar]
- Min, D.; Xiang, Z.; Liu, J.; Jameel, H.; Chiang, V.; Jin, Y.; Chang, H.-M. Improved Protocol for Alkaline Nitrobenzene Oxidation of Woody and Non-Woody Biomass. J. Wood Chem. Technol. 2014, 35, 52–61. [Google Scholar] [CrossRef]
- Marton, J. Reactions in alkaline pulping. In Lignins: Occurrence, Formation, Structure and Reactions; Sarkanen, K.V., Ludwig, C., Eds.; Wiley Interscience: New York, NY, USA, 1971; pp. 639–694. [Google Scholar]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.K.; Chang, H.; Jameel, H. Effect of hardwoods characteristics on kraft pulping process: Emphasis on lignin structure. BioResources 2011, 6, 3623–3637. [Google Scholar]
- Almeida, D.; Santos, R.B.; Hart, P.W.; Jameel, H. Hardwood pulping kinetics of bulk and residual phases. TAPPI J. 2015, 14, 652–662. [Google Scholar] [CrossRef]
- Santos, R.B.; Hart, P.; Jameel, H.; Chang, H.-M. Wood Based Lignin Reactions Important to the Biorefinery and Pulp and Paper Industries. Bioresources 2012, 8, 1456–1477. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.B.; Jameel, H.; Chang, H.-M.; Hart, P.W. Impact of lignin and carbohydrate chemical structures on kraft pulping processes and biofuel production. TAPPI J. 2013, 12, 23–31. [Google Scholar] [CrossRef]
- Theliander, H. Recovery of Cooking Chemicals: The Treatment and Burning of Black Liquor. In Pulping Chemistry and Technology; Volume 2; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter: Berlin, Germany, 2009; pp. 297–334. ISBN 9783110213416. [Google Scholar]
- Magaton, A.S.; Colodette, J.L.; Piló-Veloso, D.; Gomide, J.L. Behavior of Eucalyptus Wood Xylans across Kraft Cooking. J. Wood Chem. Technol. 2011, 31, 58–72. [Google Scholar] [CrossRef]
- Helander, M.; Mattsson, T.; Theliander, H.; Lindström, M.E. Parameters Affecting the Cross-Flow Filtration of Dissolved LignoBoost Kraft Lignin. J. Wood Chem. Technol. 2015, 36, 1–8. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H. Probing the molecular weights of sweetgum and pine kraft lignin fractions. TAPPI J. 2021, 20, 381–391. [Google Scholar] [CrossRef]
- Aminzadeh, S.; Zhang, L.; Henriksson, G. A possible explanation for the structural inhomogeneity of lignin in LCC networks. Wood Sci. Technol. 2017, 51, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Lawoko, M.; Henriksson, A.G.; Gellerstedt, G. Structural Differences between the Lignin−Carbohydrate Complexes Present in Wood and in Chemical Pulps. Biomacromolecules 2005, 6, 3467–3473. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Kirui, A.; Widanage, M.C.D.; Mentink-Vigier, F.; Cosgrove, D.J.; Wang, T. Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun. 2019, 10, 347. [Google Scholar] [CrossRef]
- Min, D.-Y.; Yang, C.; Chiang, V.; Jameel, H.; Chang, H.-M. The influence of lignin–carbohydrate complexes on the cellulase-mediated saccharification II: Transgenic hybrid poplars (Populus nigra L. and Populus maximowiczii A.). Fuel 2014, 116, 56–62. [Google Scholar] [CrossRef]
- Giummarella, N.; Pu, Y.; Ragauskas, A.J.; Lawoko, M. A critical review on the analysis of lignin carbohydrate bonds. Green Chem. 2019, 21, 1573–1595. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Xu, W.; Cheng, F.; Pranovich, A.; Smeds, A.I.; Willför, S.M.; Xu, C. Valorization of Lignin–Carbohydrate Complexes from Hydrolysates of Norway Spruce: Efficient Separation, Structural Characterization, and Antioxidant Activity. ACS Sustain. Chem. Eng. 2019, 7, 1447–1456. [Google Scholar] [CrossRef]
- Sakagami, H.; Hashimoto, K.; Suzuki, F.; Ogiwara, T.; Satoh, K.; Ito, H.; Hatano, T.; Takashi, Y.; Fujisawa, S.-I. Molecular requirements of lignin–carbohydrate complexes for expression of unique biological activities. Phytochemistry 2005, 66, 2108–2120. [Google Scholar] [CrossRef]
- De Carvalho, D.M.; Lahtinen, M.H.; Lawoko, M.; Mikkonen, K. Enrichment and Identification of Lignin–Carbohydrate Complexes in Softwood Extract. ACS Sustain. Chem. Eng. 2020, 8, 11795–11804. [Google Scholar] [CrossRef]

| Species | Parameter | Cook Time (min) | ||||
|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | ||
| Pine | H-factor | 7 | 32 | 503 | 983 | 1400 |
| Pulp yield, % | 93.2 | 69.1 | 51.4 | 47.4 | 43.5 | |
| REA, g/L as Na2O | 19.2 | 17.0 | 11.5 | 10.0 | 5.9 | |
| Solids in BL, % | 11.1 | 13.8 | 16.6 | 17.3 | 16.6 | |
| Acacia | H-factor | 0 | 19 | 227 | 469 | 688 |
| Pulp yield, % | 91.3 | 74.9 | 66.1 | 62.5 | 56.4 | |
| REA, g/L as Na2O | 26.4 | 17.3 | 11.8 | 8.9 | 7.3 | |
| Solids in BL, % | 9.0 | 11.1 | 12.8 | 13.6 | 13.8 | |
| Sweetgum | H-factor | 0 | 51 | 273 | 487 | 650 |
| Pulp yield, % | 85.5 | 62.6 | 50.7 | 48.7 | 47.6 | |
| REA, g/L as Na2O | 20.5 | 12.5 | 9.0 | 4.7 | 3.5 | |
| Solids in BL, % | 8.9 | 12.1 | 14.1 | 14.4 | 14.4 | |
| Eucalyptus | H-factor | 0 | 15 | 216 | 442 | 648 |
| Pulp yield, % | 93.8 | 67.6 | 52.0 | 48.7 | 48.1 | |
| REA, g/L as Na2O | 24.5 | 14.5 | 8.1 | 5.8 | 5.0 | |
| Solids in BL, % | 9.0 | 11.0 | 13.4 | 14.3 | 14.7 | |
| Species | Compound | Sample/Cook Time (min) | |||||
|---|---|---|---|---|---|---|---|
| Wood * | 30 | 60 | 90 | 120 | 150 | ||
| Pine | Glucan, g | 40.0 | 38.7 | 36.1 | 35.1 | 35.4 | 32.8 |
| Total Lignin, g | 31.7 | 28.7 | 19.2 | 6.7 | 3.9 | 3.3 | |
| Xylan, g | 6.1 | 5.8 | 4.9 | 3.6 | 3.5 | 3.4 | |
| Galac + Man + Arab, g | 19.7 | 16.9 | 7.8 | 7.3 | 5.9 | 5.3 | |
| Acacia | Glucan, g | 42.7 | 41.7 | 40.5 | 40.4 | 40.3 | 39.5 |
| Total Lignin, g | 27.9 | 25.1 | 20.1 | 8.1 | 6.1 | 4.1 | |
| Xylan, g | 10.0 | 8.6 | 8.1 | 7.2 | 7.3 | 6.7 | |
| Galac + Man + Arab, g | 1.3 | 1.0 | 0.6 | 0.5 | 0.0 | 0.0 | |
| Sweetgum | Glucan, g | 45.2 | 39.6 | 36.4 | 33.8 | 33.8 | 33.5 |
| Total Lignin, g | 25.9 | 21.6 | 10.8 | 2.9 | 1.4 | 1.2 | |
| Xylan, g | 14.8 | 12.3 | 9.9 | 8.7 | 8.5 | 8.6 | |
| Galac + Man + Arab, g | 6.3 | 5.2 | 3.0 | 2.4 | 2.6 | 3.0 | |
| Eucalyptus | Glucan, g | 44.6 | 41.9 | 31.9 | 31.7 | 30.6 | 30.4 |
| Total Lignin, g | 24.8 | 24.5 | 13.8 | 3.0 | 1.7 | 1.1 | |
| Xylan, g | 13.1 | 11.2 | 7.5 | 7.8 | 7.0 | 7.4 | |
| Galac + Man + Arab, g | 4.0 | 3.4 | 1.3 | 0.9 | 0.5 | 0.5 | |
| Species | Sr–V Ratio | S–G Ratio |
|---|---|---|
| Acacia | 1.2 | 1.0 |
| Sweetgum | 2.8 | 2.3 |
| Eucalyptus | 4.5 | 3.6 |
| Species | pH | Parameter | Sample / Cook Time (min) | ||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | |||
| Pine | 9.5 | Lignin in the BL | 3.0 | 12.4 | 25.0 | 27.8 | 28.4 |
| TS b precipitated | 0.4 | 4.6 | 6.9 | 19.4 | 21.0 | ||
| Total lignin | 0.3 | 4.3 | 6.4 | 16.4 | 19.4 | ||
| Carbohydrates | 0.0 | 0.0 | 0.2 | 0.5 | 0.4 | ||
| Ash | 0.0 | 0.1 | 0.1 | 0.4 | 0.5 | ||
| Pine | 2.5 | Lignin in the BL | 2.7 | 8.1 | 18.6 | 11.4 | 9.0 |
| TS b precipitated | 1.2 | 3.0 | 16.9 | 9.0 | 7.1 | ||
| Total lignin | 1.0 | 2.6 | 13.9 | 7.6 | 6.4 | ||
| Carbohydrates | 0.0 | 0.1 | 0.6 | 0.3 | 0.2 | ||
| Ash | 0.0 | 0.0 | 0.2 | 0.1 | 0.1 | ||
| Acacia | 9.5 | Lignin in the BL | 2.8 | 8.4 | 19.3 | 21.1 | 23.1 |
| TS b precipitated | 0.2 | 2.3 | 5.2 | 0.9 | 0.8 | ||
| Total lignin | 0.1 | 1.8 | 4.3 | 0.4 | 0.3 | ||
| Carbohydrates | 0.1 | 0.3 | 0.4 | 0.4 | 0.3 | ||
| Ash | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Acacia | 2.5 | Lignin in the BL | 2.8 | 6.6 | 14.9 | 20.8 | 22.7 |
| TS b precipitated | 0.5 | 2.0 | 8.3 | 17.7 | 20.5 | ||
| Total lignin | 0.4 | 1.8 | 7.2 | 15.8 | 18.7 | ||
| Carbohydrates | 0.0 | 0.1 | 0.6 | 0.8 | 0.8 | ||
| Ash | 0.0 | 0.1 | 0.1 | 0.4 | 0.2 | ||
| Sweetgum | 9.5 | Lignin in the BL | 4.3 | 15.1 | 23.0 | 24.5 | 24.7 |
| TS b precipitated | 0.6 | 5.5 | 12.4 | 14.7 | 12.3 | ||
| Total lignin | 0.1 | 3.1 | 9.0 | 10.9 | 9.4 | ||
| Carbohydrates | 0.4 | 1.7 | 2.0 | 2.1 | 1.6 | ||
| Ash | 0.0 | 0.1 | 0.3 | 0.3 | 0.3 | ||
| Sweetgum | 2.5 | Lignin in the BL | 4.2 | 12.0 | 14.0 | 13.5 | 15.3 |
| TS b precipitated | 0.8 | 4.2 | 3.6 | 4.4 | 8.4 | ||
| Total lignin | 0.3 | 3.2 | 3.2 | 4.0 | 7.8 | ||
| Carbohydrates | 0.3 | 0.4 | 0.2 | 0.2 | 0.4 | ||
| Ash | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | ||
| Eucalyptus | 9.5 | Lignin in the BL | 0.2 | 9.8 | 19.1 | 20.0 | 20.6 |
| TS b precipitated | 0.0 | 2.5 | 9.8 | 13.5 | 13.6 | ||
| Total lignin | 0.0 | 1.7 | 7.9 | 11.3 | 11.6 | ||
| Carbohydrates | 0.0 | 0.5 | 1.1 | 1.4 | 1.3 | ||
| Ash | 0.0 | 0.0 | 0.2 | 0.2 | 0.2 | ||
| Eucalyptus | 2.5 | Lignin in the BL | 0.1 | 8.1 | 11.2 | 8.7 | 9.0 |
| TS b precipitated | 0.0 | 1.2 | 4.2 | 5.2 | 5.9 | ||
| Total lignin | 0.0 | 1.0 | 3.5 | 4.7 | 5.4 | ||
| Carbohydrates | 0.1 | 0.1 | 0.3 | 0.3 | 0.3 | ||
| Ash | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardim, J.M.; Hart, P.W.; Lucia, L.A.; Jameel, H.; Chang, H.-m. The Effect of the Kraft Pulping Process, Wood Species, and pH on Lignin Recovery from Black Liquor. Fibers 2022, 10, 16. https://doi.org/10.3390/fib10020016
Jardim JM, Hart PW, Lucia LA, Jameel H, Chang H-m. The Effect of the Kraft Pulping Process, Wood Species, and pH on Lignin Recovery from Black Liquor. Fibers. 2022; 10(2):16. https://doi.org/10.3390/fib10020016
Chicago/Turabian StyleJardim, Juliana M., Peter W. Hart, Lucian A. Lucia, Hasan Jameel, and Hou-min Chang. 2022. "The Effect of the Kraft Pulping Process, Wood Species, and pH on Lignin Recovery from Black Liquor" Fibers 10, no. 2: 16. https://doi.org/10.3390/fib10020016
APA StyleJardim, J. M., Hart, P. W., Lucia, L. A., Jameel, H., & Chang, H.-m. (2022). The Effect of the Kraft Pulping Process, Wood Species, and pH on Lignin Recovery from Black Liquor. Fibers, 10(2), 16. https://doi.org/10.3390/fib10020016









