Abstract
The excellent combination of properties has seen a steep increase in the demand for titanium (Ti)-based material as biomedical implant devices. However, some features that promote biocompatibility are found to be lacking in Ti implants. The use of polymer nanofiber (NF) coating on the surfaces of the implants has been proven to remedy these setbacks. In particular, electrospun NFs are versatile as natural extracellular matrix mimics and as facilitators in the biocompatibility function of Ti-based implants. Therefore, various properties of Ti implants coated with polymer NFs and the correlations among these properties are explored in this review. Synthetic polymers are favorable in tissue engineering applications because they are biocompatible and have low toxicity and degradation rates. Several approved synthetic polymers and polymer hybrids have been electrospun onto Ti implant surfaces to successfully improve the biomedical applicability of the implants with regard to their physical (including diameter and porosity), chemical (including corrosion resistance), mechanical (including elastic modulus, strength and ductility) and biological properties (including tissue integration, antimicrobial and cytotoxicity).
1. Introduction
The demand for biocompatible titanium (Ti) and its alloys as implant materials for hard tissue replacement in the orthopedic and dental fields has seen a steep rise during the past decades, along with the significant increase in population and life expectancy [1,2,3]. In particular, implants made from commercially pure Ti (cpTi) and Ti–6Al–4V Extra Low Level of Interstitial (ELI) alloy are the most widely accepted and successfully used due to their exceptional combination of biological and mechanical properties [1,4]. The most prominent of these properties include chemical stability, fatigue resistance, high corrosion resistance and an intrinsic ability to osseointegrate without stimulating adverse immune responses [2,5]. The biocompatibility of Ti and its alloys with human tissue is mainly due to the ability to form a chemically stable protective titanium dioxide (TiO2) layer on the surface in reaction with oxygen [6]. Despite their outstanding features, which propel them to be frontrunners as biomedical materials, Ti and its alloys present some drawbacks that require mediation. Failure to thoroughly address the inadequacies of Ti-based implants escalates the requirement for revision surgery, and the penalties thereof include high economic associated costs and, possibly, death [1,7].
Features such as highly porous and interconnected mesh with a large surface-to-volume ratio promote interactions at the implant–tissue interface but are difficult to achieve in conventional metallic implants [5]. Additionally, Ti exhibits limited bioactivity due to its inherent bio-inertness. On the other hand, the surfaces of Ti-based implants attract bacterial colonization because of their biocompatibility characteristics. Consequently, preventative strategies to improve the antibacterial ability of the implants before surgery are required [1]. Surface modification of Ti and its alloys should be aimed at increasing the surface area of implants to further augment their biomechanical benefits and incorporate bactericidal effects. To achieve this, several techniques have been proposed, and the emergence of nanotechnology has expanded the scope of nanoscale topographical modifications and has drawn considerable attention to the application of polymer nanofiber (NF) coatings on Ti implant surfaces [8,9].
As NFs are becoming increasingly prominent in biomedical applications, the production of high-quality multifunctional NFs has become imperative. Among the various techniques explored to fabricate polymer NFs, the electrospinning process is favored as it is a versatile, scalable, economical and simple yet robust process and is the most widely used technique for the preparation of well-defined NFs from a wide range of biocompatible polymeric solutions [1,9,10,11,12,13]. This technique is considered the most reliable process for producing long and continuous NFs that are proficient in simulating the microstructure of the native extracellular matrix (ECM) due to their high surface area to volume ratio and relatively large internal porosity [1,11]. Furthermore, electrospun NFs are able to encapsulate supplementary material into the polymer in the most convenient manner to create coatings with adjustable physical (such as microstructure and porosity), chemical (such as type of polymers, solvent and biomolecules), mechanical (such as tensile strength, elastic modulus and ductility) and biological (such as bioactivity and antibacterial) properties [3,14].
The central requirement for successful and efficient implantation is for the implanted material to incite tissue regeneration around the implant surface by mimicking the natural ECM, which is in contact with a vast majority of the cells [15]. The ECM consists of a complex network of nanometer-sized tissue-specific molecules that serve to initiate and mediate responses that regulate cell growth, migration, differentiation, survival, tissue organization and matrix remodeling and bear most of the applied mechanical stresses [16,17]. The NF scaffold is able to serve as a synthetic ECM to support cell growth and tissue development [16,18]. As such, induction of NF polymers for coating Ti implant surfaces promotes tissue-mimicking, and in order to facilitate tissue growth, certain minimum requirements should be satisfied, namely the NF scaffold should be/have [16,18]:
- Three-dimensional (3D);
- Highly porous with an interconnected pore network;
- Fabricated using material that is biodegradable or bioresorbable;
- Controlled degradation rate to match tissue regeneration rate;
- Suitable surface chemistry for cell attachment, proliferation, and differentiation;
- Mechanical properties matching those of the tissues at the site of implantation; and
- Easily fabricated in a variety of morphologies to define the shape and size of the regenerated tissue.
Comprehending the combinatory impact of the various aspects related to the surface coating of Ti implant with electrospun NFs may significantly expedite the process of identifying and alleviating sources of implantation disruption post-surgery. Nonetheless, the literature rarely provides a holistic overview of advances towards achieving Ti implant success through surface modification nanotechniques [19]. Further research on peri-implant tissue response to surface modification of implants using novel coatings is necessary since the optimal dynamics of the implant–tissue integration remains vague [5]. Therefore, this paper aims to identify the diverse properties of various NF coats that enable them to promote the proficiency of Ti-based materials in biomedical implant applications. As such, enhancement of the NFs to aid in their biocompatibility is also discussed, and the interrelations among the properties are outlined in the current review.
2. Nanofiber Polymers for Biomedical Applications
The features of the NFs that are used to coat biomedical Ti-based implants are predominantly dependent on the type of polymer employed [2]. Applicable polymers are categorized as either natural or synthetic based on their source and composition [20,21,22]. Natural polymers, namely cellulose, collagen, gelatin, chitosan, chitin, dextrose and silk fibroin, have been electrospun into NF scaffolds [11,18,22]. Kadavil et al. [9] report on gelatin providing cellular attachment and adhesion of human stem cells, which is typical of most natural polymers. However, natural polymers are limited in their clinical application due to being immunogenic, exhibiting batch-to-batch differences, limited availability, expensive production and vulnerability to cross-contamination [9,21]. Moreover, natural polymers lack mechanical strength and have a relatively rapid degradation rate due to their hydrophilic nature, limiting their use in long-term clinical processes. These limitations of natural polymers may be remedied through the use of synthetic polymers [9,20,21].
Synthetic polymers have numerous advantages in comparison to their natural counterparts, namely cost-effectiveness and durability, and the majority of them have stable mechanical properties for applications in load-bearing tissue engineering scaffolds [9,20,21]. Owed to their ease of processing and biocompatibility, the most popular synthetic polymers include poly(ε-caprolactone) (PCL), poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), poly(glycolic acid) (PGA), poly(ethylene oxide) (PEO) and poly(vinyl alcohol) (PVA) [9,11,19]. These polymers have been approved by the United States Food and Drug Administration (FDA) for use in human medical devices, which emphasizes their in vivo applicability and that their toxicity factor has been evaluated [21,22,23,24,25,26,27].
In addition to biocompatibility, biodegradability and lack of toxicity are common properties among synthetic polymers relevant for coating surfaces of biometals [28,29]. Biodegradation may be defined by hydrolysis in physiological conditions (as in the human body) [28,29]. Boia et al. [26] greatly emphasized the slow degradation of PCL-based implants, which is supported by Perumal et al. [27], who reported that PCL has been said to gradually degrade when compared to PLGA and PLA. The degradation rate is dependent on the hydrophilicity of the monomeric units, and basically, the comparative degradation rates of the polymers may be summarized in terms of length of the degradation period as [21,27,30]: PCL > PLA > PLGA > PGA > hydrophilic polymers (such as PEO and PVA). Table 1 lists the distinguishing properties of synthetic polymers used in the biomedical field.

Table 1.
Overview of the characteristics of synthetic polymers.
2.1. Hybridization of Polymers
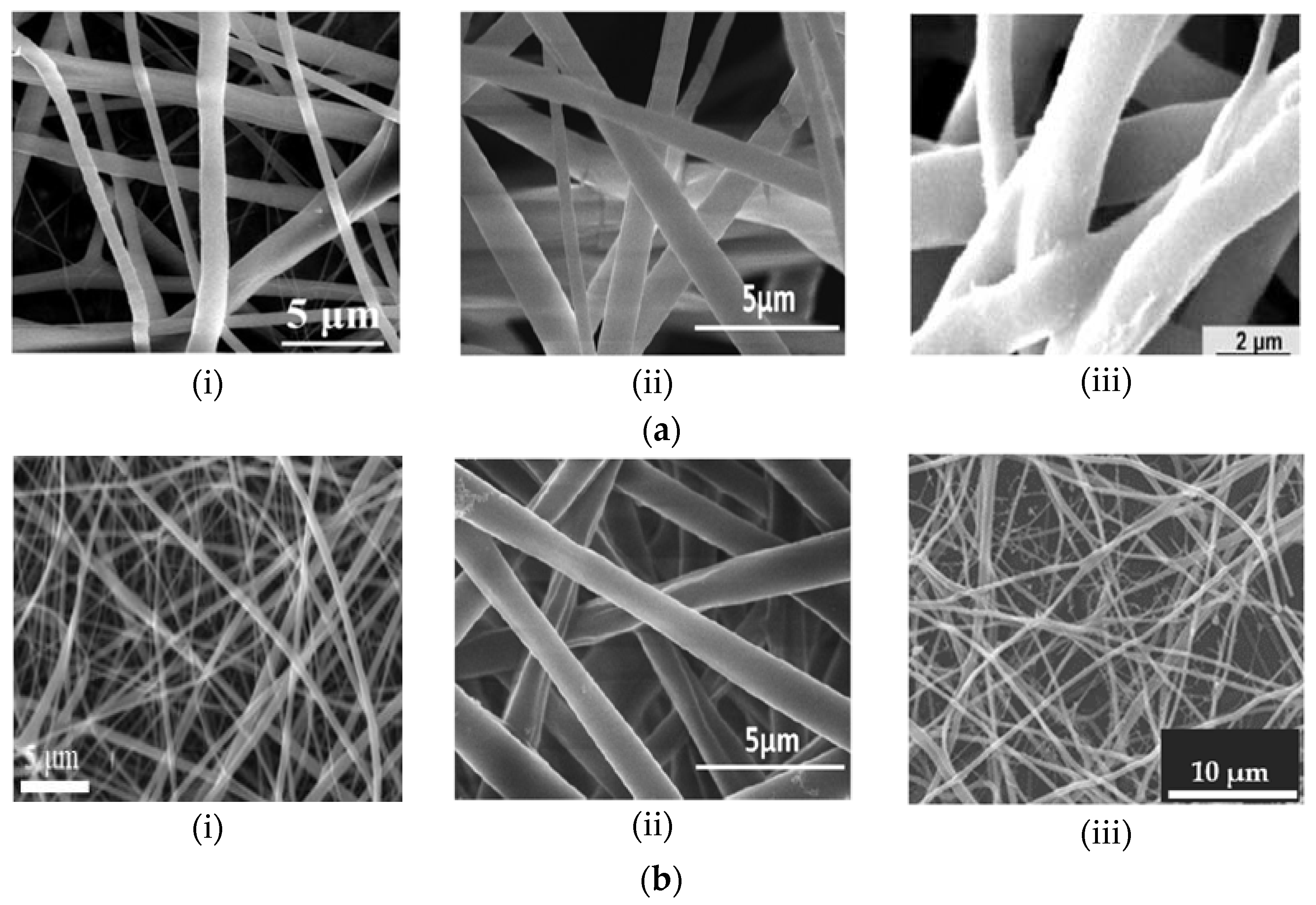
Despite the various clinical advantages of synthetic polymers, their disadvantages—for instance, lack of cell-specific recognition sites due to their smooth and hydrophobic surfaces—reduce their applications in implantable devices [22]. In addition, water-soluble synthetic polymers, such as PVA, exhibit poor mechanical properties [9]. Studies indicate that one of the most effective strategies applied to subdue the limitations of polymer groups is the production of novel composite fibers through the combination of various polymers [2,9,18,36]. The results of Jahanmard et al. [3] indicated that the biological properties of bi-layered PCL/PLGA composite NFs far exceeded those of the single layers. Synthetic polymers are often fused with natural polymers to form fibers with optimized mechanical properties, degradation rates and bioactivity while maintaining the similarity to the ECM and promoting cell attachment [9,22]. Examples of single and hybrid electrospun NFs are illustrated by the scanning electron microscope (SEM) images in Figure 1.
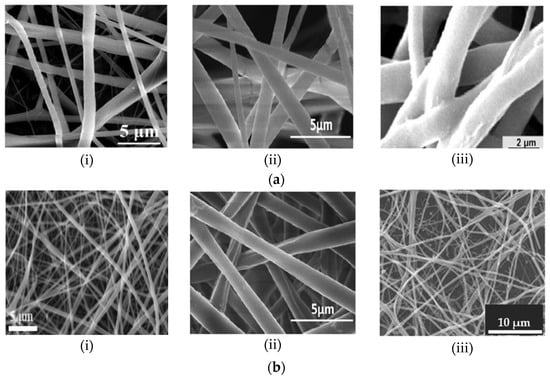
Figure 1.
Scanning electron microscope (SEM) micrograph illustrations of nanofibers (NFs) composed of (a) pure synthetic polymers (i) PCL [18], (ii) PLLA (Reprint with permission from [37]. Copyright 2013, Springer Nature) and (iii) PLGA [3]; and (b) synthetic and natural polymer composites of (i) PCL/Collagen [38], (ii) PLLA/Chitosan [37] and (iii) PEO/Chitosan [2].
2.2. Electrospinning Technique
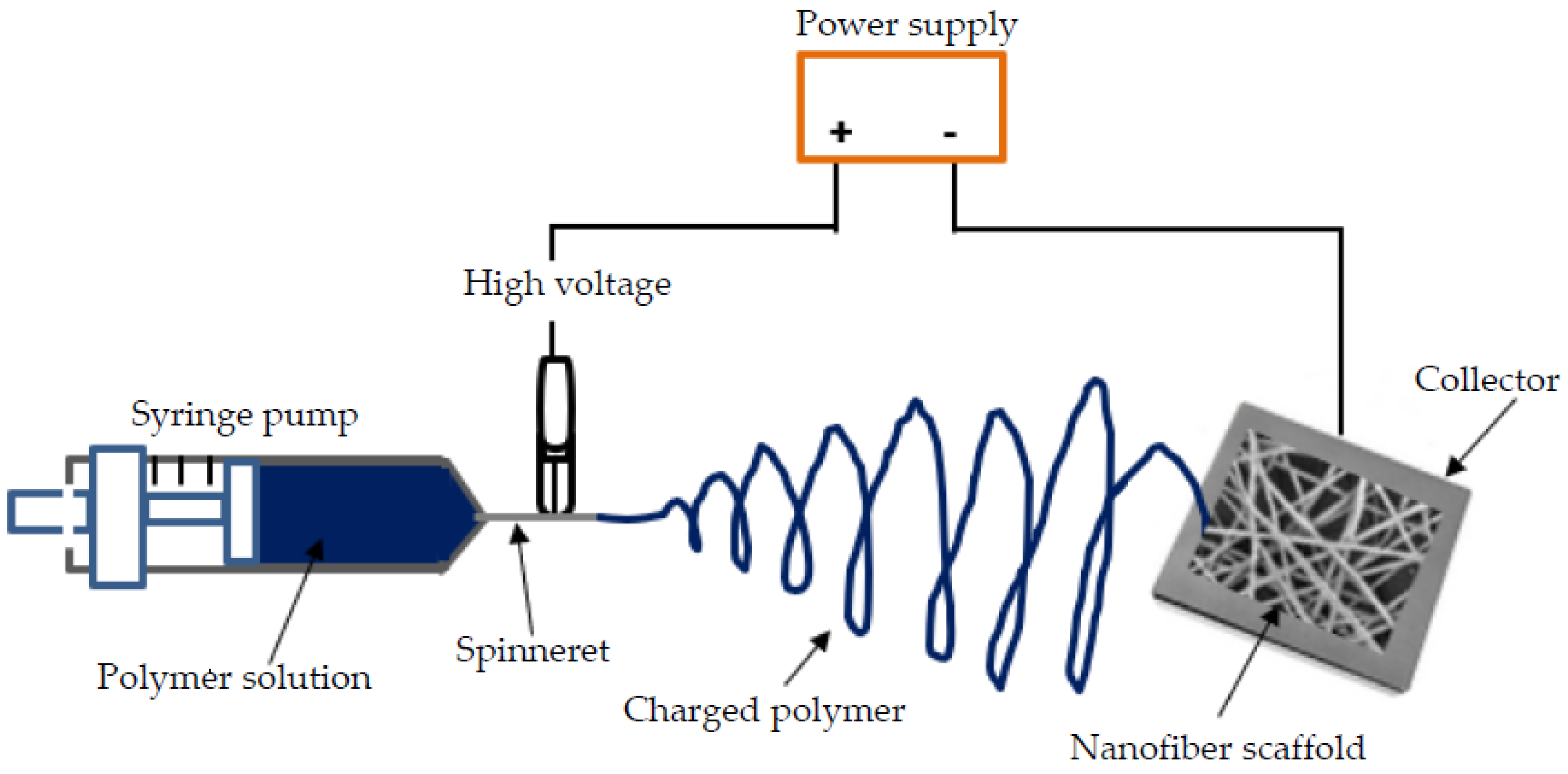
Electrospinning is a common nanotechnique used to fabricate scaffolds of aligned polymeric NFs with diameters varying between 3 nm and greater than 5 µm [14,15]. The engineered 3D porous scaffolds serve as a pattern to provide mechanical and biochemical support to the surrounding cells relative to the tissue type [1,17]. A simple electrospinning setup (Figure 2) consists of a high voltage power supply (typically between 5 to 30 kV), a piece of feeding equipment (usually a syringe), a spinneret and a collector [11,12,35]. The collector for electrospun fibers is usually on a grounded plate and is usually a metallic material; hence, coating a Ti implant using the electrospinning method is relatively straightforward [15,39,40]. Important factors that govern the quality NFs produced using the electrospinning process include solution parameters (such as polymer structure and viscosity), processing parameters (such as flow, voltage and distance) and ambient conditions [22,38]. By altering these parameters, multiple experimental arrangements of the process, including coaxial, solution and melt electrospinning, are achievable [3,18,41]. Deviations from the basic electrospinning process are necessary to modify the primary properties of NFs and realize a tunable coating towards desired structural and functional properties [3,9,18]. The flexibility of the electrospinning technique is convenient for application in Ti implants intended for the complex human body environment. Several researchers have successfully applied electrospinning to coat Ti [1,19,39,42] and Ti–6Al–4V [2,39,43,44] samples intended for use as human implants. Kadavil et al. [9] notes also that polymers such as PCL and PVA have gained popularity as readily electrospinnable polymers and have been used as a template for the preparation of non-electrospinnable polymers. A specific amount of PEO was used as a fiber-forming additive by Nitti et al. [45] to improve the electrospinnability of chitosan. Therefore, technique modifications allow for the accommodation of the various biopolymer solutions.
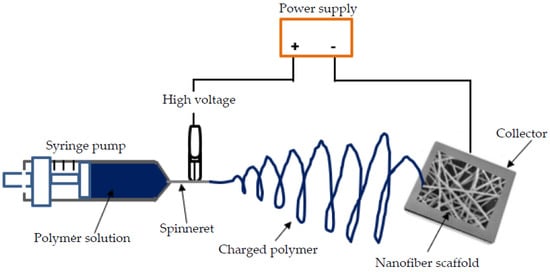
Figure 2.
Schematic representation of a basic electrospinning horizontal set-up.
3. Physical Characteristics of Electrospun Nanofibers
Nanofibers merge the nano- and macroscale worlds of implant surface modification since the diameters are in the nanometer range and the lengths may be from a few meters to kilometers [15,46]. Physical properties of polymers include dimensions, porosity, molecular weight, molar volume, density, degree of polymerization, the crystallinity of material and others [47]. The diameter of NFs is an essential physical property since the fibers are classified as nano-structured based on their diameter and contribute significantly to the adhesion of cells onto the fiber surfaces [14].
Electrospinning provides a direct method to produce long polymer fibers with diameters in the range of 40–2000 nm, and thinner fibers are achievable by modifying the typical electrospinning technique and/or manipulating the spinning parameters [32,46]. Furthermore, studies show that embedded substances, such as antibacterial agents, cause a reduction in the NF diameter even by approximately 25% in some cases as a result of the reduced polymer concentration [48]. Tian et al. [14] fabricated electrospun composite NFs comprising of PGA and collagen, with diameters of 10 µm, 3–5 µm and 500 nm, and analyzed the properties of fibroblast cells on these fibers as a function of fiber diameter and structure. The authors were able to demonstrate that the fiber composition and diameter have a direct influence on the morphology and alignment of the fibroblast cells. The cells attached and elongated on the PGA/collagen fibers with a diameter of 500 nm more readily than those seeded on fibers with larger diameters. On the other hand, Kadavil et al. [9] reported on the improved mechanical behavior of fibers due to the fibers’ diameters greatly increasing, and Yang et al. [49] found that the diameter of electrospun gelatin/PVA NFs increased as the ratio of PVA increased. Likewise, Ravichandran et al. [39] observed a higher diameter for PLGA fibers (957 nm) than PLGA/collagen (378 nm) on Ti surfaces. Morel et al. [50] found that increasing the diameter of the PLLA NFs reduced the Young’s modulus. Cipitria et al. [51] showed PCL NF meshes with an average diameter of 689 nm as having higher values of ductility, ultimate strength and Young’s modulus than those with an average diameter of 196 nm.
An increase in NF diameter generally decreases the porosity of the electrospun mat [52]. Weng and Xie [11] reported on NF dressings capable of simultaneously preventing infection and fostering cell proliferation by controlling the pore size. Highly porous electrospun nanofibrous scaffolds mimic the structure and function of the native ECM by producing interconnection pores that provide regions favored by various stem cells for attachment, growth, proliferation and differentiation [5,14,17,39,48]. The pores also provide a larger surface area for embedded fibers and increase the adhesive strength of the coating [42]. Leong et al. [53] introduced nanoporosity in electrospun PDLLA fibers by creating vapor-induced phase separation conditions during electrospinning. Although the nanoporous fiber scaffolds were mechanically weaker than the conventional solid fiber scaffolds, the porous fibers increased the surface area by an estimated 62% and had greater protein adsorption and enhanced initial cell attachment than the solid fiber scaffolds.
4. Chemical Implications of Nanofiber-Coated Ti Implants
Corrosion resulting from a chemical reaction between the implant and the surrounding tissue may compromise the success of implantation treatment. Implantable Ti-based materials are generally regarded as highly corrosion-resistant due to the formation of a passive oxide film, which largely consists of TiO2, of approximately 2–5 nm thickness [54]. Nevertheless, the oxide layer is not entirely stable and has been found to break down in the hostile body environment, resulting in the deterioration of implant efficiency due to corrosion of metals [13,55]. Surface modification is, thus, required to enhance the corrosion resistance of Ti implant surfaces [54,55]. The application of corrosion-resistant coatings on metal surfaces is a cost-effective and efficient approach for corrosion control [13].
Biodegradable polymer coatings act as corrosion inhibitors for implants by isolating the metal surfaces from sources of corrosion in the corrosive environment to prevent post-implantation corrosion [31,56]. Moreover, nanostructures have been proven to be highly effective against corrosion and, subsequently, electrospun NFs may pose as corrosion inhibiting barriers to stunt the metallic corrosion rate since porous coatings show greater stability [13]. The results of the study of Hanas and Sampath Kumar [57] confirmed that PCL NF coating fabricated by electrospinning protected metallic surfaces from pitting corrosion. Abdal-hay et al. [42] designed a biodegradable hybrid PCL/PLA membrane to coat Ti surfaces and determined the corrosion performance. Their results from the potentiodynamic polarization test in a simulated body fluid (SBF), with an ionic composition and a concentration similar to human body plasma, showed that polymer-coated Ti samples were corrosion-resistant. These results were consistent with those found during the work of AlFalah et al. [13], in which corrosion analysis of electrospun NF coating containing PCL on a metal surface indicated that the NF coating might be considered a new layer of protective coating with excellent corrosion resistance.
The effects of a corroding material advance beyond the surface of an implanted device and deteriorate other properties required for biocompatibility. Corrosion occurs when the metallic ions leach away from the implant and into the human body. Consequently, biological and mechanical parameters are affected, leading to implantation failure because when the material corrodes, it will eventually become brittle and fracture [58]. On the other hand, material fractures accelerate corrosion due to an increase in exposed surface area and loss of the protective oxide layer [58,59]. Furthermore, released metallic ions from a corroded surface may cause inflammation stimulated by a cellular response to infection [59].
5. Mechanical Properties of Nanofibers for Ti-Based Implants
Mechanical properties that are relevant for the optimum functioning of an implant include yield strength, ultimate tensile strength, elastic modulus, ductility (measured as percentage elongation or percentage reduction in area), compressive and shear stresses, Vickers hardness and fatigue resistance [60,61]. An ideal biomaterial should have an elastic modulus like that of the surrounding bone to ensure a more uniform distribution of stress at the implant–bone interface [60,62]. A combination of high strength and high ductility is favorable; however, the strength of the materials is usually inversely proportional to the ductility [63,64]. Therefore, a superior biomaterial should exhibit an excellent balance of strength and ductility [65].
Titanium is popular for biomedical implant applications because of its excellent mechanical properties and relatively low density, and thus, its NF coating should support the mechanical advantages [42,66]. Mechanical properties of a polymer define the type of NFs and the ultimate use thereof [67]. In addition, the mechanical properties of electrospun polymer NFs determine the ability of the fibers to withstand forces during surgical operations and those exerted by the physiological activities of the human body [51]. Innate drawbacks in the mechanical strength of most NF polymers may limit their use for bone-related applications. However, the limitations may be overcome by combining polymers, and electrospinning is capable of encapsulating materials in the polymers that may develop NFs with improved mechanical properties [11,14].
Miele et al. [38] investigated the tensile strength, strain at fracture point and elastic modulus of the collagen/PCL membrane. A drastic decrease in tensile strength and elastic modulus was observed with the addition of PCL. The tensile strength, elastic modulus and elongation of collagen were 4.06 MPa, 1.26 MPa and 33.2%, respectively, while those of collagen/PCL (1:1 w/w) were 2.50 MPa, 0.58 MPa and 12.8%, respectively. A reduction in the elastic modulus is favored as it should match the low elastic modulus of the bone. Yang et al. [49] fabricated electrospun nanocomposite fiber by blending hydrophilic polymers gelatin and PVA and discovered a direct correlation between an increase in the PVA ratio and augmented tensile strength and elongation for the gelatin/PVA NFs. For both the studies of Miele et al. [38] and Yang et al. [49], the natural polymers collagen and gelatin, respectively, presented rather poor mechanical properties during preparation and testing. Thus, the addition of the synthetic counterparts assisted in improving the mechanical properties of the NF membranes.
Tian et al. [14] also postulated that the improved retention of mechanical properties of PGA in comparison to collagen could enhance the shapeablility of bioabsorbable PGA/collagen composites. Khandaker et al. [43] further conducted in vivo mechanical tests with the electrospun collagen/PCL NF coated on Ti–6Al–4V ELI surfaces and observed a positive influence of NF treated surfaces on the mechanical fixation between Ti and bone interfaces. Alternatively, Şimşek et al. [19] used ultraviolet (UV)-induced crosslinking with an insoluble polymer mat to stabilize a highly soluble PEO NF coating for Ti surfaces. Table 2 shows the mechanical properties of Ti alloys and natural human tissue.

Table 2.
Mechanical properties of Ti implant material and human tissue.
Another essential factor to consider with regard to NF-coated Ti implants is the surface morphology of the implants, particularly pertaining to the roughness and the wettability (or hydrophilicity). Appropriate surface roughness may produce beneficial mechanical interlocking at the initial adhesion stage and aid in further cell adhesion [76,77,78]. Khandaker et al. [44] found that coating Ti with a collagen/PCL NF mesh improved the surface roughness. Moreover, rough-textured surfaces are able to stimulate cell attachment, differentiation and the formation of ECM [39]. Enhanced wettability behavior may also support scaffold cell adhesion, proliferation and bioactivity [27].
The contact angle indicates the degree of wettability, such that a lower contact angle value (<90°) indicates higher wettability which is related to an improvement in hydrophilicity [27]. Surface pre-treatment—for example, by mechanical polishing and chemical etching—contributes to the reduction in the contact angle of NF-coated Ti implants [39]. Şimşek et al. [19] measured water contact angles of Ti surfaces and found that the values had decreased after coating with PEO NFs. Similarly, Ravichandran et al. [39] found that hydrophilicity was maximized when the contact angle was reduced to zero with the inclusion of collagen when electrospinning PLGA/collagen NFs onto Ti and Ti alloy substrates. Miele et al. [38] showed the wettability of electrospun PCL being improved with the addition of hydrophilic collagen and was attributed to the reduction in the interfacial tension between the water and the solid surface of the NFs. The necessity to incorporate the hydrophilic polymer is supported by the results of Kiran et al. [6], which displayed a contact angle of approximately 140° for cpTi samples coated with electrospun pure PCL, resulting in a hydrophobic surface. Ti implant biocompatibility may be improved by increasing the surface roughness while decreasing the contact angle [78].
Adhesion of Nanofibers on Ti-Based Substrates
Rigid bonding between NF coatings and Ti implants (that is, interfacial adhesion) is compulsory to prevent any detectable disruption, delamination and folding of the NF coatings on the Ti implant surface during implantation [79]. The adhesion of a polymer coating on the Ti substrate contributes significantly towards protecting the underlying substrate from an accelerated accumulation of water molecules and corrosive ions at the substrate surface [42]. According to Chen et al. [80], adhesion is the main characteristic of the substrate/coating system as it determines the durability and longevity of the system in applications.
The adhesion strength at the polymer and metallic interface is influenced by:
- A polymer with a molecular structure that can provide more electrostatic interaction on the Ti substrate surface and result in increased adhesion strength [42,79,81].
- Surface treatment (usually mechanically or chemically) of the Ti substrate prior to coating is known to increase the surface free energy of the substrate [6,81,82].
- The composition of the coatings and the concentration of the polymer in the NF solution are crucial determining factors of NF adhesion strength. Imbuing the NF polymer solution with an additive (such as nanoparticles of an antibacterial agent) may increase the adhesion strength of the interface by reducing the surface tension of the NF film [81,82].
- High porosity of an NF membrane may cause a faster diffusion of water molecules into the coating. Consequently, the diffusion may cause the layer nearest to a metallic surface to delaminate or completely release from the substrate when the water reaches the implant surface [42].
- The high surface area and the high volume-to-mass ratio of NFs may contribute to the bonding of NFs to the Ti implant surface [79].
- A coating technique during which solvent evaporation may occur could result in poor adhesion performance of the polymer coat [42].
- The application of an interface layer between the polymer NFs and the Ti substrate may result in a tight attachment of otherwise poorly adhering NFs. The interfacial layer (which may be, for example, a polymer hybrid combination or an adhesive) should exhibit ideal adhesion characteristics with an affinity for both the NF film and the substrate surface [42].
Table 3 lists commonly used test methods for evaluating adhesion and the related American Society for Testing and Materials (ASTM) and International Organization for Standardization (ISO) standards.

Table 3.
Standards for adhesion measurement techniques for metallic substrates [83,84,85].
Abdal-hay et al. [42] evaluated the adhesion strength of Ti-coated samples in the dry and wet conditions using the pull-off adhesion test. The Ti specimens were first dip-coated in PLA solution followed by electrospinning of a PCL solution onto Ti substrates with a semi-dried PLA coating layer. In wet conditions, the coated samples were immersed in standard SBF. The results for the dry and wet adhesion tests were, respectively, 2.67 and 0.8 MPa for the PCL layer and 3.8 and 1.9 MPa for the PLA/PCL hybrid layer. The better adhesion of the PLA/PCL layer was attributed to the use of the PLA as an interfacial layer and the minimization of solvent evaporation and water diffusion to the implant surface at the initial dip-coating step [42].
Similarly, Kiran et al. [6] determined the adhesion strength of PCL/hydroxyapatite coating on pre-treated cpTi by means of a cross-cut tape test. The coating achieved excellent adhesion properties and was classified as 4 B, with less than 3% of the coating area removed per ASTM D3359 standard. The adhesion properties may be attributed to the surface treatment and to the organic/inorganic interface of PCL/hydroxyapatite [6]. Song et al. [79] conducted a scratch test to investigate the adhesion of the electrospun PCL NFs on Ti pins and confirmed a strong bonding strength of the NF coating with the pins. In addition, their ex vivo porcine bone implantation model demonstrated that NF coating was non-delaminated during implantation, and over 80% of the PCL NFs remained bound to the Ti implant surface after 30 min of ultrasound sonication. The authors, however, acknowledged that the exact mechanisms of interfacial adhesion between the NF coating and Ti implant surface remain vague.
6. Biological Properties of Nanofiber-Coated Ti Implants
Titanium as an implant material is used in the biomedical field owing to its superior properties over other commonly used biomaterials. However, they are also bio-inert and unable to bond efficiently with living cells directly after implantation into the human body [5,55]. Since their surfaces are vital in governing the response of the biological environment to artificial devices, coatings of Ti implants are being studied extensively as surface modification methods in order to promote bioactivity and enhance tissue integration [5,55]. Electrospun NFs show a potential for growth factors or signaling molecules for tissue regeneration [11].
6.1. Biomineralization Studies
The dynamic process of the development and growth of the apatite layer on a Ti substrate is an indication of its bioactivity and is generally investigated through biomineralization [1]. Moreover, minerals of amorphous calcium phosphate are the main constituents of the human bone and are highly biodegradable, biocompatible and bioactive [27]. Kiran et al. [1] illustrated mineralization by evaluating the apatite-forming ability of cpTi and Ti surfaces coated with pure PCL and PCL/TiO2 nanocomposite samples. The substrates were immersed in SBF for 21 days. Substantial mineralization was observed in PCL/TiO2 nanocomposite samples, while the control substrates exhibited insignificant mineralization.
Correspondingly, Abdal-hay et al. [42] applied a novel bicomponent PCL/PLA membrane onto Ti substrates using electrospinning and found apatite forming on the coated Ti samples but not on uncoated Ti samples when the samples were placed in SBF. For their mineralization studies, Ravichandran et al. [39] coated cpTi and Ti–6Al–4V disks with electrospun PLGA and PLGA/collagen NFs. The amount of ECM secreted by human stem cells was qualitatively analyzed using Alizarin red staining, and cell mineralization increased within a 21-day incubation period on the fiber-coated Ti surfaces. Song et al. [79] used a rat tibia implantation model to observe a progressive in situ mineralization within a PCL/PVA NF coating layer and presented indications of cell proteins and a measurable amount of calcium phosphate.
6.2. Osseointegration and Soft Tissue Attachment
Successful implantation of Ti implants involves the in vivo bio-response of the implants to the ECM during the healing process [5]. In addition, 3D electrospun NFs exhibit a high surface-to-volume ratio, which promotes soft and hard tissue attachment by further facilitating nutrient transport, cell binding and migration functions [11]. The direct and functional integration between living bone and surface of a Ti implant, otherwise coined osseointegration, determines the success of implantation [5,66,86]. For an implant to be successfully osseointegrated, primary stability—defined as the absence of micro-motion at the osseous site between the implant surface and the surrounding osseous tissue—should be established [5,39]. To assess biocompatibility Ti implants, Abdal-hay et al. [42] deposited a layer of PCL/PLA hybrid coating on the Ti surfaces, and the results showed superior performance of polymer-coated Ti samples by promoting osteoblast adherence, proliferation and survival.
The ECM of the natural bone is mostly secreted by osteogenic cells, and the bone contains bio-composite porous 3D collagen fibers [27]. Ti implants generally take approximately 3 to 4 months to integrate with the human tissue [5], while the overall mean survival rate for a dental implant is reported to be 2 to 16 years [39]. Therefore, the degradation time of the ultrafine osteogenic fibers should coincide with the regeneration and/or healing process to improve the probability of long-term implant stability [5,21]. Thus, orthopedic and dental implant surfaces should support the adhesion and overgrowth of cells [3]. Ravichandran et al. [39] used human mesenchymal stem cells (hMSCs), which are able to differentiate into soft and hard tissues, on cpTi and Ti–6Al–4V discs with PLGA and PLGA/collagen NFs by electrospinning and conducted various cell interaction assays. These included cell attachment to determine attachment efficiency of the samples for up to 60 min, cell proliferation using a colorimetric assay for up to 21 days and osteogenic differentiation of the cell by measuring the alkaline phosphatase activity also for up to 21 days. The in vitro results indicated that the nanofibrous coating on the Ti implant surfaces has the potential to enhance osseointegration.
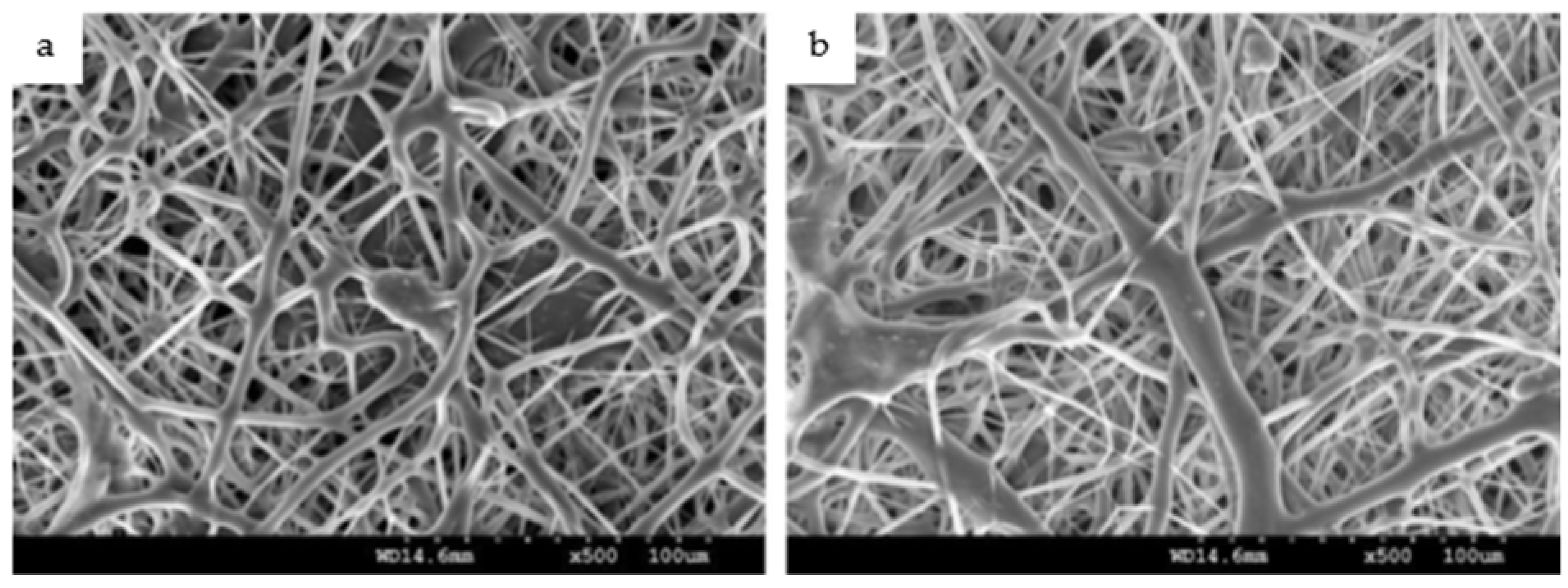
Microscopic images obtained during the investigations of Das et al. [5] revealed distinguishable differences in the surface topography between the uncoated and NF-coated Ti implant at various magnifications. The purpose of their investigation was to evaluate the osteogenic NF-coated Ti implants on rabbit study models. The surfaces of the Ti implants were coated with a bio-composite incorporating PCL using a modified electrospinning setup. The findings confirmed the hypothesis of the study that nanofibrous coating favors the dynamics of osseointegration. Similarly, the in vivo mechanical tests conducted by Khandaker et al. [43] using electrospun collagen/PCL NF coated on Ti–6Al–4V (ELI) revealed that all coated samples bonded with bone and none showed the existence of fibers on the Ti implant surfaces after 6 weeks of implantation. During a 3-week period of in vitro culture, rat osteoblast cells (R-OST-583) were able to persist, proliferate and differentiate on the Ti substrate in the presence and absence of the NF coating [43]. Kiran et al. [1] employed PCL/TiO2 nanocomposites, which were electrospun onto Ti surfaces, to demonstrate homogenous cell adhesion and proliferation of human fetal osteoblastic cell lines (hFOB) over the coated surfaces, as shown in Figure 3.
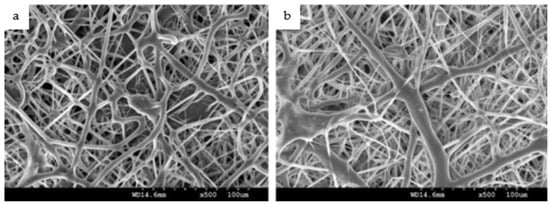
Figure 3.
SEM images of human fetal osteoblastic cell lines seeded on Ti substrates coated with PCL/TiO2 NFs after incubation for (a) 1 day and (b) 3 days [1].
The literature has also shown undesirable effects on cell adhesion associated with polymer NF coating. During the in vivo study of Zhang et al. [48], X-rays revealed radiographic signs of osteolysis and serious soft tissue swelling in rabbits implanted with Ti implants coated with the polymer PLGA using the electrospinning nanotechnique. Likewise, Boschetto et al. [2] found that uncoated Ti–6Al–4V discs presented larger mineralized areas of SaOS-2 human osteosarcoma cells associated with bone formation as compared to those coated with electrospun chitosan/PEO NFs, and osteoblast differentiation markers used to detect the bone formation process were more prevalent on the bare Ti–6Al–4V substrates than on those coated with the NFs. These NFs had to be infused with bioactive glasses to display the most homogeneous distribution of the osteoblast differentiation markers associated with mineralized matrix enhancement of all samples analyzed. These results were supported by the SEM images obtained by Şimşek et al. [19], which revealed a substantially reduced attachment of fibroblastic MC3T3-E1 preosteoblasts on cpTi strips coated with cross-linked PEO NFs when compared to the uncoated strips. The reduced cellular attachment persisted for 28 days, and the authors postulated that the poor affinity for the preosteoblastic cells, which are necessary for osseointegration, may be attributed to the hydrophilicity of the PEO polymer [19].
6.3. Antimicrobial Properties
Biomaterials used as medical implant materials are expected to display antibacterial activity for efficient biological activity [9]. Surfaces of Ti-based implants are susceptible to bacterial-associated infections during the invasive surgeries for which they are purposed [2]. As the amount of bone replacement surgeries increases, so do the incidences of implant-associated infection, which could lead to biofilm formation and devastating side effects [2,19,48]. Until recently, addressing antibacterial activity on the surface before the implantation was not considered part of biocompatibility enhancement modifications. Implant loosening resulting in re-surgeries has emphasized post-surgery complications resulting from bacterial infection, particularly with established and inherently treatment-resistant biofilms formed by microorganisms on the surfaces of biomedical implants [1]. Over the decades, several preventative and treatment strategies have been explored, and the majority generally suggest enriching implant surfaces with antibacterial agents, including antibiotic and metal-based antimicrobial agents, before surgery [2,66].
Another solution suggested for obtaining an antibacterial surface without sacrificing bioactivity is based on the use of coatings, hence the rapidly increasing popularity of NF coating of metallic substrates using the electrospinning process [2]. Şimşek et al. [19] significantly reduced attachment of both biofilm-forming and non-biofilm-forming strains of Staphylococcus epidermidis by coating Ti surfaces with PEO NFs using the electrospinning technique. Bacteria of the Staphylococcus genus, particularly Staphylococcus aureus and S. epidermidis, are responsible for a significant proportion of implant-related infections [19,48]. Hydrophilic chains of biocompatible polymers, such as poly(ethylene glycol) (PEG) or PEO-based coatings, provided a promising approach to inhibit bacterial attachment by means of the water layer covering the Ti substrate surface and introducing a high activation barrier against bacterial adhesion [19]. Boschetto et al. [2] electrospun chitosan blended with PEO onto Ti–6Al–4V ELI discs to improve the bioactivity and the antimicrobial properties. All their analyses, namely microbial viability assay, colony-forming unit counts and fluorescence microscopic observations, showed a significantly lower density of bacterial cells of S. epidermidis on the NF-coated surface as compared to the uncoated Ti–6Al–4V samples after 48 h.
In addition to the distinct ability of electrospun NF polymers to mimic natural matrices, they are able to retain various groups of antibacterial agents, including antibiotic drugs (for example, vancomycin, rifampicin and gentamicin) and metal/metal oxide-based antimicrobial agents (for example, silver (Ag), copper (Cu) and TiO2) for a controlled release and the longevity of implantation post-surgery without triggering the immune system to respond [1,9,11,66]. The ability of NFs to entrap and locally administer the release of antimicrobial agents is especially exceptional since the NF scaffold shares the cell attachment-promoting behavior of Ti, which induces the “race to the surface” feature between bacterial and mammalian cells [1]. Since the attachment of bacteria to the surface of a polymer may result in the formation of biofilms, biofilm-resistant polymers are essential for applications in the medical field. Polymers infused with antibacterial agents may promote prevention against bacterial adhesion and biofilm formation [9].
Zhang et al. [48] employed in vitro and in vivo experimental set-ups to evaluate the antimicrobial effect of Ti implants coated with electrospun PLGA and PLGA/vancomycin NFs against an S. aureus strain. There was no obvious effect of the PLGA-coated implants on the bacterial growth in vitro, while the PLGA/vancomycin group showed clear inhibition of bacterial growth. Furthermore, the in vivo assay showed signs of infection in the groups implanted with PLGA-coated Ti, and drug-loaded NFs did not stimulate immune reactions 4 weeks after implantation. Sadri et al. [87] also illustrated the antibacterial effectiveness of vancomycin-loaded chitosan/PEO coating against the adhesion of S. aureus. The occurrence of antibiotic-resistant bacteria, as with methicillin/oxacillin-resistant staphylococci, is another concern, which necessitates increments of antibiotics concentrations [3,7].
Jahanmard et al. [3] found that although antibiotics reduced both the planktonic and implant-adherent bacteria by at least 100-fold for up to 28 days, higher concentrations of antibiotics induce unwanted changes in NF morphology by forming micro-particles during the electrospinning process. Alternatively, Kiran et al. [1] carried out an antibacterial assay of Ti plates coated with PCL/TiO2 (2 and 5 wt %) against the bacterium S. aureus. The electrospun NF mat consisting of only PCL and 2% TiO2 showed a relative increase in the bacterial count compared to the control, and 5% TiO2 achieved antibacterial activity without significant toxicity. Aadil et al. [88] electrospun NF mat containing PVA and Ag nanoparticles, and the results revealed that the mat was effective against Gram-negative bacterium Escherichia coli and Gram-positive bacterium Bacillus circulans with disc diffusion inhibition zones of 1.1 and 1.3 cm, respectively. Similarly, des Ligneris et al. [89] successfully determined the antibacterial effect of PVA/Cu composite NFs was against E. coli.
6.4. Cytotoxicity
Titanium, Ti alloys and synthetic polymers are generally non-toxic [29]. Literature has illustrated through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity assay that synthetic polymers are not adversely toxic to various cells, including osteoblasts and fibroblasts [24]. However, the obligation to function in blended forms with additional materials (such as antimicrobials) in order to fulfil the biocompatibility responsibilities often introduces the toxicity factor to implants and coatings when the additional material is administered in bulk dosages.
Kiran et al. [1] observed the MTT assay for toxicity on surface-coated cpTi substrates for day 1 and day 3 using human fetal osteoblastic cell lines. The surfaces had been coated with PCL/TiO2 NF by electrospinning. Compared to cpTi and PCL NF mat, initial attachment and proliferation of PCL/TiO2 nanocomposites supported the growth of the cells and mediated their proliferation by up to approximately 38%. However, cytotoxic was observed at a higher TiO2 nanoparticle content. Conversely, Zhang et al. [48] measured the cell viability of osteoblastic cells on Ti implants coated with PLGA and PLGA/vancomycin NFs. The results of their Cell Counting Kit-8 (CCK-8) cytotoxicity evaluation validated the cell-promoting effect of all NF-coated implants and thus showed the absence of cell toxicity over a 28-day test period. This was attributed to the slow and controlled release of vancomycin by NFs for the PLGA/vancomycin group.
7. Correlation between the Various Properties for Implant Stability
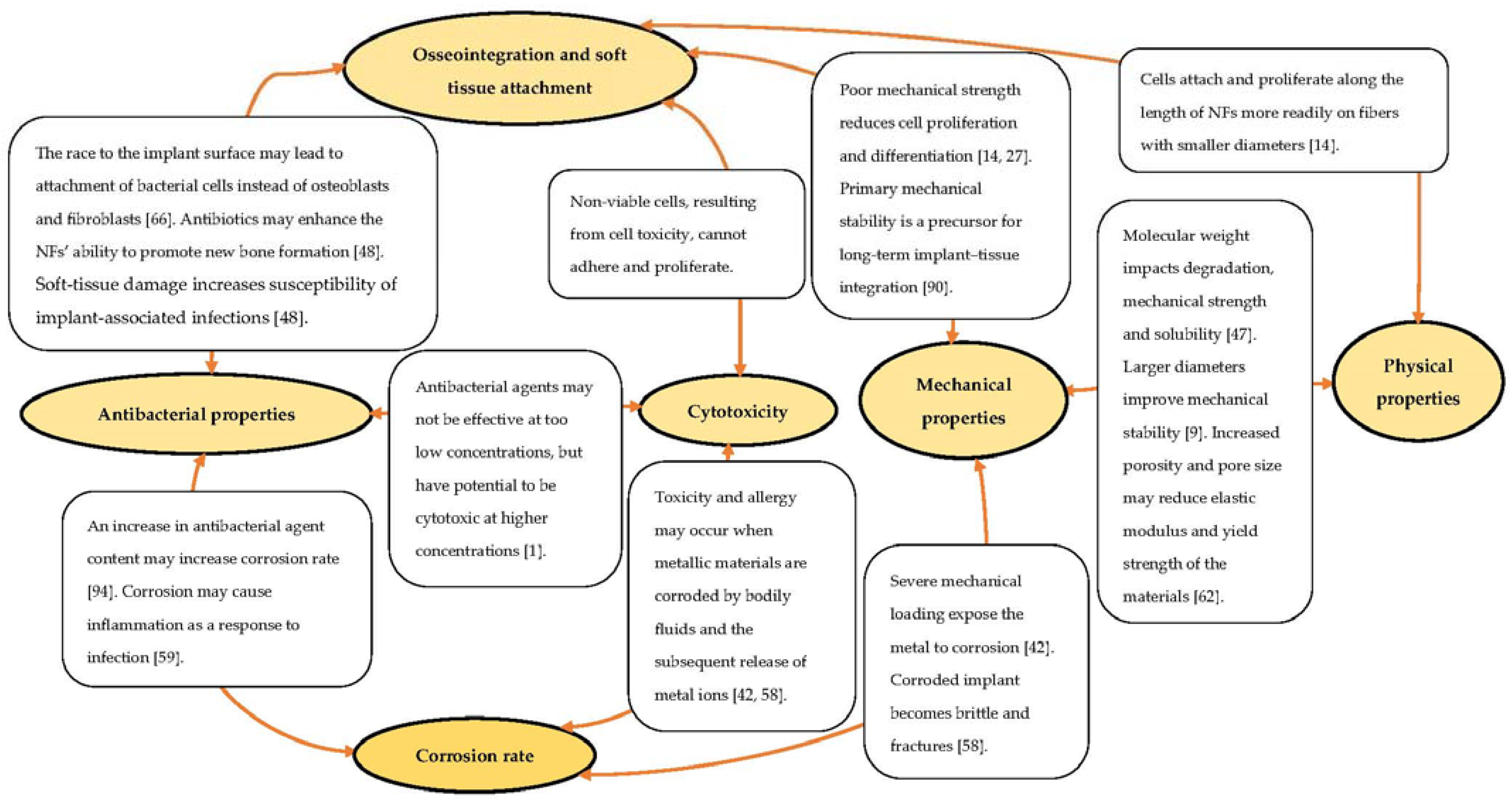
The stability of implants is a prerequisite characteristic for osseointegration and achieving long-term in vivo functioning [90]. Nonetheless, studies evaluating the stability of Ti implants coated with polymer NFs are scarce. Implant stability is defined as a measure of primary and secondary stability and is essential for influencing the outcome of implant treatment. It is, thus, useful as a tool for the objective prediction of implant success by indicating the most suitable interval for implantation [62,91]. Primary stability is determined by mechanical properties, including tensile and shear stresses at the implant–tissue interface, and secondary stability is identified as biological stability that is attainable through the process of osseointegration and soft tissue integration of the implant [62,92,93]. Several studies have illustrated a generally statistically significant correlation (p < 0.01; r > 0.5) between primary mechanical and secondary biological stability using invasive and non-invasive quantitative methods [90,91,92,93,94]. Figure 4 highlights some interrelations of the various properties that contribute to the failure of Ti implants in clinical applications to emphasize the recent advances towards the interplay between the various aspects presented in the current literature review.
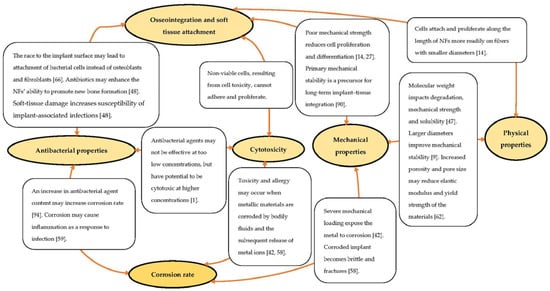
Figure 4.
Correlation of the various aspects relatable to implantation failure.
8. Future Recommendations
Research on the various aspect, individually or in combinations, mandatory for the functionalization of polymer NF-coated Ti biomedical implants is scarce. Thus, future recommendations are as follows:
- Comprehensive observations of the mechanisms at the implant–tissue and implant–coating interfaces should be prioritized.
- Further research is necessary to clarify the nature of the ECM interaction with electrospun NFs at the nanoscale.
- In vitro interactions between the tissue and the implant should be continually observed once the biodegradable NFs have been completely degraded in simulated human body conditions.
- Additional polymeric biomaterials should be evaluated for approval to be utilized in conjunction with implants to overcome the limitation of polymers when addressing critical issues, for instance, tensile strength and Young’s modulus, for load-bearing bone implants.
- With the various polymers available and human body complexities, polymer combinations, along with the nature and concentrations of additives required, should be explored.
- The various demands on NF coating toward the surface modification of Ti implants are mutually dependent and should be investigated holistically for the implants to attain clinical and commercial viability.
- In essence, the development of smart, functional materials exhibiting a synergy of chemical, physical, mechanical and biological properties should be the priority of implant-related studies.
9. Conclusions
Titanium and its alloys have increasingly been applied as medical implants over the decades because of their mechanical and biological advantages over fellow implantable materials. Likewise, the problems associated with metallic implants have also persisted since the discovery of biomaterials for orthopedic and dental implant applications. The root of most of these issues lies with the understanding of implant–tissue interactions at the nanoscale since the native tissue consists of complex nanostructures with unique arrangements of fibrous shapes. Thus, ECM-mimicking materials have prompted the need for further investigations. As such, nanotechnology has been found to provide effective means for manufacturing such materials. Electrospinning, in particular, offers advantages for the preparation of nanofibrous coating on Ti implant surfaces that resemble the fibrillary architecture of the ECM in native tissues.
In addition, most synthetic electrospun NFs, particularly composed of a mixture of polymers, are capable of exhibiting multiple biocompatible features. Furthermore, the accurate composition of the polymer NF coating may be able to provide the Ti implant surface with a tunable combination of physical, chemical, mechanical and biological properties to fulfil the implantation requirements. These requirements include mechanical stability with decelerated degradation rates of the NF coating while maximizing vigorous implant–tissue interactions and upholding the innate corrosion resistance and non-toxicity of Ti-based implants. Electrospinning of polymer NF scaffolds that are able to house and administer the dosages of various elements, including antibacterial and cell simulating agents, over a prolonged period are essential towards the realization of successful implantation. Implant success will, in turn, reduce the need for revision surgery, which would subsequently result in diminished surgical cost and implant-related mortality.
Author Contributions
Conceptualization, N.N., T.C.D. and O.d.S.; resources, N.N. and T.C.D.; writing—original draft preparation, N.N.; writing—review and editing, N.N., T.C.D. and O.d.S.; funding acquisition, T.C.D. and O.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Collaborative Programme in Additive Manufacturing (CPAM) (Contract No CSIR-NLC-CPAM-15-MOA-CUT-01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kiran, A.S.K.; Kumar, T.S.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and bioactive surface modifications of titanium implants by PCL/TiO2 nanocompo-site coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef] [Green Version]
- Boschetto, F.; Doan, H.N.; Vo, P.P.; Zanocco, M.; Zhu, W.; Sakai, W.; Adachi, T.; Ohgitani, E.; Tsutsumi, N.; Mazda, O.; et al. Antibacterial and osteoconductive effects of chitosan/polyethylene oxide (PEO)/bioactive glass nanofibers for orthopedic applications. Appl. Sci. 2020, 10, 2360. [Google Scholar] [CrossRef] [Green Version]
- Jahanmard, F.; Croes, M.; Castilho, M.; Majed, A.; Steenbergen, M.J.; Lietaert, K.; Vogely, H.C.; van der Wal, B.C.H.; Stapel, D.A.C.; Malda, J.; et al. Bactericidal coating to prevent early and delayed implant-related infections. J. Control. Release 2020, 326, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Zaman, H.A.; Sharif, S.; Idris, M.H.; Kamarudin, A. Metallic biomaterials for medical implant applications: A review. Appl. Mech. Mater. 2015, 735, 19–25. [Google Scholar] [CrossRef]
- Das, S.; Dholam, K.; Gurav, S.; Bendale, K.; Ingle, A.; Mohanty, B.; Chaudhari, P.; Bellare, J.R. Accentuated osseointegration in osteogenic nanofibrous coated titanium implants. Sci. Rep. 2019, 9, 17638. [Google Scholar] [CrossRef] [Green Version]
- Kiran, A.S.K.; Kumar, T.S.S.; Perumal, G.; Sanghavi, R.; Doble, M.; Ramakrish-na, S. Dual nanofibrous bioactive coating and antimicrobial surface treatment for infection resistant titanium implants. Prog. Org. Coat. 2018, 121, 112–119. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Bosco, R.; Van Den Beucken, J.; Leeuwenburgh, S.; Jansen, J. Surface engineering for bone implants: A trend from passive to active surfaces. Coatings 2012, 2, 95–119. [Google Scholar] [CrossRef] [Green Version]
- Kadavil, H.; Zagho, M.; Elzatahry, A.; Altahtamouni, T. Sputtering of electrospun polymer-based nanofibers for biomedical applications: A perspective. Nanomaterials 2019, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Li, H.M.; Zhang, Q.G.; Guo, N.N.; Zhu, A.M.; Liu, Q.L. Ultrafine polystyrene nanofibers and its application in nanofibrous membranes. Chem. Eng. Sci. 2015, 264, 329–335. [Google Scholar] [CrossRef]
- Weng, L.; Xie, J. Smart electrospun nanofibers for controlled drug release: Recent advances and new perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qiu, Y.; Wang, H.; Chen, Y.; Jin, S.; Chen, S. Preparation of nanofibers with renewable polymers and their application in wound dressing. Int. J. Polym. Sci. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- AlFalah, M.G.K.; Kamberli, E.; Abbar, A.H.; Kandemirli, F.; Saracoglu, M. Corrosion performance of electrospinning nanofiber ZnO-NiO-CuO/polycaprolactone coated on mild steel in acid solution. Surf. Interfaces 2020, 21, 100760. [Google Scholar] [CrossRef]
- Tian, F.; Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Yokoyama, Y.; Estrada, G.G.; Kobayashi, H. Quantitative analysis of cell adhesion on aligned micro- and nanofibers. J. Biomed. Mater. Res. A 2008, 84, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.N.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.K.; Tuan, R.S. Tissue engineering with mesenchymal stem cells. IEEE Eng. Med. Biol. Mag. 2003, 22, 51–56. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [Green Version]
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Şimşek, M.; Aldemir, S.D.; Gümüşderelioğlu, M. Anticellular PEO coatings on titanium surfaces by sequential electrospinning and crosslinking processes. Emergent Mater. 2019, 2, 169–179. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative re-view of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Chong, S.-F.; Smith, A.A.A.; Zelikin, A.N. Microstructured, functional PVA hydrogels through bioconjugation with oligopeptides under physiological conditions. Small 2013, 9, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Briceño, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar]
- Montoro, S.R.; Medeiros, S.d.F.; Alves, G.M. Nanostructured hydrogel. In Nanostructured Polymer Blends; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; Elsevier Inc.: Oxford, UK, 2014; pp. 325–355. [Google Scholar]
- Boia, R.; Dias, P.A.N.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrósio, A.F.; Bragad, M.E.M.; et al. Po-rous poly(ε-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control Release 2019, 316, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Perumal, G.; Sivakumar, P.M.; Nandkumar, A.M.; Doble, M. Synthesis of magnesium phosphate nanoflakes and its PCL composite electrospun nanofiber scaffolds for bone tissue regeneration. Mater. Sci. Eng. C 2020, 109, 110527. [Google Scholar] [CrossRef]
- Chen, P. A Preliminary Discourse on Adhesion of Nanofibers Derived from Electrospun Polymers. Ph.D. Thesis, University of Akron, Akron, OH, USA, 2013. [Google Scholar]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.I.; Kim, B.-S.; Kang, S.W.; Kwon, J.H.; Lee, Y.M.; Kim, S.H.; Kim, Y.H. In vivo biocompatibilty and degradation behavior of elastic poly(L-lactide-co-epsilon-caprolactone) scaffolds. Biomaterials 2004, 25, 5939–5946. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Oh, T.H. Biopolymer coatings for biomedical applications. Polymers 2020, 12, 3061. [Google Scholar] [CrossRef]
- Ravichandran, R. Biomimetic Surface Modification of Dental Implant for Enhanced Osseointegration. Master’s Thesis, National University of Singapore, Singapore, 2009. [Google Scholar]
- Wang, Y.; Li, M.; Rong, J.; Nie, G.; Qiao, J.; Wang, H.; Wu, D.; Su, Z.; Niu, Z.; Huang, Y. Enhanced orientation of PEO polymer chains induced by nanoclays in electrospun PEO/clay composite nanofibers. Colloid Polym. Sci. 2013, 291, 1541–1546. [Google Scholar] [CrossRef]
- Helmus, M.N.; Gibbons, D.F.; Cebon, D. Biocompatibility: Meeting a key functional requirement of next-generation medical devices. Toxicol. Pathol. 2008, 36, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Tao, J. Effects of Molecular Weight and Solution Concentration on Electrospinning of PVA. Master’s Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, 2003. [Google Scholar]
- Al Aboody, M.S. Electrospun fabrication and direct coating of bio-degradable fibrous composite on orthopedic titanium implant: Synthesis and characterizations. Mater. Res. Express 2021, 8, 015307. [Google Scholar] [CrossRef]
- Chen, S.; Hao, Y.; Cui, W.; Chang, J.; Zhou, Y. Biodegradable electrospun PLLA/chitosan membrane as guided tissue regeneration membrane for treating periodontitis. J. Mater. Sci. 2013, 48, 6567–6577. [Google Scholar] [CrossRef] [Green Version]
- Miele, D.; Catenacci, L.; Rossi, S.; Sandri, G.; Sorrenti, M.; Terzi, A.; Giannini, C. Collagen/PCL nanofibers electrospun in green solvent by DOE assisted process. An in-sight into collagen contribution. Materials 2020, 13, 4698. [Google Scholar] [CrossRef]
- Ravichandran, R.; Ng, C.C.H.; Liao, S.; Pliszka, D.; Raghunath, M.; Ramakrishna, S.; Chan, C.K. Biomimetic surface modification of titanium surfaces for early cell capture by advanced electrospinning. Biomed. Mater. 2012, 7, 015001. [Google Scholar] [CrossRef]
- Teo, W.-E. Electrospun Coated Metal Implants. Available online: http://electrospintech.com/coatedmetal.html (accessed on 14 January 2022).
- Lee, B.-Y.; Behler, K.; Kurtoglu, M.E.; Wynosky-Dolfi, M.A.; Rest, R.F.; Gogotsi, Y. Titanium dioxide-coated nanofibers for advanced filters. J. Nanoparticle Res. 2010, 12, 2511–2519. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Hwang, M.-G.; Lim, J.K. In vitro bioactivity of titanium implants coated with bicomponent hybrid biodegradable polymers. J. Sol-Gel Sci. Technol. 2012, 64, 756–764. [Google Scholar] [CrossRef]
- Khandaker, M.; Riahinezhad, S.; Sultana, F.; Morris, T.; Wolf, R.; Vaughan, M. Effect of collagen-polycaprolactone nanofibers matrix coating on the In vitro cytocompatibility and in vivo bone responses of titanium. J. Med. Biol. Eng. 2018, 38, 197–210. [Google Scholar] [CrossRef]
- Khandaker, M.; Riahinezhad, S.; Williams, W.R.; Wolf, R. Microgroove and collagen-poly(ε-caprolactone) nanofiber mesh coating improves the mechanical stability and osseointegration of titanium implants. Nanomaterials 2017, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Nitti, P.; Gallo, N.; Natta, L.; Scalera, F.; Palazzo, B.; Sannino, A.; Gervaso, F. Influence of nanofiber orientation on morphological and mechanical properties of electrospun chitosan mats. J. Healthc. Eng. 2018, 2018, 3651480. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wei, B.; Xu, Y.; Gu, Q.; Wang, L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of im-plant-associated infections. Int. J. Nanomed. 2014, 9, 3027–3036. [Google Scholar]
- Yang, D.; Li, Y.; Nie, J. Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydr. Polym. 2007, 69, 538–543. [Google Scholar] [CrossRef]
- Morel, A.; Domaschke, S.; Urundolil Kumaran, V.; Alexeev, D.; Sadeghpour, A.; Ramakrishna, S.N.; Ferguson, S.J.; Rossi, R.M.; Mazza, E.; Ehret, A.E.; et al. Correlating diameter, mechanical and structural properties of poly(L-lactide) fibres from needleless electrospinning. Acta Biomater. 2018, 8, 169–183. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef] [Green Version]
- Colmenares-Roldán, G.J.; Quintero-Martínez, Y.; Agudelo-Gómez, L.M.; Rodríguez-Vinasco, L.F.; Hoyos-Palacio, L.M. Influence of the molecular weight of polymer, solvents and operational condition in the electrospinning of polycaprolactone. Rev. Fac. Ing. Univ. Antioq. 2017, 84, 35–45. [Google Scholar] [CrossRef]
- Leong, M.F.; Chian, K.S.; Mhaisalkar, P.S.; Ong, W.F.; Ratner, B.D. Effect of electrospun poly(D,L-lactide) fibrous scaffold with nanoporous surface on attachment of por-cine esophageal epithelial cells and protein adsorption. J. Biomed. Mater. Res. A 2009, 89, 1040–1048. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface modifications of titanium materials for developing corrosion behavior in human body environment: A review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Sasikumar, Y.; Karuppusamy, I.; Nallaiyan, R. Surface modification methods for titanium and its alloys and their corrosion behavior in biological environment: A review. J. Bio-Tribo-Corros. 2019, 5, 36. [Google Scholar] [CrossRef]
- Shaterian, M.; Khoobi, A.; Enhessari, M.; Ozaee, K. A new strategy based on thermodiffusion of ceramic nanopigments into metal surfaces and formation of anti-corrosion coatings. Microporous Mesoporous Mater. 2015, 218, 62–68. [Google Scholar] [CrossRef]
- Hanas, T.; Sampath Kumar, T.S. Tailoring biodegradation of fine grained AZ31 alloy implants by nanofibrous coatings. Mater. Today Proc. 2017, 4, 6697–6703. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Pat. Mater. Sci. 2010, 2, 40–54. [Google Scholar]
- Gilbert, J.L.; Kubacki, G.W. Oxidative stress, inflammation, and the corrosion of metallic biomaterials: Corrosion causes biology and biology causes corrosion. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Elsevier Inc.: London, UK, 2016; pp. 59–88. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases. 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Fernandes, D.J.; de Souza, F.M.; Monteiro, E.d.S.; de Biasi, R.S. Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J. Mater. Res. Technol. 2019, 8, 1060–1069. [Google Scholar] [CrossRef]
- Grzeskowiak, R.M.; Schumacher, J.; Dhar, M.S.; Harper, D.P.; Mulon, P.-Y.; Anderson, D.E. Bone and cartilage interfaces with orthopedic implants: A literature review. Front. Surg. 2020, 7, 601244. [Google Scholar] [CrossRef]
- Nakano, T. Mechanical properties of metallic biomaterials. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing Limited: Oxford, UK, 2010; pp. 71–98. [Google Scholar]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Niinomi, M. Recent progress in research and development of metallic structural biomaterials with mainly focusing on mechanical biocompatibility. Mater. Trans. 2018, 59, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dzogbewu, T.C.; du Preez, W.B. Additive manufacturing of titanium-based implants with metal-based antimicrobial agents. Metals 2021, 11, 453. [Google Scholar] [CrossRef]
- Whisnant, D. Polymer Chemistry: Mechanical Properties. Available online: https://eng.libretexts.org/Bookshelves/Materials_Science/Supplemental_Modules_(Materials_Science)/Polymer_Chemistry/Polymer_Chemistry%3A_Mechanical_Properties (accessed on 21 October 2021).
- Fernandes, D.J.; Elias, C.N.; Valiev, R.Z. Properties and performance of ultrafine grained titanium for biomedical applications. Mater. Res. 2015, 18, 1163–1175. [Google Scholar] [CrossRef]
- American Elements. Titanium—Commercially Pure (CP). Available online: https://www.americanelements.com/titanium-commercially-pure-cp-7440-32-6 (accessed on 14 January 2022).
- AZoMaterials. Titanium (Ti)—Properties, Applications. Available online: https://www.azom.com/properties.aspx?ArticleID=712 (accessed on 30 August 2021).
- Hosseini, S. Fatigue of Ti-6Al-4V. In Biomedical Engineering—Technical Applications in Medicine; Hudak, R., Penhaker, M., Majernik, J., Eds.; BoD—Books on Demand: Norderstedt, Germany, 2012; pp. 75–92. [Google Scholar]
- MatWeb. Titanium, Ti. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=66a15d609a3f4c829cb6ad08f0dafc01 (accessed on 30 August 2021).
- AZoMaterials. Titanium Alloys—Ti6Al4V Grade 5. Available online: https://www.azom.com/properties.aspx?ArticleID=1547 (accessed on 8 June 2021).
- MatWeb. Titanium Ti-6Al-4V ELI (Grade 23), Annealed. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=c4297fb8f1094da189732c224e3be1ed (accessed on 30 August 2021).
- Poinern, G.E.J.; Brundavanam, R.K.; Fawcett, D. Nanometre scale hydroxyapatite ceramics for bone tissue engineering. Am. J. Biomed. Eng. 2013, 3, 148–168. [Google Scholar]
- Boyan, B.D.; Lossdörfer, S.; Wang, L.; Zhao, G.; Lohmann, C.H.; Cochran, D.L. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur. Cells Mater. 2003, 6, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bumgardner, J.D.; Cavin, R.; Carnes, D.L.; Ong, J.L. Osteoblast precursor cell attachment on heat-treated calcium phosphate coatings. J. Dent. Res. 2003, 82, 449–453. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Seta, J.; Chen, L.; Bergum, C.; Zhou, Z.; Kanneganti, P.; Kast, R.E.; Auner, G.W.; Shen, M.; Markel, D.C.; et al. Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection. Biomed. Mater. 2017, 12, 45008. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, K.; Lu, X.; Lam, Y.C. A review on the mechanical methods for evaluating coating adhesion. Acta Mech. 2014, 225, 431–452. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Tian, N.; Dong, X.; Cheng, C.-K. Implant coating manufactured by micro-arc oxidation and dip coating in resorbable polylactide for antimicrobial applica-tions in orthopedics. Coatings 2019, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Prolongo, S.G.; Gude, M.R.; Ureña, A. Nanoreinforced adhesives. In Nanofibers; Kumar, A., Ed.; IntechOpen Limited: London, UK, 2010; pp. 39–68. [Google Scholar]
- Maxwell, A.S. Review of Test Methods for Coating Adhesion. Available online: https://eprintspublications.npl.co.uk/2077/1/MATC49.pdf (accessed on 20 September 2021).
- American Society for Testing and Materials (ASTM). Standard Test Method for Evaluation of Scratch Resistance of Polymeric Coatings and Plastics Using an Instrumented Scratch Machine. Available online: https://www.astm.org/Standards/D7027.htm (accessed on 6 September 2021).
- ASTM. Standard Practice for Instrumented Indentation Testing. Available online: https://www.astm.org/Standards/E2546.htm (accessed on 6 September 2021).
- Raita, Y.; Komatsu, K.; Hayakawa, T. Pilot study of gingival connective tissue responses to 3-dimensional collagen nanofiber-coated dental implants. Dent. Mater. J. 2015, 34, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Sadri, M.; Pashmforosh, N.; Samadieh, S. Implants modified with polymeric nanofibers coating containing the antibiotic vancomycin. Nanomed. Res. J. 2017, 2, 208–215. [Google Scholar]
- Aadil, K.R.; Mussatto, S.I.; Jha, H. Synthesis and characterization of silver nanoparticles loaded poly(vinyl alcohol)-lignin electrospun nanofibers and their antimicrobial activity. Int. J. Biol. Macromol. 2018, 120, 763–767. [Google Scholar] [CrossRef] [Green Version]
- des Ligneris, E.; Dumée, L.F.; Al-Attabi, R.; Castanet, E.; Schütz, J.; Kong, L. Mixed matrix poly(vinyl alcohol)-copper nanofibrous anti-microbial air-microfilters. Membranes 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swami, V.; Vijayaraghavan, V.; Swami, V. Current trends to measure implant stability. J. Indian Prosthodont. Soc. 2016, 16, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, M.F.J.; Al-Jumaily, H.A. The relationship between primary (mechanical) and secondary (biological) implant stability: A new measurement technique. J. Med. Dent. Sci. 2020, 8, 11–15. [Google Scholar]
- Vollmer, A.; Saravi, B.; Lang, G.; Adolphs, N.; Hazard, D.; Giers, V.; Stoll, P. Factors influencing primary and secondary implant stability—A retrospective cohort study with 582 implants in 272 patients. Appl. Sci. 2020, 10, 8084. [Google Scholar] [CrossRef]
- Monje, A.; Ravidà, A.; Wang, H.-L.; Helms, J.A.; Brunski, J.B. Relationship be-tween primary/mechanical and secondary/biological implant stability. Int. J. Oral Maxillofac. Implants. 2019, 34, s7–s23. [Google Scholar] [CrossRef]
- Chen, J.; Peng, W.; Zhu, L.; Tan, L.; Etim, I.P.; Wang, X.; Yang, K. Effect of copper content on the corrosion behaviors and antibacterial properties of binary Mg-Cu alloys. Mater. Technol. 2018, 33, 145–152. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).