Fiber Spinning from Cellulose Solutions in Imidazolium Ionic Liquids: Effects of Natural Antioxidants on Molecular Weight, Dope Discoloration, and Yellowing Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Aging of Spinning Dopes
2.2. Dry-Jet Wet Spinning
2.3. Rheology
2.4. Molecular Weight Distribution
2.5. Accelerated Aging of the Fibers
3. Results and Discussion
3.1. Side Reactions in the Spinning Dope: Solvent-Derived vs. Autoxidative Processes
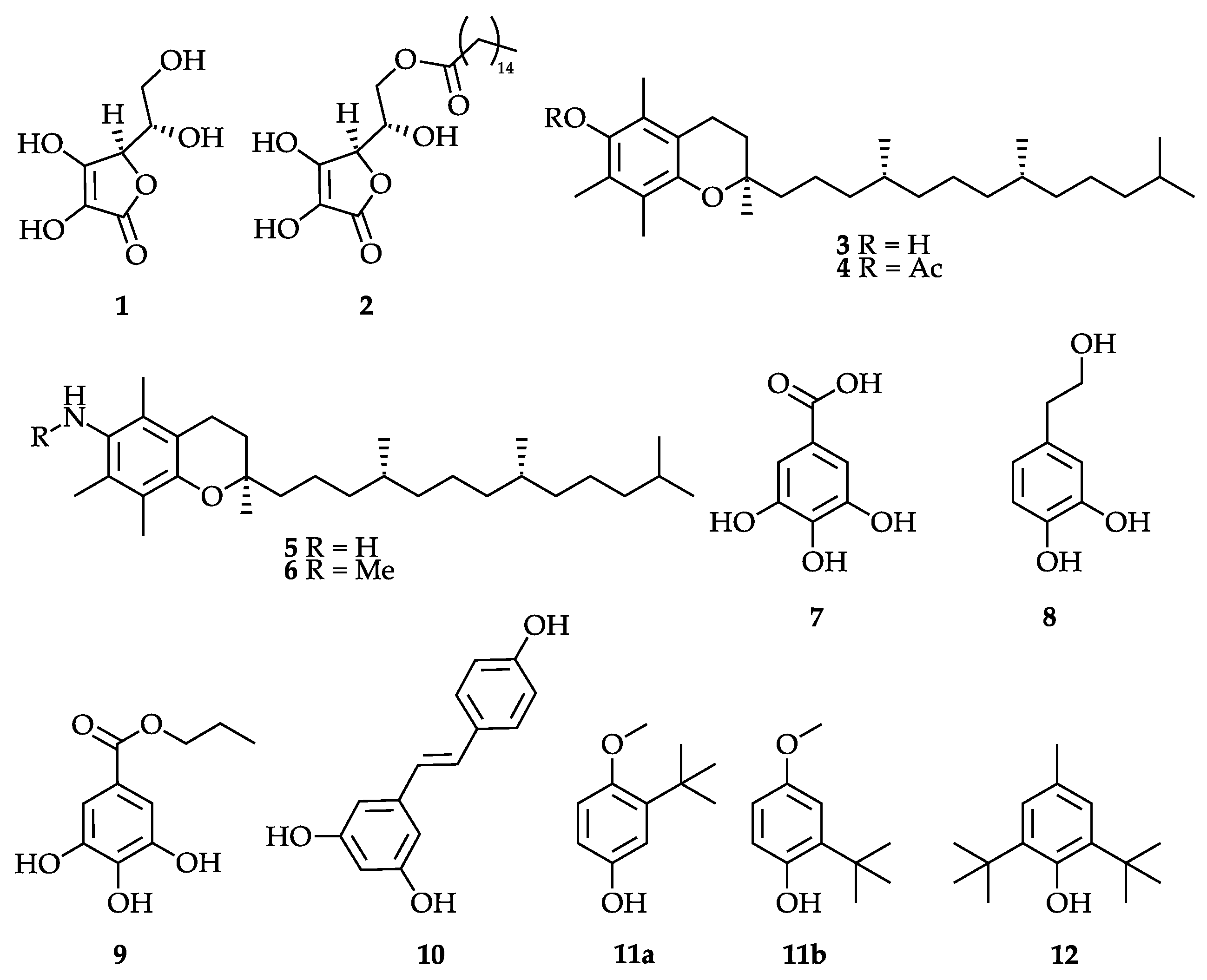
3.2. Selection of Antioxidants
3.3. Cellulose Degradation in the Spinning Dope
- (1)
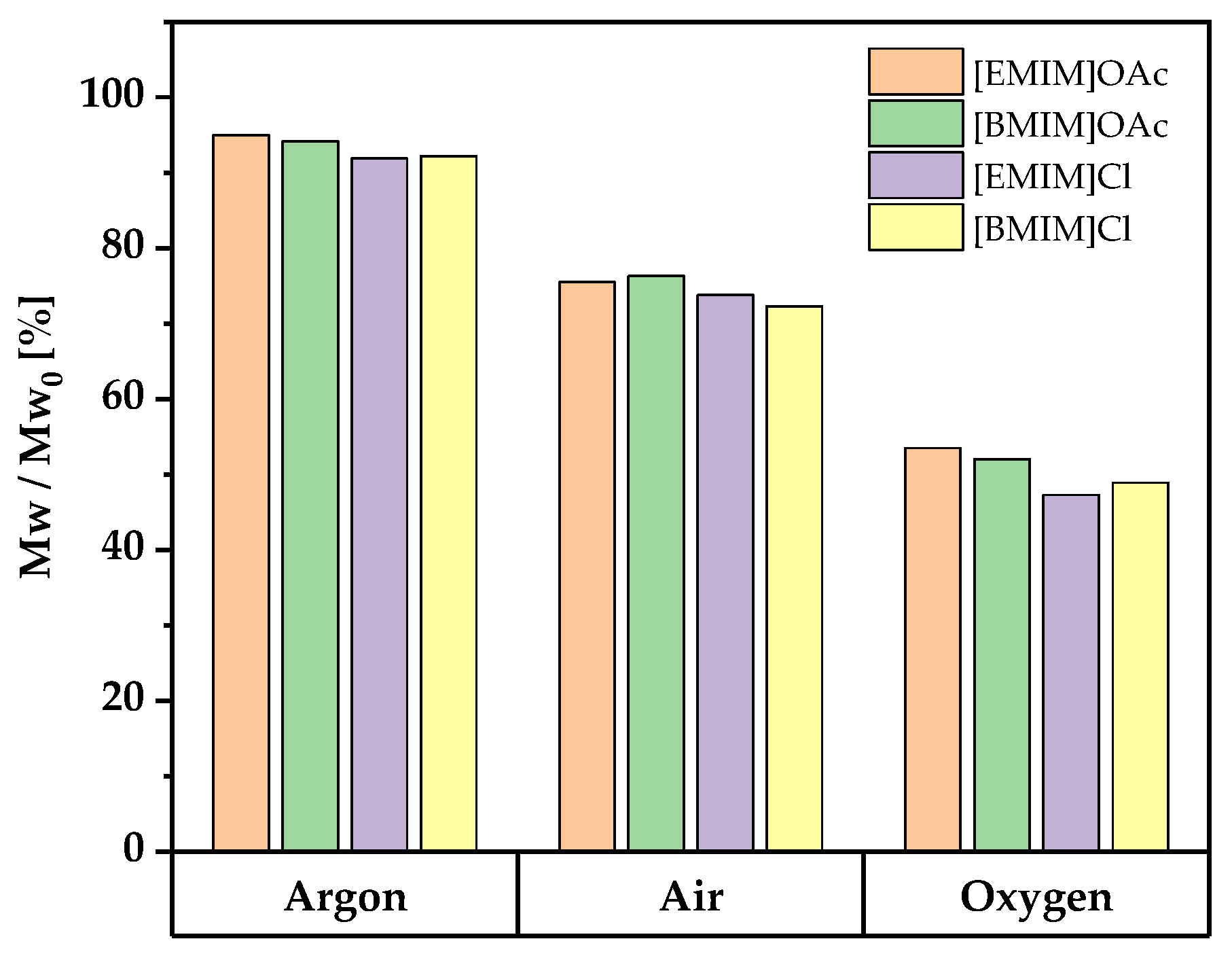
- All employed antioxidants had a protective effect on cellulose integrity. The molar mass loss was in all cases smaller than without additive. In some cases, the protective effect was better than working under an inert argon atmosphere (cf. Figure 1), which demonstrated that the protective atmosphere was able to limit, but not fully eliminate the autoxidation processes. Cellulose degradation during the first hour of swelling at room temperature was not detectable (data not shown), which indicated that the side reactions are mainly connected with elevated temperatures.
- (2)
- All antioxidants used were chemically compatible with the ionic liquids and with the cellulose solute. No violent reactions, signs of pronounced degradation of solvent or solute, phase separations, or precipitates were observed. The effects of antioxidant addition on dope viscosity and spinnability were insignificant, which can be related to the very low content of the additives (2 wt% rel. to the dissolved pulp (5 wt%) corresponding to 0.1 wt% of the dope).
- (3)
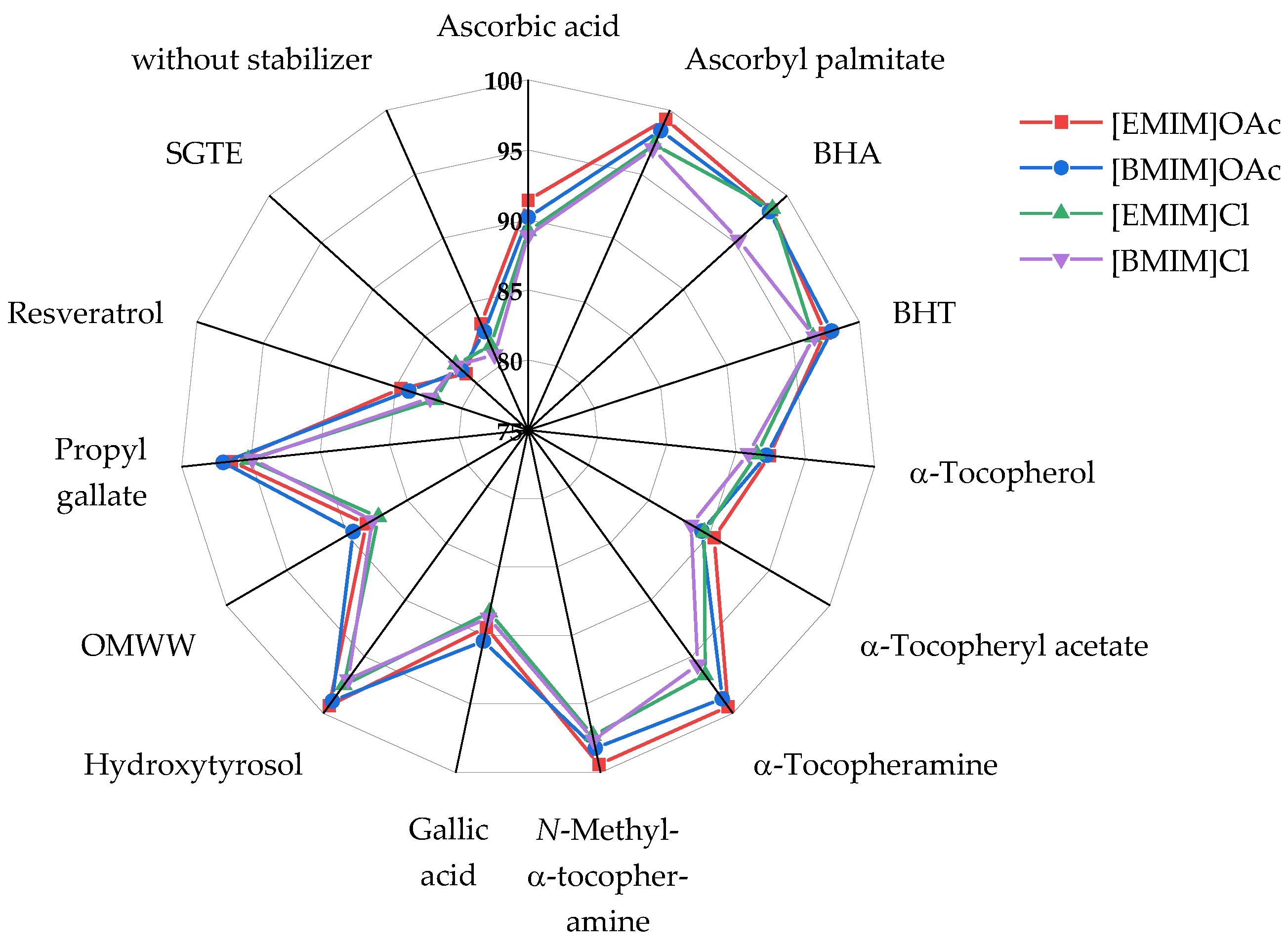
- The differences between the four ionic liquids were minor. For a given antioxidant, the same effectiveness category (A, B, or C, see below) was obtained for all four ILs, without a single exception. On average, degradation in the two imidazolium chloride ILs was 2–3% (relative molar loss) stronger than in the imidazolium acetate ILs, independent of the antioxidant used.
- (4)
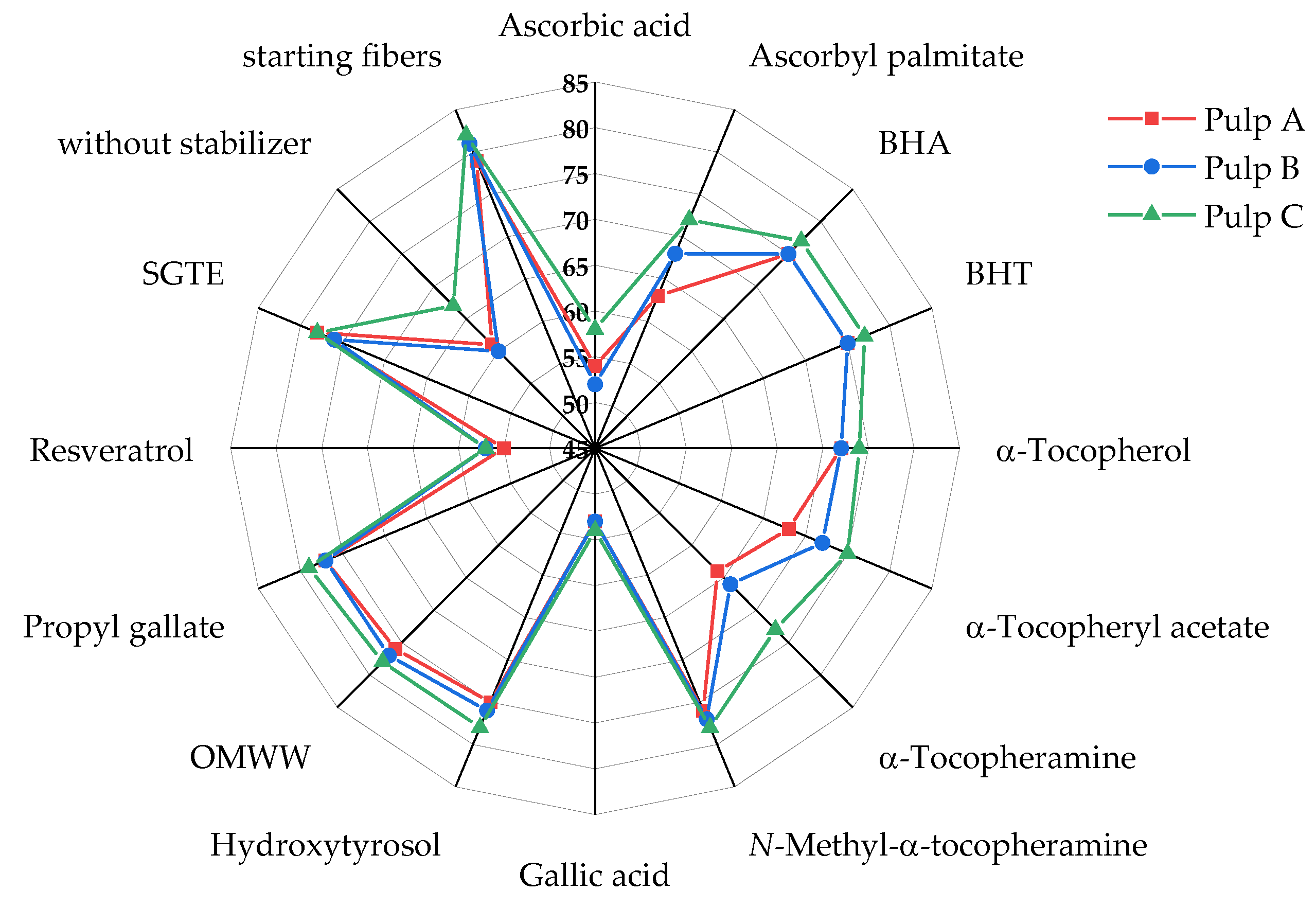
- The results were very similar for all three pulps used. The relative molar mass losses for the pulps at a given pair of antioxidant and IL differed by less than 4%. The values in Figure 2, determined for pulp B, are thus also fully representative for pulps A and C, so it appears possible to draw a general conclusion from the test set.
3.4. Discoloration of the IL Spinning Dope
3.5. Yellowing Behavior of the Spun Fibers upon Accelerated Aging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sixta, H.; Iakovlev, M.; Testova, L.; Roselli, A.; Hummel, M.; Borrega, M.; van Heiningen, A.; Froschauer, C.; Schottenberger, H. Novel concepts of dissolving pulp production. Cellulose 2013, 20, 1547–1561. [Google Scholar] [CrossRef]
- Sixta, H.; Michud, A.; Hauru, L.; Asaadi, S.; Ma, Y.; King, A.W.T.; Kilpeläinen, I.; Hummel, M. Ioncell-F: A high-strength regenerated cellulose fibre. Nordic Pulp Pap. Res. J. 2015, 30, 43–57. [Google Scholar] [CrossRef]
- Stepan, A.M.; Michud, A.; Hellstén, S.; Hummel, M.; Sixta, H. IONCELL-P&F: Pulp Fractionation and Fiber Spinning with Ionic Liquids. Ind. Eng. Chem. Res. 2016, 55, 8225–8233. [Google Scholar]
- Asaadi, S.; Kakko, T.; King, A.W.T.; Kilpeläinen, I.; Hummel, M.; Sixta, H. High-Performance Acetylated Ioncell-F Fibers with Low Degree of Substitution. ACS Sustain. Chem. Eng. 2018, 6, 9418–9426. [Google Scholar] [CrossRef]
- Guizani, C.; Larkiala, S.; Moriam, K.; Sawada, D.; Elsayed, S.; Rantasalo, S.; Hummel, M.; Sixta, H. Air gap spinning of a cellulose solution in [DBNH][OAc] ionic liquid with a novel vertically arranged spinning bath to simulate a closed loop operation in the Ioncell® process. J. Appl. Polym. Sci. 2021, 138, 49787. [Google Scholar] [CrossRef]
- Zhang, J.; Yamagishi, N.; Gotoh, Y.; Potthast, A.; Rosenau, T. High Performance Cellulose Fibers Regenerated from 1-Butyl-3-methylimidazolium Chloride Solution: Effects of Viscosity and Molecular Weight. J. Appl. Polym. Sci. 2020, 137, 48681–48688. [Google Scholar] [CrossRef]
- Zhang, J.; Kitayama, H.; Potthast, A.; Rosenau, T.; Gotoh, Y. Non-woven fabrics of fine regenerated cellulose fibers prepared from ionic-liquid solution via wet type solution blow spinning. Carbohydr. Polym. 2019, 226, 115258. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, T.; Potthast, A.; Kosma, P.; Chen, C.L.; Gratzl, J.S. Autocatalytic Decomposition of N-Methylmorpholine-N-oxide Induced by Mannich Intermediates. J. Org. Chem. 1999, 64, 2166–2167. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Milacher, W.; Adorjan, I.; Hofinger, A.; Kosma, P. Discoloration of cellulose solutions in N-methyl-morpholine-N-oxide (Lyocell). Part 2: Isolation and Identification of Chromophores. Cellulose 2005, 12, 197–208. [Google Scholar] [CrossRef]
- Chrapava, S.; Touraud, D.; Rosenau, T.; Potthast, A.; Kunz, W. The investigation of the influence of water and temperature on the LiCl/DMAc/cellulose system. Phys. Chem. Chem. Phys. 2003, 5, 1842–1847. [Google Scholar] [CrossRef]
- Liebner, F.; Ebner, G.; Becker, E.; Potthast, A.; Rosenau, T. Thermal aging of 1-alkyl-3-methylimidazolium ionic liquids and its effect on dissolved cellulose. Holzforschung 2010, 64, 161–166. [Google Scholar] [CrossRef]
- Ebner, G.; Schiehser, S.; Potthast, A.; Rosenau, T. Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett. 2008, 49, 7322–7324. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Henniges, U.; Schiehser, S.; Kosma, P.; Saake, B.; Lebioda, S.; Radosta, S.; Vorwerg, W.; Wetzel, H.; et al. Comparison testing of methods for gel permeation chromatography of cellulose: Coming closer to a standard protocol. Cellulose 2015, 22, 1591–1613. [Google Scholar] [CrossRef]
- Sammons, R.J.; Collier, J.R.; Rials, T.G.; Petrovan, S. Rheology of 1-butyl-3-methylimidazolium chloride cellulose solutions. I. Shear rheology. J. Appl. Polym. Sci. 2008, 110, 1175. [Google Scholar] [CrossRef]
- Zaccaron, S.; Ahn, K.; Henniges, U.; Potthast, A.; Rosenau, T. An improved, less erroneous protocol for the classical “cuen”, “cuoxam” or “cadoxen” viscosity measurements of pulps. Cellulose 2022, 29, 3733–3744. [Google Scholar] [CrossRef]
- ISO 2470-1; Measurement of Diffuse Blue Reflectance Factor—Part 1: Indoor Daylight Conditions (ISO Brightness). International Organization for Standardization: Geneva, Switzerland, 2009.
- Brightness loss of pulp. In TAPPI Method UM 200; TAPPI: Peachtree Corners, GA, USA, 2012.
- Standard Testing Methods. In Paptac E.4P; Pulp and Paper Technical Association of Canada: Brossard, QC, Canada, 2014.
- Packer, L.; Fuchs, J. Vitamin C in Health and Disease; Marcel Dekker Inc.: New York, NY, USA, 1997. [Google Scholar]
- Clemetson, C.A.B. Vitamin C; CRC Press: Boca Raton, FL, USA, 2017; Volume 1. [Google Scholar]
- Parsons, E. Ascorbic Acid: Properties, Synthesis and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 1–285. [Google Scholar]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin c—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.M.; León, P.G.; Flores, S.K.; Pérez, C.D.; De’Nobili, M.D. Development of polysaccharide networks for L-(+)-ascorbic acid stabilization into antioxidant/nutritional supplier-edible films. In Handbook of Carbohydrate Polymers: Development, Properties and Applications; Ito, R., Matsuo, Y., Eds.; Nova Science Publisher Inc.: London, UK, 2010; pp. 393–418. [Google Scholar]
- Abbas, S.; da Wei, C.; Hayat, K.; Xiaoming, Z. Ascorbic Acid: Microencapsulation Techniques and Trends—A Review. Food Rev. Int. 2012, 28, 343–374. [Google Scholar] [CrossRef]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer selection for hot-melt extrusion coupled to fused deposition modelling in pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Antioxidants and its properties as affected by extrusion process: A review. Recent Pat. Food Nutr. Agric. 2015, 7, 108–114. [Google Scholar] [CrossRef]
- Mohamed, H.M. Green, environment-friendly, analytical tools give insights in pharmaceuticals and cosmetics analysis. Trends Analyt. Chem. 2015, 66, 176–192. [Google Scholar] [CrossRef]
- Yavir, K.; Konieczna, K.; Marcinkowski, K.; Kloskowski, A. Ionic liquids in the microextraction techniques: The influence of ILs structure and properties. Trends Analyt. Chem. 2020, 130, 115994. [Google Scholar] [CrossRef]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Najafi-Taher, R.; Derakhshan, M.A.; Faridi-Majidi, R.; Amani, A. Preparation of an ascorbic acid/PVA-chitosan electrospun mat: A core/shell transdermal delivery system. RSC Adv. 2015, 5, 50462–50469. [Google Scholar] [CrossRef]
- Dias, J.R.; Antunes, F.E.; Bártolo, P.J. Influence of the rheological behaviour in electrospun PCL nanofibres production for tissue engineering applications. Chem. Eng. Trans 2013, 32, 1015–1020. [Google Scholar]
- Zhao, X.; Lui, Y.S.; Toh, P.W.J.; Loo, S.C.J. Sustained release of hydrophilic L-ascorbic acid 2-phosphate magnesium from electrospun polycaprolactone scaffold—A study across blend, coaxial, and emulsion electrospinning techniques. Materials 2014, 7, 7398–7408. [Google Scholar] [CrossRef] [Green Version]
- Rwei, S.P.; Lin, W.-P. Synthesis and characterization of adipic acid/polyethylene glycol/poly(ethylene terephthalate) copolyester fiber. Text. Res. J. 2015, 85, 1691–1703. [Google Scholar] [CrossRef]
- Maxim, M.L.; White, J.F.; Block, L.E.; Gurau, G.; Rogers, R.D. Advanced biopolymer composite materials from ionic liquid solutions. ACS Symp. Ser. 2012, 1117, 167–187. [Google Scholar]
- Skvortsov, I.Y.; Chernikova, E.V.; Kulichikhin, V.G.; Varfolomeeva, L.A.; Kuzin, M.S.; Toms, R.V.; Prokopov, N.I. The effect of the synthetic procedure of acrylonitrile-acrylic acid copolymers on rheological properties of solutions and features of fiber spinning. Materials 2020, 13, 3454. [Google Scholar] [CrossRef]
- Sauberlich, H.E. Pharmacology of vitamin C. Ann. Rev. Nutr. 1994, 14, 371–391. [Google Scholar] [CrossRef]
- Waterman, K.C.; Adami, R.C.; Alsante, K.M.; Hong, J.; Landis, M.S.; Lombardo, F.; Roberts, C.J. Stabilization of pharmaceuticals to oxidative degradation. Pharm. Develop. Technol. 2002, 7, 1–32. [Google Scholar] [CrossRef]
- Wagner, K.H.; Feigl, P.; Elmadfa, I. Ascorbyl palmitate and its synergism to tocopherols. Forum Nutr. 2003, 56, 347–348. [Google Scholar]
- Doktorova, S.; Souto, E.B. Nanostructured lipid carrier-based hydrogel formulations for drug delivery: A comprehensive review. Expert Opin. Drug Deliv. 2009, 6, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Xu, R.; Hadinoto, K. Towards sustainability: New approaches to nano-drug preparation. Curr. Pharm. Des. 2013, 19, 6229–6245. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report on the safety assessment of Ascorbyl Palmitate, Ascorbyl Dipalmitate, Ascorbyl Stearate, Erythorbic Acid, and Sodium Erythorbate. Int. J. Toxicol. 1999, 18, 1–26. [Google Scholar] [CrossRef]
- Wagner, K.H.; Elmadfa, I. Nutrient antioxidants and stability of frying oils (tocochromanols, ß-carotene, phylloquinone, ubiquinone 50, and ascorbyl palmitate). In Frying of Food: Oxidation, Nutrient and Non-Nutrient Antioxidants, Biologically Active Compounds and High Temperatures, 2nd ed.; Boskou, D., Elmadfa, I., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 177–197. [Google Scholar]
- Du, L.; Xu, H.Z.; Li, T.; Zhang, Y.; Zou, F.Y. Fabrication of ascorbyl palmitate loaded poly(caprolactone)/silver nanoparticle embedded poly(vinyl alcohol) hybrid nanofibre mats as active wound dressings: Via dual-spinneret electrospinning. RSC Adv. 2017, 7, 31310–31318. [Google Scholar] [CrossRef] [Green Version]
- Miri, M.A.; Habibi Najafi, M.B.; Movaffagh, J.; Ghorani, B. Encapsulation of Ascorbyl Palmitate in Zein by Electrospinning Technique. J. Polym. Env. 2021, 29, 1089–1098. [Google Scholar] [CrossRef]
- Preedy, V.R.; Watson, R.R. Encyclopedia of Vitamin E; CABI Publishing: Oxford, UK, 2007. [Google Scholar]
- Catala, A. Tocopherol: Sources, Uses and Health Benefits; Nova Science Pub Inc.: Hauppauge, NY, USA, 2012. [Google Scholar]
- Rosenau, T.; Ebner, G.; Stanger, A.; Perl, S.; Nuri, L. From a Theoretical Concept to Biochemical Reactions: Strain Induced Bond Localization (SIBL) in Oxidation of Vitamin E. Chem. Eur. J. 2005, 11, 280–287. [Google Scholar] [CrossRef]
- Patel, A.; Liebner, F.; Netscher, T.; Mereiter, K.; Rosenau, T. Nitration of non-alpha tocopherols—Products and mechanistic considerations. J. Org. Chem. 2007, 72, 6504–6512. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Elder, T.; Kosma, P. Novel Tocopheryl Compounds XIII. Stabilization and first direct spectroscopic evidence of the ortho-quinone methide derived from vitamin E. Org. Lett. 2002, 4, 4285–4288. [Google Scholar] [CrossRef]
- Dahe, G.J.; Teotia, R.S.; Kadam, S.S.; Bellare, J.R. The biocompatibility and separation performance of antioxidative polysulfone/vitamin E TPGS composite hollow fiber membranes. Biomaterials 2011, 32, 352–365. [Google Scholar] [CrossRef]
- Wu, H.-L.; Bremner, D.H.; Li, H.-Y.; Shi, Q.-Q.; Wu, J.-Z.; Xiao, R.-Q.; Zhu, L.-M. A novel multifunctional biomedical material based on polyacrylonitrile: Preparation and characterization. Mat. Sci. Eng. C 2016, 62, 702–709. [Google Scholar] [CrossRef] [Green Version]
- Ghaheh, F.S.; Khoddami, A.; Alihosseini, F.; Jing, S.; Ribeiro, A.; Cavaco-Paulo, A.; Silva, C. Antioxidant cosmetotextiles: Cotton coating with nanoparticles containing vitamin E. Process Biochem. 2017, 59, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Chi, Y.-S.; Obendorf, S.K. Preventing discoloration of squalene-soiled cotton fabrics with antioxidants. J. Surfact. Deterg. 1998, 1, 523–527. [Google Scholar] [CrossRef]
- Yokota, S.; Kitaoka, T.; Opietnik, M.; Rosenau, T.; Wariishi, H. Synthesis of Gold Nanoparticles for In Situ Conjugation with Structural Carbohydrates. Angew. Chem. Int. Ed. Engl. 2008, 47, 9866–9869. [Google Scholar] [CrossRef]
- Zingg, J.M. Molecular and cellular activities of vitamin E analogues. Mini Rev. Med. Chem. 2007, 7, 543–558. [Google Scholar] [CrossRef]
- Nam, Y.S.; Kim, J.W.; Park, J.; Shim, J.; Lee, J.S.; Han, S.H. Tocopheryl acetate nanoemulsions stabilized with lipid-polymer hybrid emulsifiers for effective skin delivery. Colloids Surf. B Biointerfaces 2012, 94, 51–57. [Google Scholar] [CrossRef]
- Wei, Y.S.; Niu, Z.C.; Wang, F.Q.; Feng, K.; Zong, M.H.; Wu, H. A novel Pickering emulsion system as the carrier of tocopheryl acetate for its application in cosmetics. Mat. Sci. Eng. C 2020, 109, 110503. [Google Scholar] [CrossRef]
- Pérez, E.; Martín, L.; Rubio, C.; Urieta, J.S.; Piera, E.; Caballero, M.A.; Téllez, C.; Coronas, J. Encapsulation of α-tocopheryl acetate into zeolite y for textile application. Ind. Eng. Chem. Res. 2010, 49, 8495–8500. [Google Scholar] [CrossRef]
- Wu, X.M.; Branford-White, C.J.; Yu, D.G.; Chatterton, N.P.; Zhu, L.M. Preparation of core-shell PAN nanofibers encapsulated α-tocopherol acetate and ascorbic acid 2-phosphate for photoprotection. Colloids Surf. B Biointerfaces 2011, 82, 247–252. [Google Scholar] [CrossRef]
- Smith, L.I.; Renfrow, W.B.; Opie, J.W. The Chemistry of Vitamin E. XXXVIII. α-Tocopheramine, a New Vitamin E Factor. J. Am. Chem. Soc. 1942, 64, 1082–1084. [Google Scholar] [CrossRef]
- Itoh, S.; Nagaoka, S.I.; Mukai, K.; Ikesu, S.; Kaneko, Y. Kinetic study of quenching reactions of singlet oxygen and scavenging reactions of free radicals by α-, β-, λ- and σ-tocopheramines in ethanol solution and micellar dispersion. Lipids 1994, 29, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, T.; Hofinger, A.; Potthast, A.; Kosma, P. A general, selective high-yield N-demethylation procedure for tertiary amines by solid reagents in a convenient column chromatography-like setup. Org. Lett. 2004, 6, 541–544. [Google Scholar] [CrossRef]
- Bieri, J.G.; Prival, E.L. Vitamin E Activity and Metabolism of N-Methyltocopheramines. Biochemistry 1967, 6, 2153–2158. [Google Scholar] [CrossRef]
- Murphy, P.A.; Lin, J.S.; Olcott, H.S.; Windle, J.J. Oxidation of N-methyl-γ-tocopheramine to a nitroxide. Lipids 1976, 11, 296–298. [Google Scholar] [CrossRef]
- Igarashi, O. Oxidation products of N-methyl-2,2,7,8-tetramethyl-6-amino-chroman, a model compound of N-methyl-γ-tocopheramine with potassium ferricyanide. J. Nutr. Sci. Vitaminol. 1977, 23, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.A.; Collins, P.B. Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications; Nova Publishers: Hauppauge, NY, USA, 2013; pp. 1–350. [Google Scholar]
- Haslam, E.; Cai, Y. Plant polyphenols (vegetable tannins): Gallic acid metabolism. Nat. Prod. Rep. 1994, 11, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Haslam, E.; Lilley, T.H. Interactions of natural phenols with macromolecules. Progr. Clin. Biol. Res. 1986, 213, 53–65. [Google Scholar]
- Daglia, M.; di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chimnoi, N.; Techasakul, S.; Supaphol, P. Gallic acid-loaded electrospun poly(L-lactic acid) fiber mats and their release characteristic. Macromol. Chem. Phys. 2009, 210, 814–822. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Electrospun characteristics of gallic acid-loaded poly vinyl alcohol fibers: Release characteristics and antioxidant properties. J. Sci. Adv. Mat. Devices 2018, 3, 175–180. [Google Scholar] [CrossRef]
- Phiriyawirut, M.; Phachamud, T. Suitable electrospinning condition for gallic acid-loaded cellulose acetate fiber. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 210–220. [Google Scholar]
- Britton, J.; Davis, R.; O’Connor, K.E. Chemical, physical and biotechnological approaches to the production of the potent antioxidant hydroxytyrosol. Appl. Microbiol. Biotechnol. 2019, 103, 5957–5974. [Google Scholar] [CrossRef]
- Quiles, J.L.; Ramírez-Tortosa, M.C.; Yaqoob, P. Olive Oil and Health; CABI Publishing: Wallingford, UK, 2006. [Google Scholar]
- Emília Juan, M.; Wenzel, U.; Daniel, H.; Planas, J.M. Cancer Chemopreventive Activity of Hydroxytyrosol: A Natural Antioxidant from Olives and Olive Oil. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2010; pp. 1295–1300. [Google Scholar]
- Contesini, F.J.; de Castro, R.J.S.; Júnior, J.V.M.; Teixeira, C.B. Human metabolism of polyphenols from extra virgin olive oil. In Olive Consumption and Health; Savalas, C.A., Nicolau, S.M., Eds.; Nova Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 249–258. [Google Scholar]
- Peltzer, M.; Navarro, R.; López, J.; Jiménez, A. Evaluation of the melt stabilization performance of hydroxytyrosol (3,4-dihydroxy-phenylethanol) in polypropylene. Polym. Degrad. Stab. 2010, 95, 1636–1641. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Dugo, L.; Fanali, C.; Tripodo, G.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Effect of hydroxytyrosol methyl carbonate on the thermal, migration and antioxidant properties of PVA-based films for active food packaging. Polym. Int. 2016, 65, 872–882. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Fanali, C.; Dugo, L.; Giovanna Belluomo, M.; Torre, L.; Kenny, J.M.; Santi, L.; Bernini, R. Hydroxytyrosol as active ingredient in poly(vinyl alcohol) films for food packaging applications. J. Renew. Mat. 2017, 5, 81–95. [Google Scholar] [CrossRef]
- Haddar, W.; Baaka, N.; Meksi, N.; Ticha, M.B.; Guesmi, A.; Mhenni, M.F. Use of ultrasonic energy for enhancing the dyeing performances of polyamide fibers with olive vegetable water. Fibers Polym. 2015, 16, 1506–1511. [Google Scholar] [CrossRef]
- Bayraktar, O. Silk fibroin nanofibers loaded with hydroxytyrosol from hydrolysis of oleuropein in olive leaf extract. Text. Leather Rev. 2018, 1, 90–98. [Google Scholar] [CrossRef]
- Kaleh, Z.; Geißen, S.U. Selective isolation of valuable biophenols from olive mill wastewater. J. Env. Chem. Eng. 2016, 4, 373–384. [Google Scholar] [CrossRef]
- He, J.; Alister-Briggs, M.; Lyster, T.D.; Jones, G.P. Stability and antioxidant potential of purified olive mill wastewater extracts. Food Chem. 2012, 131, 1312–1321. [Google Scholar] [CrossRef]
- Kamini, N.R.; Edwinoliver, N.G.; Thirunavukarasu, K.; Gowthaman, M.K.; Rose, C. Utilization of olive oil and its by-products for industrial applications. In Olive Oil and Health; Corrigan, J.D., Ed.; Nova Science Pub Inc.: Hauppauge, NY, USA, 2010; pp. 197–220. [Google Scholar]
- Adegoke, G.O.; Vijay Kumar, M.; Gopala Krishna, A.G.; Varadaraj, M.C.; Sambaiah, K.; Lokesh, B.R. Antioxidants and Lipid Oxidation in Foods—A Critical Appraisal. J. Food Sci. Technol. 1998, 35, 283–298. [Google Scholar]
- Becker, L. Final report on the amended safety assessment of propyl gallate. Int. J. Toxicol. 2007, 26, 89–118. [Google Scholar] [CrossRef]
- Ding, T.; Li, T.; Li, J. Preparation of Coaxial Polylactic Acid-Propyl Gallate Electrospun Fibers and the Effect of Their Coating on Salmon Slices during Chilled Storage. ACS Appl. Mat. Interfaces 2019, 11, 6463–6474. [Google Scholar] [CrossRef] [PubMed]
- Wendler, F.; Meister, F.; Heinze, T. Studies on the thermostability of modified Lyocell dopes. Macromol. Symp. 2005, 223, 213–224. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Resveratrol in Health and Disease; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Rayess, Y.E. Wine: Phenolic Composition, Classification and Health Benefits; Nova Science Pub Inc.: Hauppauge, NY, USA, 2014. [Google Scholar]
- Delmas, D. Resveratrol: Sources, Production and Health Benefits; Nova Science Pub Inc.: Hauppauge, NY, USA, 2013. [Google Scholar]
- Alonso, C.; Martí, M.; Martínez, V.; Rubio, L.; Parra, J.L.; Coderch, L. Antioxidant cosmeto-textiles: Skin assessment. Eur. J. Pharm. Biopharm. 2013, 84, 192–199. [Google Scholar] [CrossRef]
- Pinho, E.; Henriques, M.; Oliveira, R.; Dias, A.; Soares, G. Development of biofunctional textiles by the application of resveratrol to cotton, bamboo, and silk. Fibers Polym. 2010, 11, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Han, L.; Sun, Q.; Xia, W.; Zhou, Q.; Zhang, Z.; Song, X. Controlled release of resveratrol and xanthohumol via coaxial electrospinning fibers. J. Biomat. Sci. 2020, 31, 456–471. [Google Scholar] [CrossRef]
- Silva, J.; Mesquita, R.; Pinho, E.; Caldas, A.; Oliveira, M.E.C.D.R.; Lopes, C.M.; Lúcio, M.; Soares, G. Incorporation of lipid nanosystems containing omega-3 fatty acids and resveratrol in textile substrates for wound healing and anti-inflammatory applications. SN Appl. Sci. 2019, 1, 1007. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.B.; Moo Lee, Y.; Seop Lee, W.; Mu Jo, S.; Kim, B.C. Double crystallization behavior in dry-jet wet spinning of cellulose/N-methylmorpholine-N-oxide hydrate solutions. Eur. Polym. J. 2002, 38, 109–119. [Google Scholar] [CrossRef]
- McKinley, H.; Jamieson, M. Handbook of Green Tea and Health Research; Nova Science Pub Inc.: Hauppauge, NY, USA, 2009. [Google Scholar]
- Deriso, C.H.; Hsu, S. Green Tea and Beyond; Nova Science Pub Inc.: Hauppauge, NY, USA, 2011. [Google Scholar]
- Rai, N.; Anand, J. Antioxidant Properties and Health Benefits of Green Tea; Nova Science Pub Inc.: Hauppauge, NY, USA, 2021. [Google Scholar]
- Demeule, M.; Michaud-Levesque, J.; Annabi, B.; Gingras, D.; Boivin, D.; Jodoin, J.; Lamy, S.; Bertrand, Y.; Béliveau, R. Green tea catechins as novel antitumor and antiangiogenic compounds. Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 441–463. [Google Scholar] [CrossRef]
- Alehosseini, A.; Gómez-Mascaraque, L.G.; Martínez-Sanz, M.; López-Rubio, A. Electrospun curcumin-loaded protein nanofiber mats as active/bioactive coatings for food packaging applications. Food Hydrocoll. 2019, 87, 758–771. [Google Scholar] [CrossRef]
- Sadri, M.; Arab-Sorkhi, S.; Vatani, H.; Bagheri-Pebdeni, A. New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers Polym. 2015, 16, 1742–1750. [Google Scholar] [CrossRef]
- Kim, S.H. Dyeing characteristics and UV protection property of green tea dyed cotton fabrics—Focusing on the effect of chitosan mordanting condition. Fibers Polym. 2006, 7, 255–261. [Google Scholar] [CrossRef]
- Pusporini, P.; Edikresnha, D.; Sriyanti, I.; Suciati, T.; Munir, M.M.; Khairurrijal, K. Electrospun polyvinylpyrrolidone (PVP)/green tea extract composite nanofiber mats and their antioxidant activities. Mat. Res. Express 2018, 5, 054001. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Hoang, N.P.; Cao, C.B. Fabrication, evaluation of drug loading capability and characterization of 3D-nano-cellulose network materials produced by bacteria of fermented aqueous green tea extractin selected culture media. Int. J. Appl. Pharm. 2020, 12, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E [Toxikologie der synthetischen Antioxidantien BHA und BHT im Vergleich mit dem natürlichen Antioxidans Vitamin E]. Z. Lebensm. Unters. Forsch. 1993, 196, 329–338. [Google Scholar] [CrossRef]
- Xu, C.; Li, C.Y.T.; Kong, A.N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; Kashanian, S. A review on DNA interaction with synthetic phenolic food additives. Food Res. Int. 2010, 43, 1223–1230. [Google Scholar] [CrossRef]
- Clayson, D.B.; Iverson, F.; Nera, E.A.; Lok, E. The importance of cellular proliferation induced by BHA and BHT. Toxicol. Ind. Health 1993, 9, 231–242. [Google Scholar] [CrossRef]
- Korntner, P.; Hosoya, T.; Dietz, T.; Eibinger, K.; Reiter, H.; Spitzbart, M.; Röder, T.; Borgards, A.; Kreiner, W.; Mahler, A.K.; et al. Chromophores in lignin-free cellulosic materials belong to three compound classes. Chromophores in cellulosics, XII. Cellulose 2015, 22, 1053–1062. [Google Scholar] [CrossRef]

| Pulp | Mn (kDa) | Mw (kDa) | DP | Ɖ | °ISO |
|---|---|---|---|---|---|
| A | 49.7 | 125.9 | 776 | 2.54 | 79 |
| B | 62.6 | 200.6 | 1272 | 3.29 | 81 |
| C | 99.3 | 362.7 | 2237 | 3.65 | 82 |
| Stabilizer | CAS | Molar Mass (g mol−1) | E-Number | References |
|---|---|---|---|---|
| Ascorbic acid (1) | 50-81-7 | 176.13 | E 300 | General: [19,20,21], mechanism: [22], in polymer processing: [23,24,25,26], in ionic liquids: [27,28], in fiber spinning: [29,30,31,32,33,34,35] |
| Ascorbyl palmitate (2) | 137-66-6 | 414.53 | E 304 | General: [36], mechanism: [37,38], transport: [39,40], safety: [41], stability: [42], in fibers: [43,44] |
| α-Tocopherol (3) | 10191-41-0 | 430.71 | E 307 | General: [45,46], mechanism: [47,48,49], in fibers: [50,51,52,53,54] |
| α-Tocopheryl acetate (4) | 7695-91-2 | 472.76 | General: [55], transport and stability: [56,57], in fibers: [58,59] | |
| α-Tocopheramine (5) | 7666-00-4 | 429.70 | General: [60], mechanism: [61] | |
| N-Methyl-α-tocopheramine (6) | 4869-06-1 | 443.73 | Synthesis: [62], mechanism/metabolism: [63], products: [64,65] | |
| Gallic acid (7) | 149-91-7 | 170.12 | General: [66], metabolism: [67], in polymers: [68,69], biological action: [70], in fibers: [71,72,73] | |
| Hydroxytyrosol (8) | 10597-60-1 | 154.16 | General: [74,75], biology: [76,77], in polymers: [78,79,80], in fibers: [81,82] | |
| OMWW lyophilizate | Compound mixture | General: [83,84,85], otherwise see hydroxytyrosol (8), which is the main active component | ||
| Propyl gallate (9) | 121-79-9 | 212.20 | E 310 | General: [86,87], application in fibers: [88,89] |
| Resveratrol (10) | 501-36-0 | 228.25 | General: [90,91,92], in fibers and textiles: [93,94,95,96,97] | |
| SGTE lyophilizate (mixture) | Compound mixture | General: [98,99], biological action: [100,101] in fibers: [102,103,104,105,106] | ||
| BHA (11) | 25013-16-5 | 180.24 | E 320 | General: [107], toxicology: [108,109,110] |
| BHT (12) | 128-37-0 | 220.35 | E 321 | As above for BHA (11) |
| Stabilizer | Cellulose Degradation | Dope Color | Yellowing upon Aging | Remarks |
|---|---|---|---|---|
| Ascorbic acid (1) | B | C | C | Inferior at chromophore formation |
| Ascorbyl palmitate (2) | A | C | B | Inferior at chromophore formation |
| α-Tocopherol (3) | B | A | B | Very similar to acetate (4) |
| α -Tocopheryl acetate (4) | B | A | B | Very similar to parent phenol (3) |
| α -Tocopheramine (5) | A | B | B | Good cellulose protection |
| N-Methyl-α -tocopheramine (6) | A | A | A | Superior overall performance, better than parent amine (5) |
| Gallic acid (7) | B | C | C | Inferior overall performance |
| Hydroxytyrosol (8) | A | A | A | Superior overall performance |
| OMWW lyophilizate (mixture) | B | B | A | Mixture, highly economical, better than tocopherols |
| Propyl gallate (9) | A | B | A | Good besides dope color, better than parent acid (7) |
| Resveratrol (10) | C | B | C | Inferior overall performance |
| SGTE lyophilizate (mixture) | C | B | A | Mixture, little protective effect |
| BHA (11) | A | A | B | Fossil-based, used for comparison |
| BHT (12) | A | A | B | Fossil-based, used for comparison |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hettegger, H.; Zhang, J.; Koide, M.; Rinner, U.; Potthast, A.; Gotoh, Y.; Rosenau, T. Fiber Spinning from Cellulose Solutions in Imidazolium Ionic Liquids: Effects of Natural Antioxidants on Molecular Weight, Dope Discoloration, and Yellowing Behavior. Fibers 2022, 10, 50. https://doi.org/10.3390/fib10060050
Hettegger H, Zhang J, Koide M, Rinner U, Potthast A, Gotoh Y, Rosenau T. Fiber Spinning from Cellulose Solutions in Imidazolium Ionic Liquids: Effects of Natural Antioxidants on Molecular Weight, Dope Discoloration, and Yellowing Behavior. Fibers. 2022; 10(6):50. https://doi.org/10.3390/fib10060050
Chicago/Turabian StyleHettegger, Hubert, Jiaping Zhang, Mitsuharu Koide, Uwe Rinner, Antje Potthast, Yasuo Gotoh, and Thomas Rosenau. 2022. "Fiber Spinning from Cellulose Solutions in Imidazolium Ionic Liquids: Effects of Natural Antioxidants on Molecular Weight, Dope Discoloration, and Yellowing Behavior" Fibers 10, no. 6: 50. https://doi.org/10.3390/fib10060050
APA StyleHettegger, H., Zhang, J., Koide, M., Rinner, U., Potthast, A., Gotoh, Y., & Rosenau, T. (2022). Fiber Spinning from Cellulose Solutions in Imidazolium Ionic Liquids: Effects of Natural Antioxidants on Molecular Weight, Dope Discoloration, and Yellowing Behavior. Fibers, 10(6), 50. https://doi.org/10.3390/fib10060050







