Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Melt Compounding and Melt Spinning into Continuous Filaments
2.3. Characterisation of Compounded Pellets
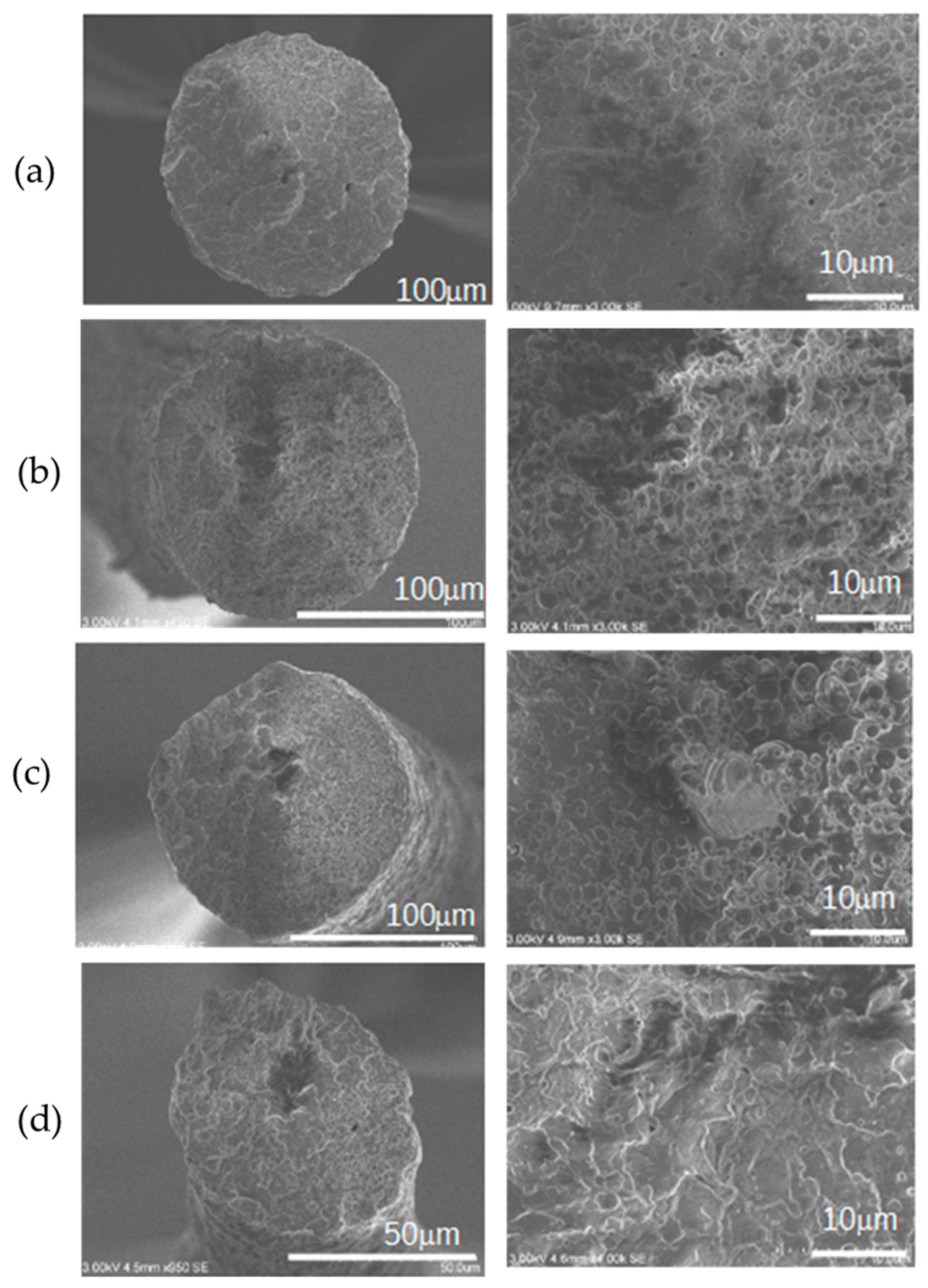
2.4. Characterisation of Melt-Spun Filaments from Pellet-Coated Blends Regarding Mechanical and Morphological Properties
2.5. Pre-Treatment of TcC/PA1010 with Cross-Linkers and Thermal Stabilisation
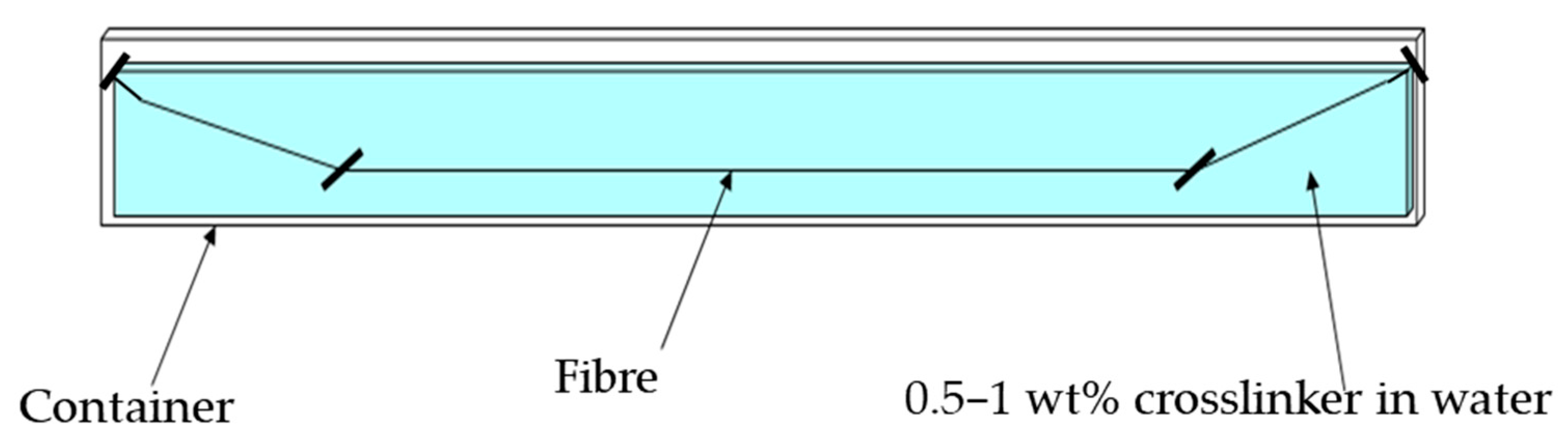
2.5.1. Filament Extrusion and Immersion
2.5.2. Thermal Stabilisation
3. Results
3.1. Effect of Cross-Linkers on Physico-Chemical Changes in Lignin/PA1010 Blends (Melt Compounded)
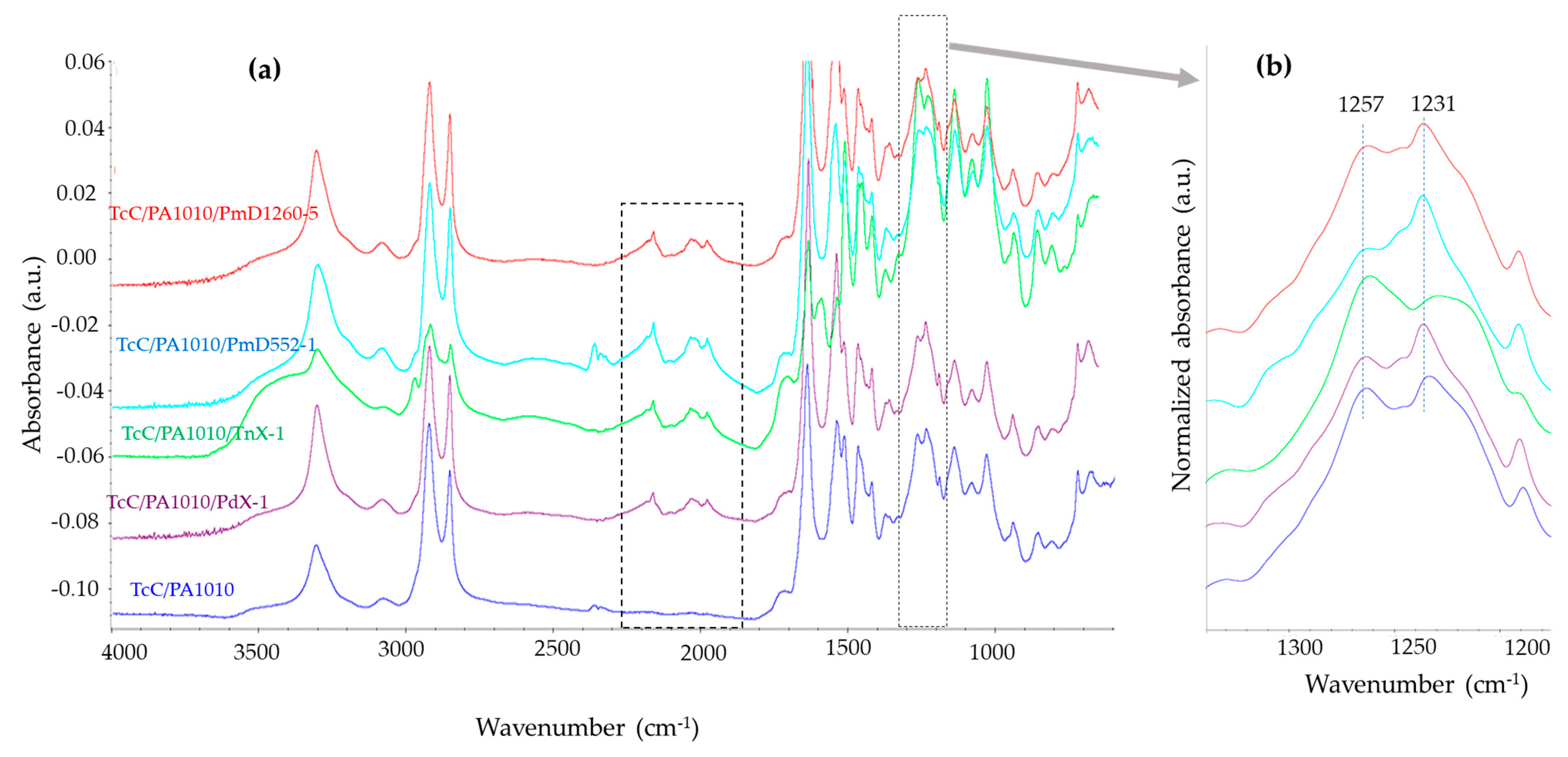
3.1.1. FTIR Spectroscopic Analysis
3.1.2. Differential Scanning Calorimetric Analysis
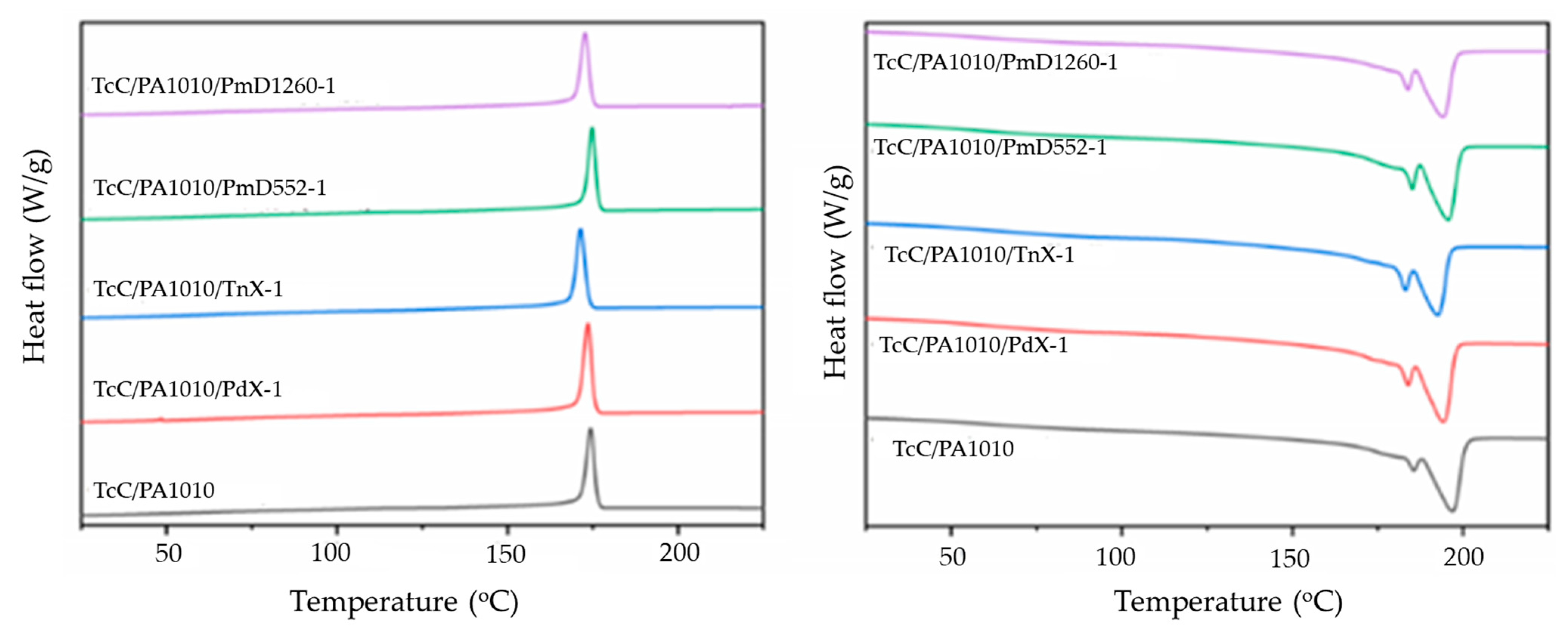
3.1.3. Dynamic Mechanical Analysis (DMA)
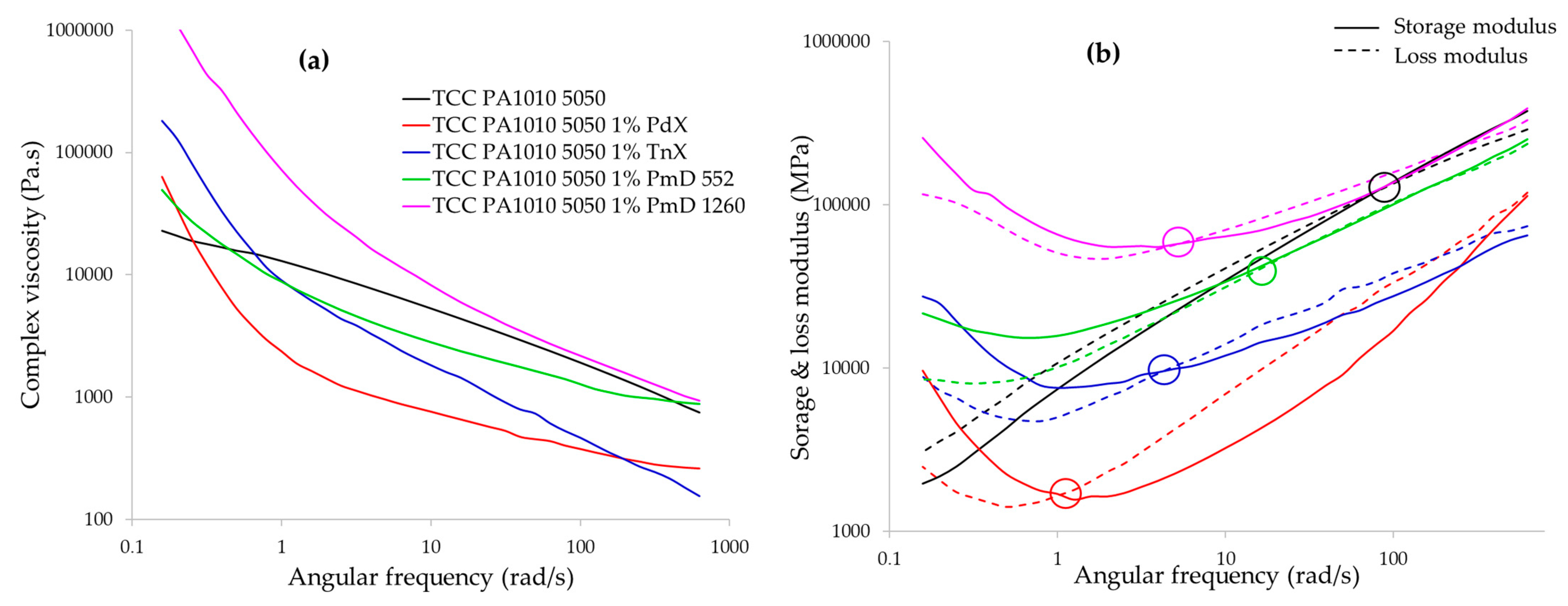
3.1.4. Rheological Behaviour
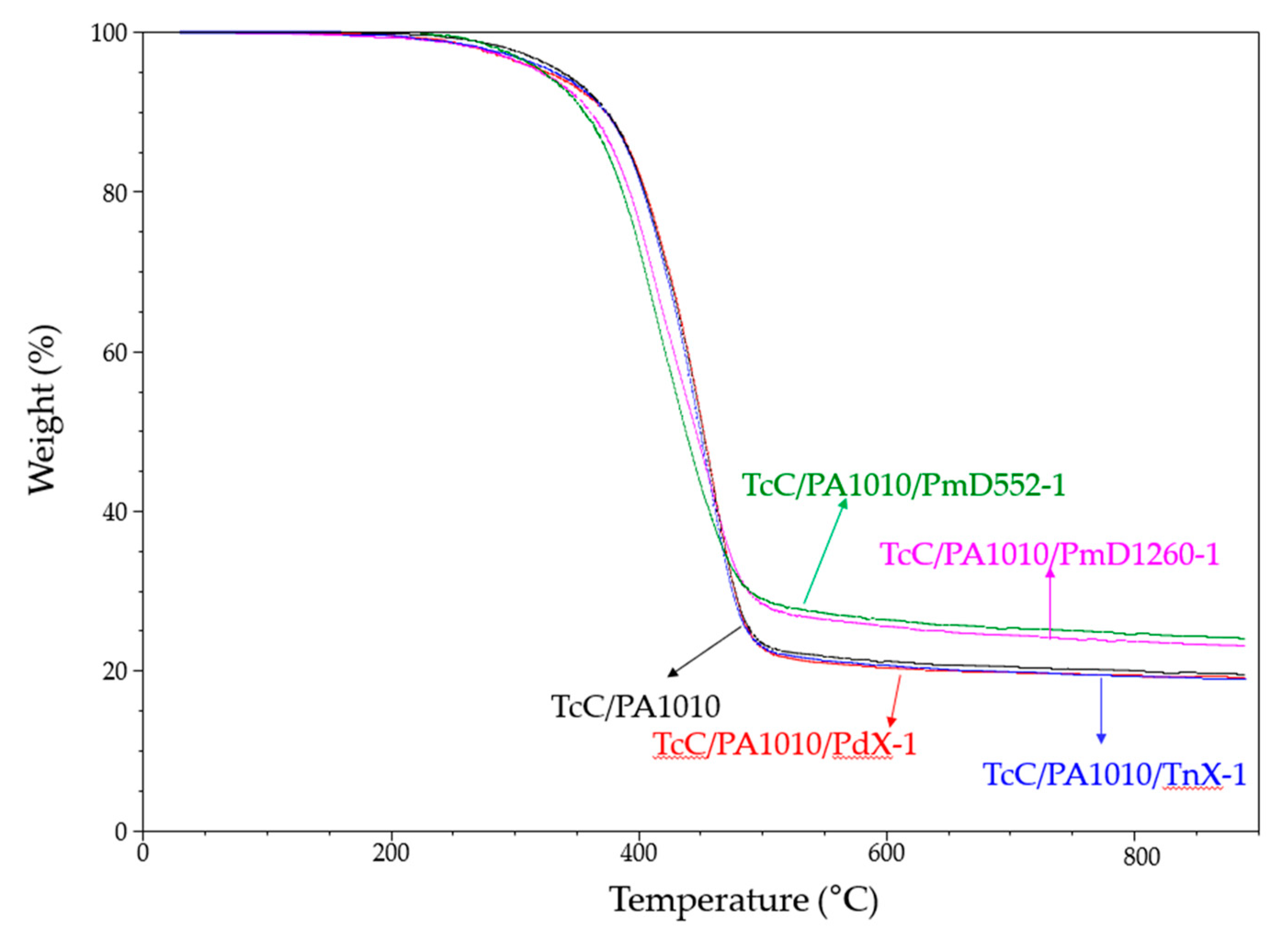
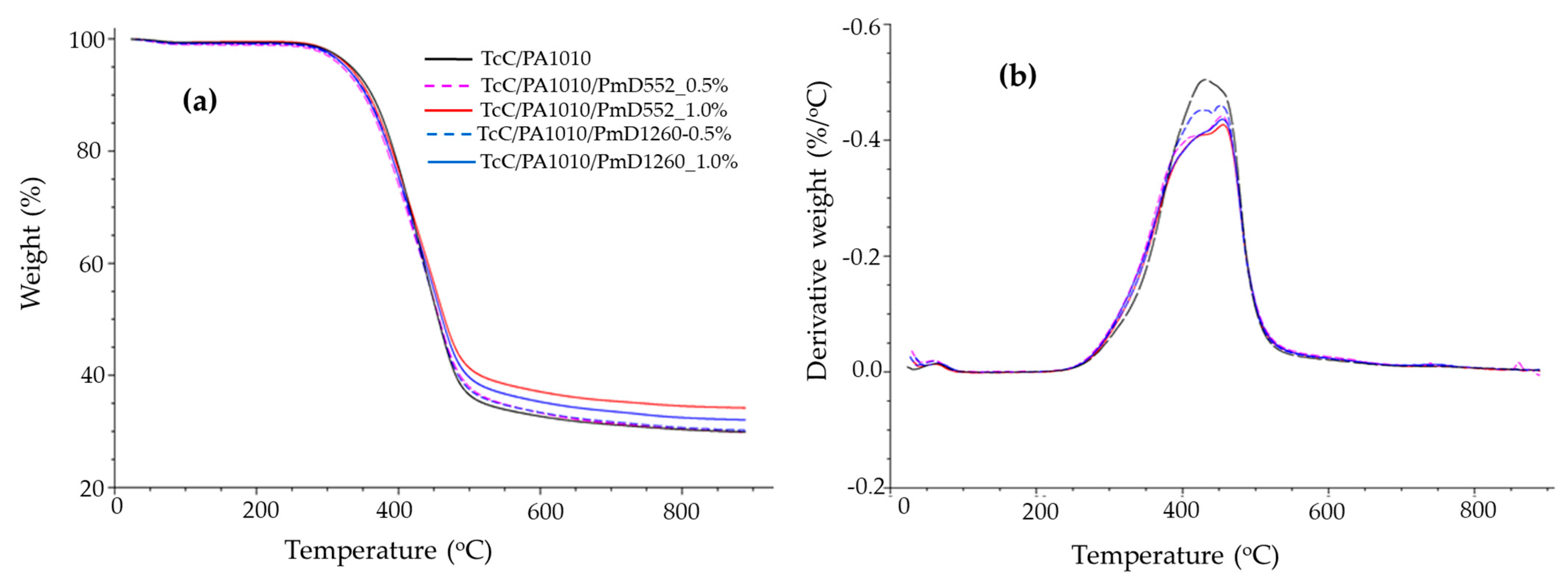
3.1.5. Thermogravimetric Analysis
3.2. Effect of Cross-Linkers Introduced Using Pellet-Coating TcC/PA1010 Compounded Pellets on the Physico-Mechanical Properties of Derived Filaments
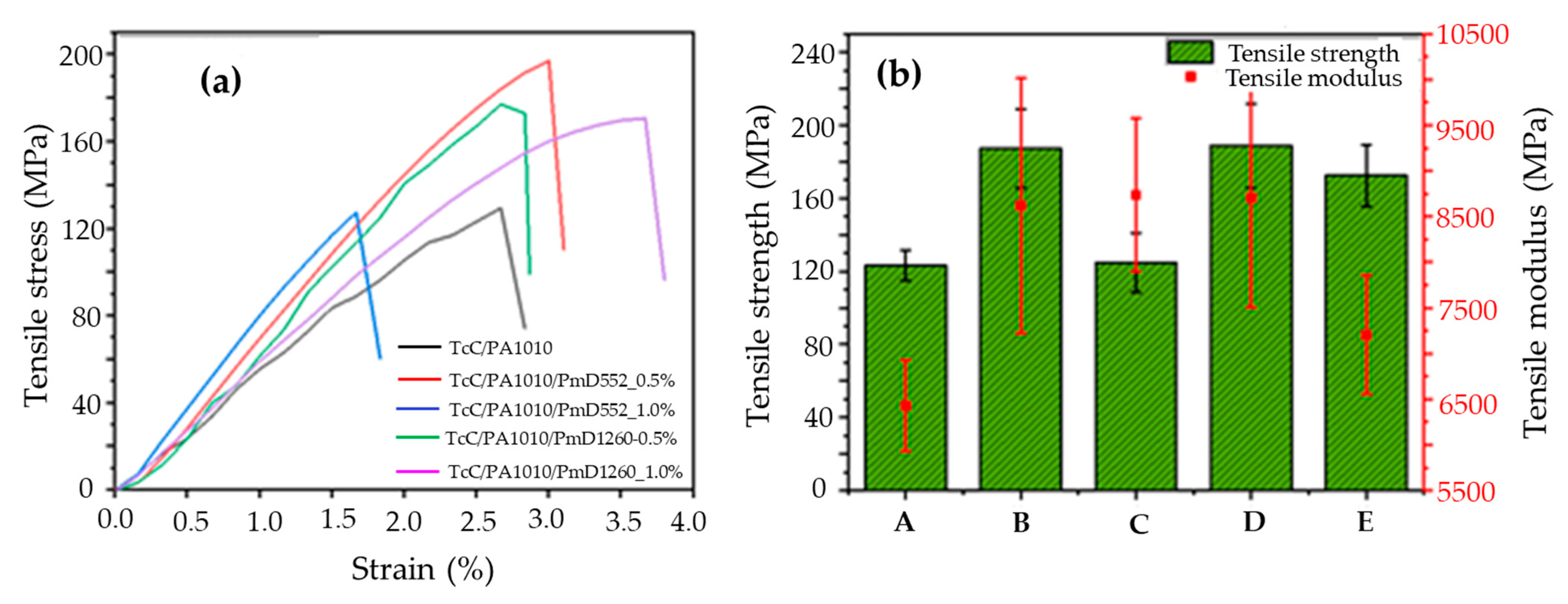
3.3. Effect of Cross-Linkers Introduced by Pre-Treating TcC/PA1010 Filaments on the Mechanical Properties of Thermally Stabilised Filaments
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogale, A.A.; Zhang, M.; Jin, J. Recent advances in carbon fibers derived from biobased precursors. J. Appl. Polym. Sci. 2016, 133, 43794. [Google Scholar] [CrossRef] [Green Version]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem Int. Ed. Engl. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J. Precursors and Manufacturing of Carbon Fibers. In Carbon Fibers; Springer Series in Materials Science; Springer: Singapore, 2018; Volume 210. [Google Scholar] [CrossRef]
- Le, N.-D.; Varley, R.J.; Hummel, M.; Trogen, M.; Byrne, N. A review of future directions in the development of sustainable carbon fiber from bio-based precursors. Mater Today Sustain. 2022, 20, 100251. [Google Scholar] [CrossRef]
- Brown, K.R.; Harrell, T.M.; Skrzypczak, L.; Scherschel, A.; Wu, H.F.; Li, X. Carbon fibers derived from commodity polymers: A review. Carbon 2022, 196, 422–439. [Google Scholar] [CrossRef]
- Thunga, M.; Chen, K.; Grewell, D.; Kessler, M.R. Bio-renewable precursor fibers from lignin/polylactide blends for conversion to carbon fibers. Carbon 2014, 68, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.A.; Rials, T.G. Recent advances in low-cost carbon fiber manufacture from lignin. J. Appl. Polym. Sci. 2013, 130, 713–728. [Google Scholar] [CrossRef]
- Bengtsson, A.; Bengtsson, J.; Sedin, M.; Sjöholm, E. Carbon fibers from lignin-cellulose precursors: Effect of stabilization conditions. ACS Sustain. Chem. Eng. 2019, 7, 8440–8448. [Google Scholar] [CrossRef] [Green Version]
- Muthuraj, R.; Horrocks, A.R.; Kandola, B.K. Hydroxypropyl-modified and organosolv lignin/bio-based polyamide blend filaments as carbon fibre precursors. J. Mater. Sci. 2020, 55, 7066–7083. [Google Scholar] [CrossRef]
- Beaucamp, A.; Wang, Y.; Culebras, M.; Collins, M.N. Carbon fibres from renewable resources: The role of the lignin molecular structure in its blendability with biobased poly(ethylene terephthalate). Green Chem. 2019, 21, 5063–5072. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Kraft lignin/poly (ethylene oxide) blends: Effect of lignin structure on miscibility and hydrogen bonding. J. Appl. Polym. Sci. 2005, 98, 1437–1444. [Google Scholar] [CrossRef]
- Muthuraj, R.; Hajee, M.; Horrocks, A.R.; Kandola, B.K. Biopolymer blends from hardwood lignin and bio-polyamides: Compatibility and miscibility. Int. J. Biol. Macromol. 2019, 132, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Hosseinaei, O.; Harper, D.P.; Bozell, J.J.; Rials, T.G. Improving processing and performance of pure lignin carbon fibers through hardwood and herbaceous lignin blends. Int. J. Mol. Sci. 2017, 18, 1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, S.; Kadla, J.F. The formation of strong intermolecular interactions in immiscible blends of poly(vinyl alcohol) (PVA) and lignin. Biomacromolecules 2003, 4, 61–567. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Lignin-based carbon fibers: Effect of synthetic polymer blending on fiber properties. J. Polym. Environ. 2005, 13, 97–105. [Google Scholar] [CrossRef]
- Muthuraj, R.; Hajee, M.; Horrocks, A.R.; Kandola, B.K. Effect of compatibilizers on lignin/bio-polyamide blend carbon precursor filament properties and their potential for thermostabilisation and carbonization. Polym. Test 2021, 95, 107133. [Google Scholar] [CrossRef]
- Culebras, M.; Beaucamp, A.; Wang, Y.; Clauss, M.; Frank, E.; Collins, M.N. Bio-based structurally compatible polymer blends based on lignin and thermoplastic elastomer polyurethane as carbon fiber precursors. ACS Sustain. Chem. Eng. 2018, 6, 8816–8825. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrol. 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Souto, F.; Calado, V.; Pereira, N. Lignin-based carbon fiber: A current overview. Mater. Res. Express 2018, 5, 072001. [Google Scholar] [CrossRef]
- Brodin, I.; Ernstsson, M.; Gellerstedt, G.; Sjöholm, E. Oxidative stabilisation of kraft lignin for carbon fibre production. Holzforschung 2012, 66, 141–147. [Google Scholar] [CrossRef]
- Kleinhans, H. Evaluation of the Carbonization of Thermo-Stabilized Lignin Fibers into Carbon Fibers. Master’s Thesis, Linköping University,, Linköping, Sweden, 2015. [Google Scholar]
- Bova, T.; Tran, C.D.; Balakshin, M.Y.; Chen, J.; Capanema, E.A.; Naskar, A.K. An approach towards tailoring interfacial structures and properties of multiphase renewable thermoplastics from lignin–nitrile rubber. Green Chem. 2016, 18, 5423–5437. [Google Scholar] [CrossRef]
- Toriz, G.; Denes, F.; Young, R.A. Lignin-polypropylene composites. Part 1: Composites from unmodified lignin and polypropylene. Polym. Comp. 2002, 23, 806–813. [Google Scholar] [CrossRef]
- Nam, B.-U.; Son, Y. Enhanced impact strength of compatibilized poly(lactic acid)/polyamide 11 blends by a crosslinking agent. J. Appl. Polym. Sci. 2020, 137, 49011. [Google Scholar] [CrossRef]
- Bondan, F.; Soares, M.R.F.; Bianchi, O. Effect of dynamic crosslinking on phase morphology and mechanical properties of polyamide 6,12/ethylene vinyl acetate copolymer blends. Polym. Bull. 2014, 71, 151–166. [Google Scholar] [CrossRef]
- Culebras, M.; Sanchis, M.J.; Beaucamp, A.; Carsí, M.; Kandola, B.K.; Horrocks, A.R.; Panzetti, G.; Birkinshaw, C.; Collins, M.N. Understanding the thermal and dielectric response of organosolv and modified kraft lignin as a carbon fibre precursor. Green Chem. 2018, 20, 4461–4472. [Google Scholar] [CrossRef]
- Available online: https://www.yumpu.com/en/document/view/11795302/initiators-and-reactor-additives-for-thermoplastics-akzonobel (accessed on 1 December 2022).
- EMS-CHEMIE AG. Primid ® XL-552. Datasheet:1-2. Available online: https://www.emsgriltech.com/fileadmin/ems-griltech/documents/Datasheet/Technical_Datasheet_XL-552.pdf (accessed on 1 December 2022).
- EMS-CHEMIE AG. Primid ® QM-1260 Datasheet:1-2. Available online: https://www.emsgriltech.com/fileadmin/ems-griltech/documents/Datasheet/Technical_Datasheet_QM-1260.pdf (accessed on 1 December 2022).
- Bol, C. Cross-Linking of Polyamide Materials. MSc Thesis, Politecnico Di Milano, Milano, Italy, 2016. [Google Scholar]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Luo, S.; Cao, J.; McDonald, A.G. Esterification of industrial lignin and its effect on the resulting poly(3-hydroxybutyrate-co-3-hydroxyvalerate) or polypropylene blends. Ind. Crop. Prod. 2017, 97, 281–291. [Google Scholar] [CrossRef] [Green Version]
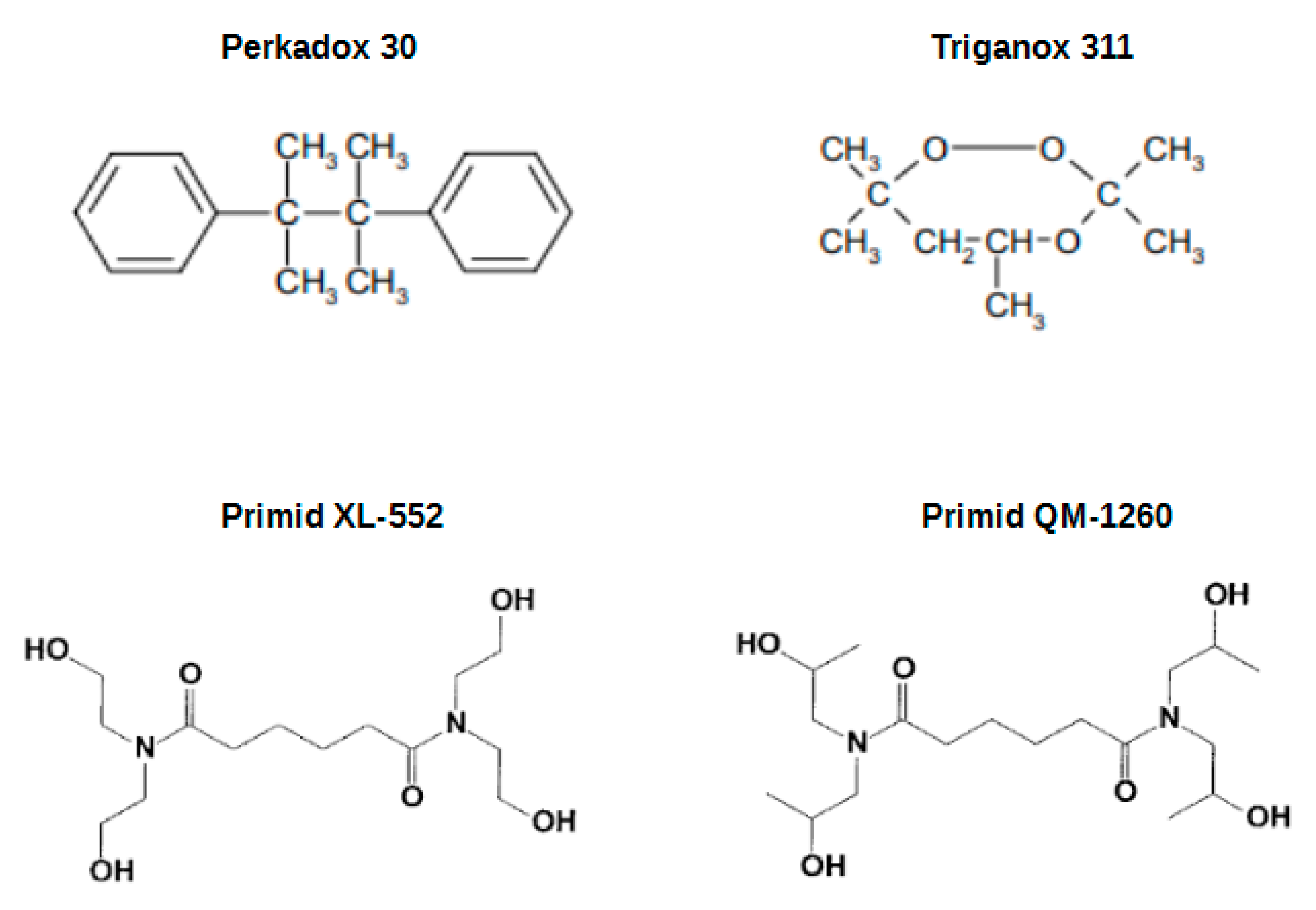
| Sample | Cross-Linker in TcC/PA1010 50:50 wt% | Processability | |
|---|---|---|---|
| Type | Conc (pph) | ||
| TcC/PA1010 | - | - | Easy |
| TcC/PA1010/PdX-1 | Perkadox 30 | 1 | Moderate |
| TcC/PA1010/PdX-2 | Perkadox 30 | 2 | Moderate |
| TcC/PA1010/PdX-3 | Perkadox 30 | 3 | Moderate |
| TcC/PA1010/TnX-1 | Triganox 311 | 1 | Moderate |
| TcC/PA1010/TnX-2 | Triganox 311 | 2 | Difficult |
| TcC/PA1010/TnX-3 | Triganox 311 | 3 | Difficult |
| TcC/PA1010/PmD552-1 | Primid XL-552 | 1 | Easy |
| TcC/PA1010/PmD552-5 | Primid XL-552 | 5 | Easy |
| TcC/PA1010/PmD552-10 | Primid XL-552 | 10 | Moderate |
| TcC/PA1010/PmD1260-1 | Primid QM-1260 | 1 | Easy |
| TcC/PA1010/PmD1260-5 | Primid QM-1260 | 5 | Easy |
| TcC/PA1010/PmD1260-10 | Primid QM-1260 | 10 | Moderate |
| Sample | Spinnability | Brittleness/Strength |
|---|---|---|
| TcC/PA1010 | Good | Strong |
| TcC/PA1010/PdX-0.5_PC | Poor | Very brittle |
| TcC/PA1010/TnX-0.5_PC | Good | Strong |
| TcC/PA1010/PmD552-0.5_PC | Good | Strong |
| TcC/PA1010/PmD1260-0.5_PC | Good | Strong |
| Samples | DSC | DMA | TGA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | Tg (°C) | TOnset (°C) | TMax (°C) | CY* at 880 °C (%) | |||
| TcC/PA1010 | 186,197 | 33.6 | 174 | 34.6 | 68, 146 | 337 | 457 | 19 (17.0) | ||
| TcC/PA1010/PdX-1 | 184,194 | 37.7 | 174 | 35.2 | *, 147 | 328 | 458 | 19 (16.8) | ||
| TcC/PA1010/PdX -2 | 184,194 | 35.3 | 174 | 37.5 | *, 145 | 317 | 456 | 20 (16.7) | ||
| TcC/PA1010/PdX-3 | 183,193 | 33.8 | 172 | 34.8 | *, 144 | 299 | 454 | 18 (16.5) | ||
| TcC/PA1010/TnX-1 | 183,193 | 34.4 | 171 | 35.1 | *, 146 | 331 | 449 | 19 (17.0) | ||
| TcC/PA1010/TnX-2 | 184,193 | 33.8 | 172 | 34.1 | *, 145 | 311 | 456 | 18 (16.7) | ||
| TcC/PA1010/TnX-3 | 183,193 | 32.5 | 171 | 33.9 | *, 146 | 310 | 449 | 21 (16.5) | ||
| TcC/PA1010/PmD552-1 | 185,196 | 35.9 | 175 | 33.6 | 66, 152 | 322 | 412 | 24 (16.8) | ||
| TcC/PA1010 /PmD552-5 | 186,195 | 28.6 | 175 | 27.8 | 67, 126 | 330 | 447 | 23 (16.2) | ||
| TcC/PA1010/PmD552-10 | 187,194 | 26.6 | 175 | 26.4 | 68, 109 | 325 | 452 | 21 (15.3) | ||
| TcC/PA1010 /PmD1260-1 | 184,194 | 33.1 | 173 | 31.3 | 68, 150 | 322 | 416 | 23 (16.8) | ||
| TcC/PA1010/PmD1260-5 | 185,195 | 32.6 | 173 | 29.8 | 69, 134 | 325 | 455 | 19 (16.2) | ||
| TcC/PA1010/PmD1260-10 | 187,195 | 28.9 | 174 | 29.1 | 70, 112 | 306 | 441 | 21 (15.3) | ||
| Sample | Tensile Strength (MPa) | Tensile Modulus (GPa) | Strain-at-Break (%) |
|---|---|---|---|
| TcC/PA1010 | 39 ± 9 | 2.0 ± 0. 6 | 25 |
| TcC/PA1010/TnX-0.5_PC | 42 ± 10 | 2.3 ± 0.3 | 3 |
| TcC/PA1010/PmD552-0.5_PC | 34 ± 9 | 1.9 ± 0.3 | 2 |
| TcC/PA1010/PmD1260-0.5_PC | 33 ± 8 | 2.3 ± 0.3 | 8 |
| Sample | TOnset (°C) | TMax (°C) | Char Yield at 880 °C (%) |
|---|---|---|---|
| TcC/PA1010 | 316 | 427 | 30.7 |
| TcC/PA1010-PmD 552_0.5% | 320 | 405,453 | 30.4 |
| TcC/PA1010-PmD 552_1% | 331 | 420,456 | 34.4 |
| TcC/PA1010-PmD 1260_0.5% | 326 | 422,451 | 30.7 |
| TcC/PA1010-PmD 1260_1% | 327 | 426,456 | 32.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandola, B.K.; Hewage, T.A.M.; Hajee, M.; Horrocks, A.R. Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres. Fibers 2023, 11, 16. https://doi.org/10.3390/fib11020016
Kandola BK, Hewage TAM, Hajee M, Horrocks AR. Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres. Fibers. 2023; 11(2):16. https://doi.org/10.3390/fib11020016
Chicago/Turabian StyleKandola, Baljinder K., Trishan A. M. Hewage, Muhammed Hajee, and A. Richard Horrocks. 2023. "Effect of Cross-Linkers on the Processing of Lignin/Polyamide Precursors for Carbon Fibres" Fibers 11, no. 2: 16. https://doi.org/10.3390/fib11020016





