Fiber Spinning of Polyacrylonitrile Terpolymers Containing Acrylic Acid and Alkyl Acrylates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Solutions
- For solutions with a polymer concentration of 1–5 wt%, the mixture was stirred for 24 h at 50 °C using a magnetic stirrer. For concentrations below 1 wt%, the solution was sequentially diluted inside a Ubbelohde capillary viscometer at 25 °C to determine the intrinsic viscosity.
- Highly viscous solutions with a polymer content greater than 5 wt% were prepared using a paddle mixer with a J-shaped rotor. Mixing was conducted at 60 rpm for 24 h at 70 °C.
- High-viscosity spinning solutions with concentrations above 20 wt% were prepared using a rotor speed of 10 rpm for 72 h at 70 °C.
2.2.2. Rheology
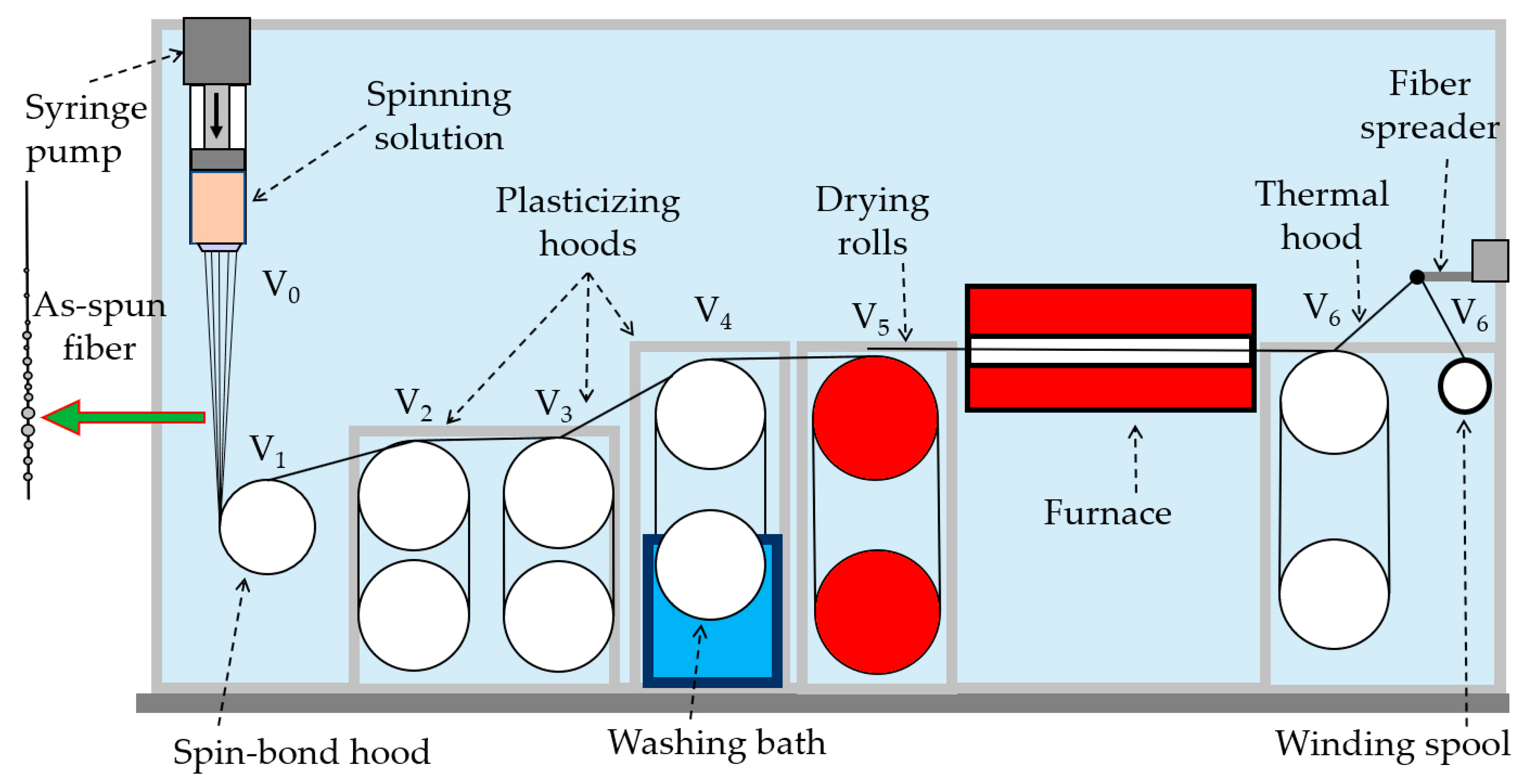
2.2.3. Fiber Spinning
2.2.4. Fiber Characterization
3. Results and Discussion
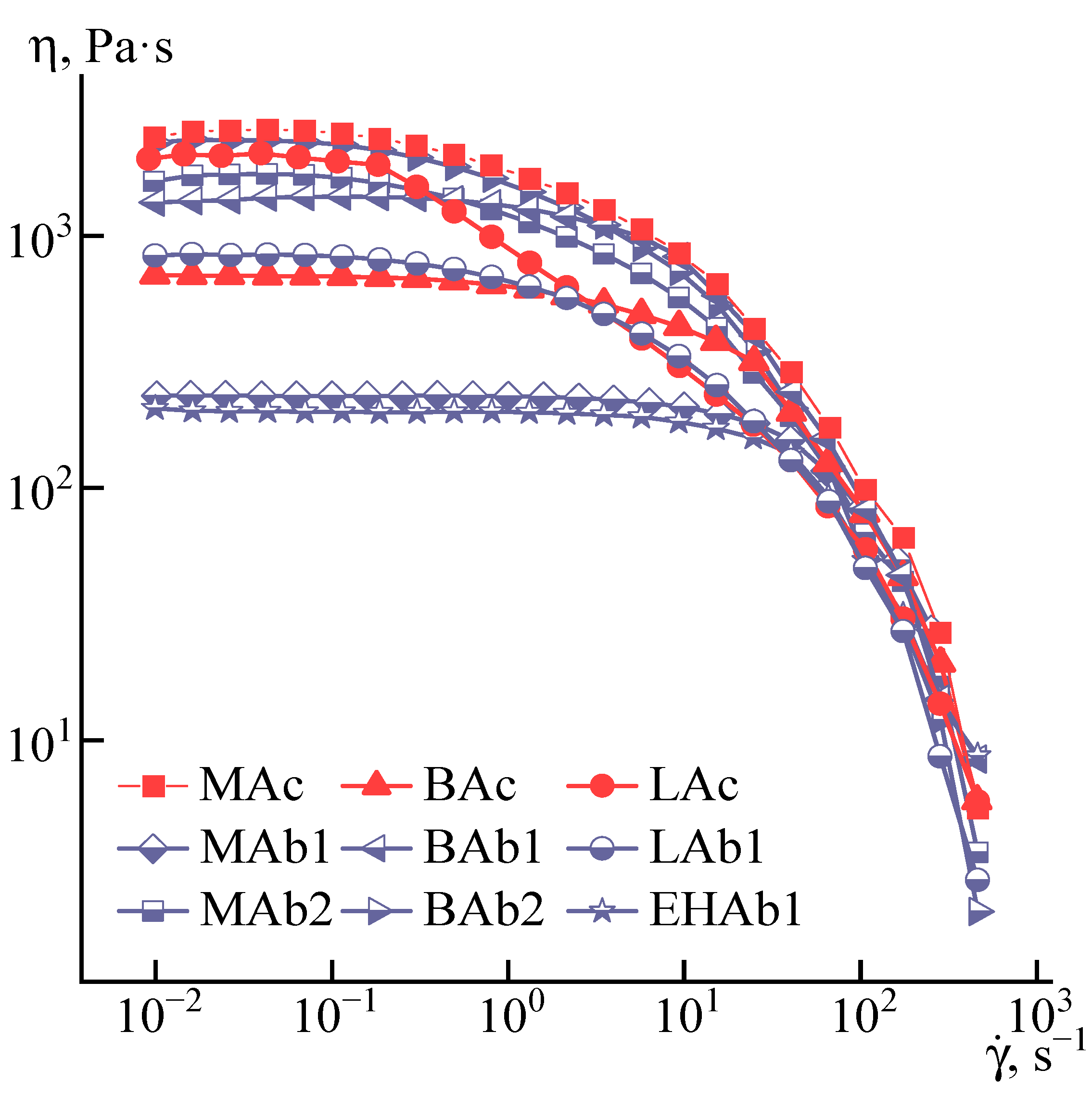
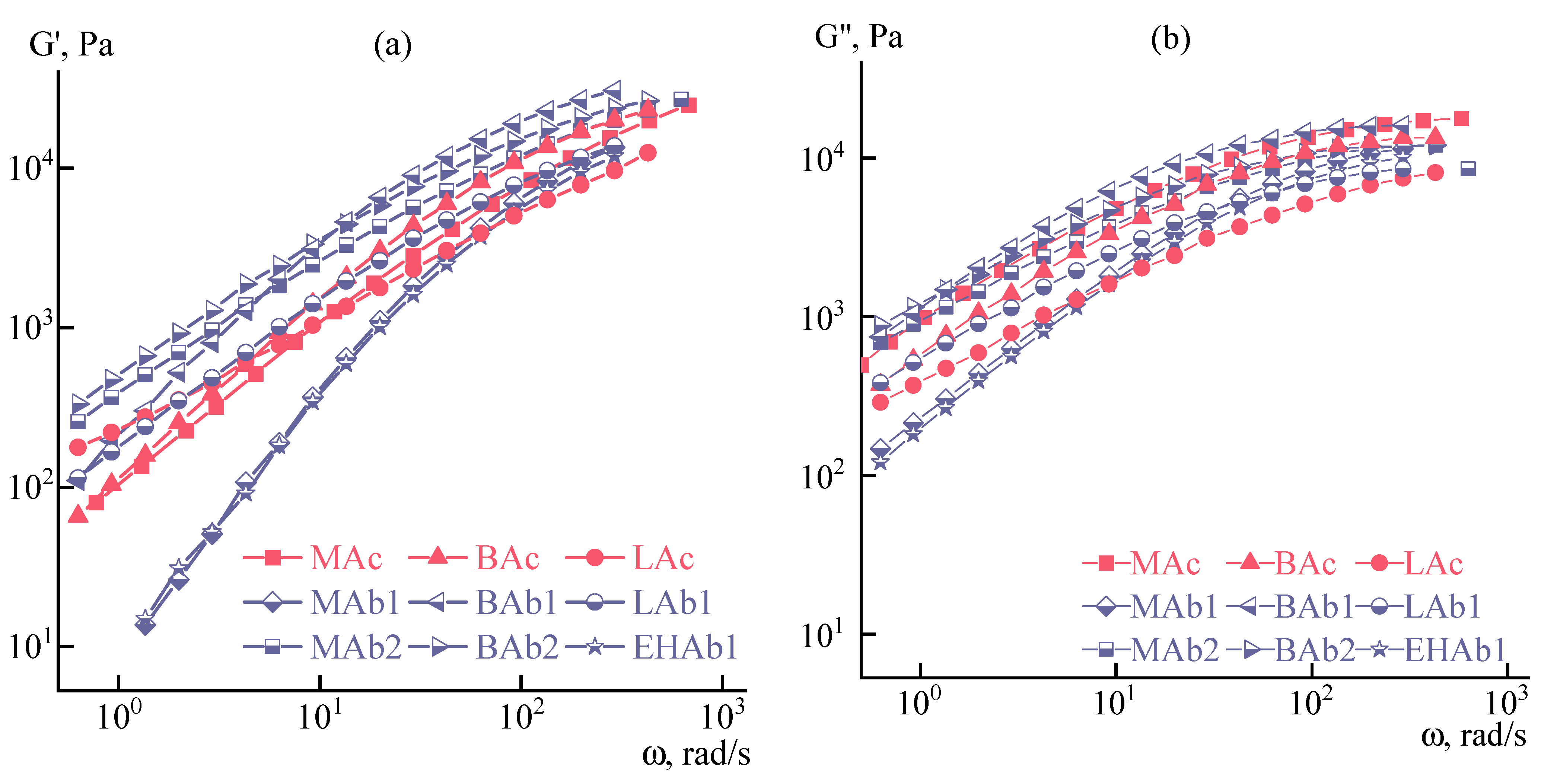
3.1. Solutions’ Rheology
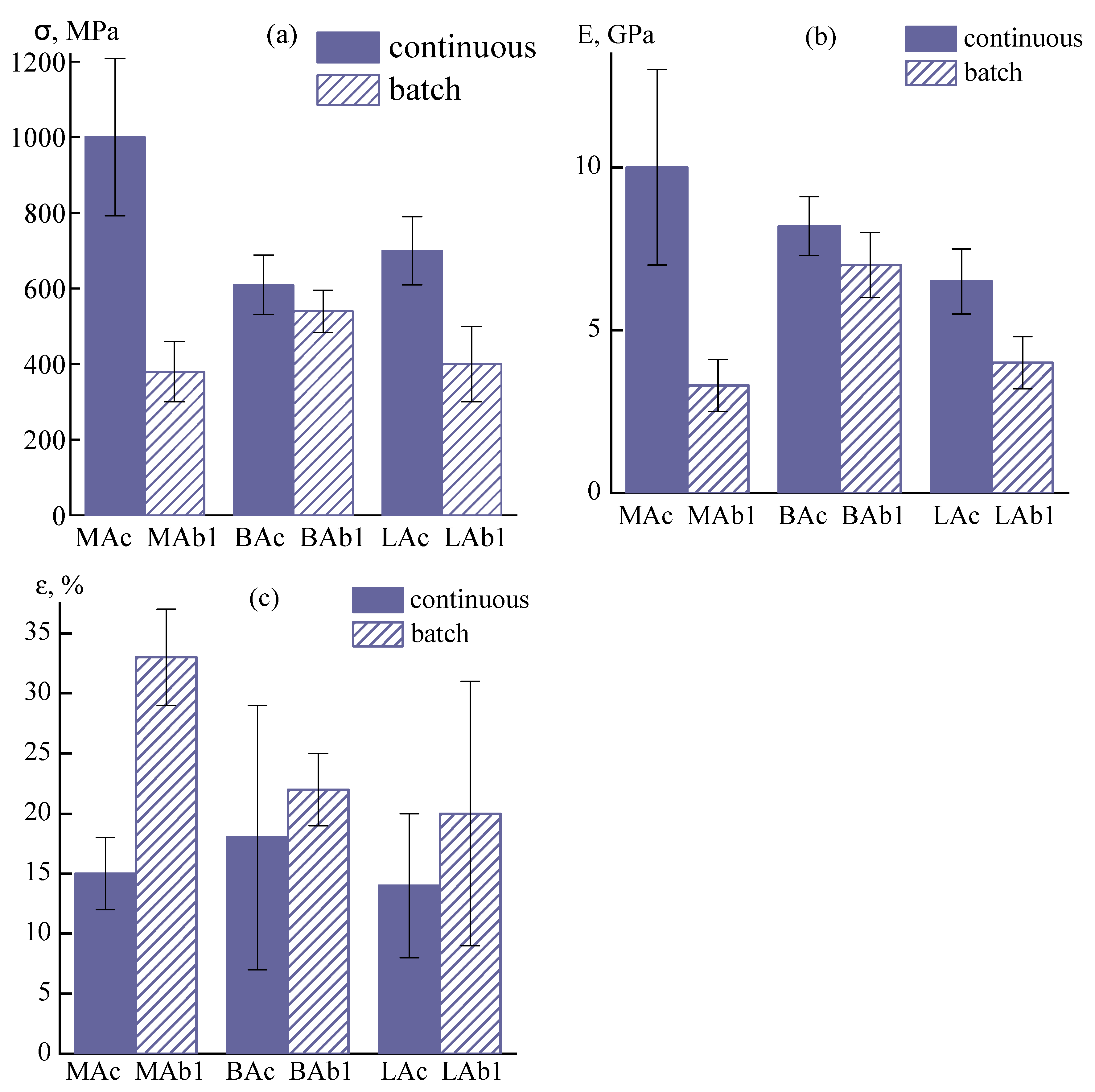
3.2. Fiber Spinning
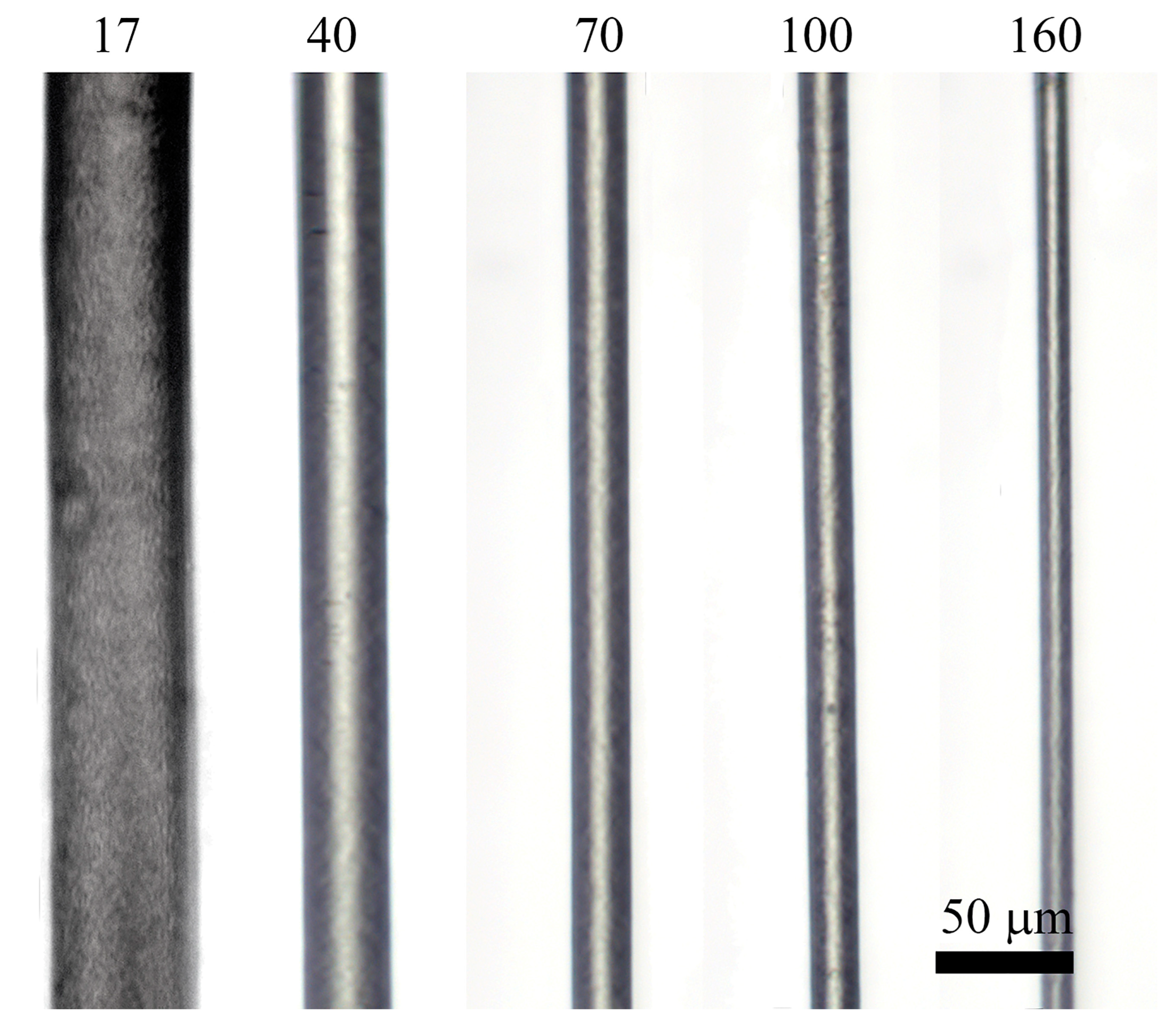
3.2.1. Spinneret Drawing Influence
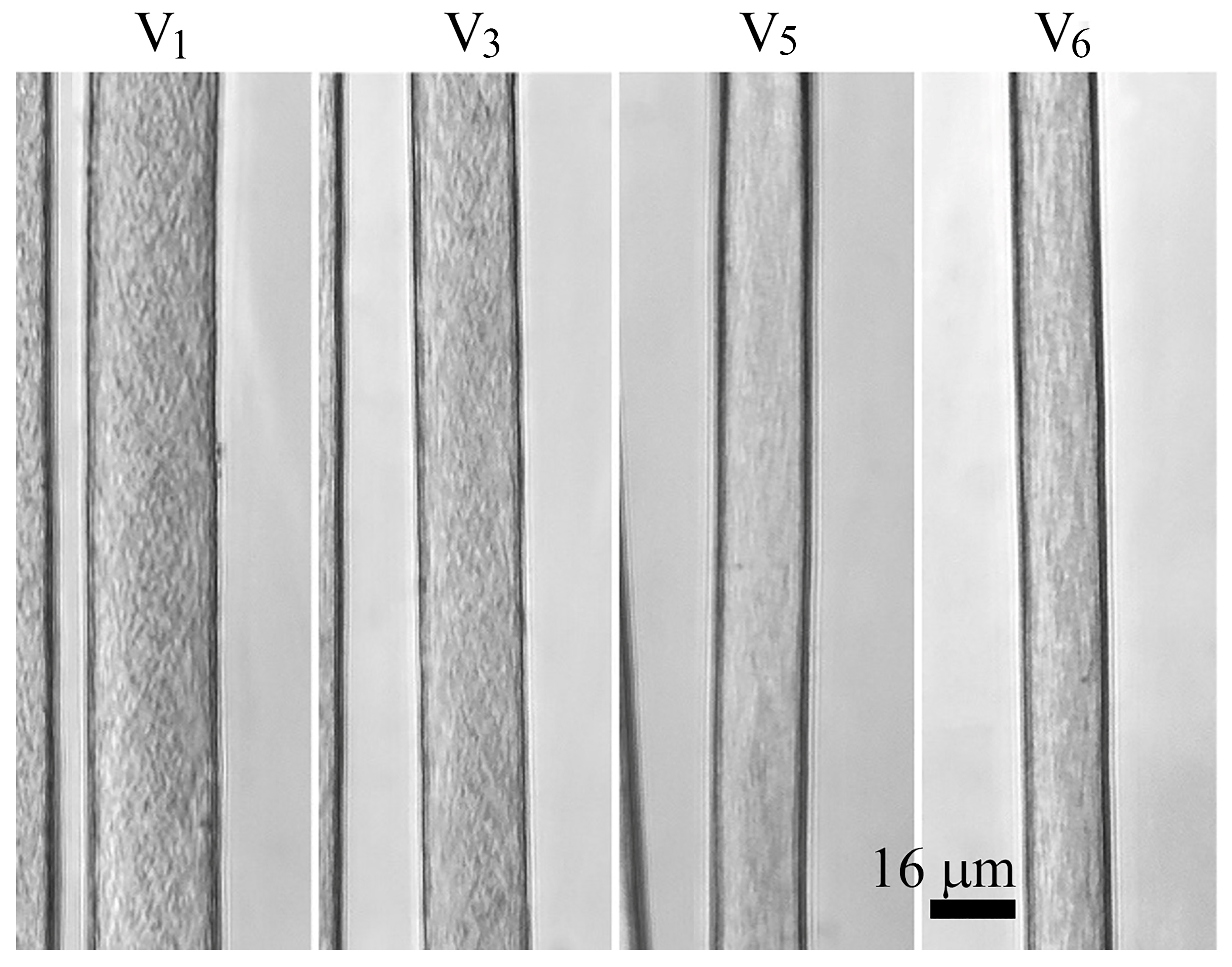
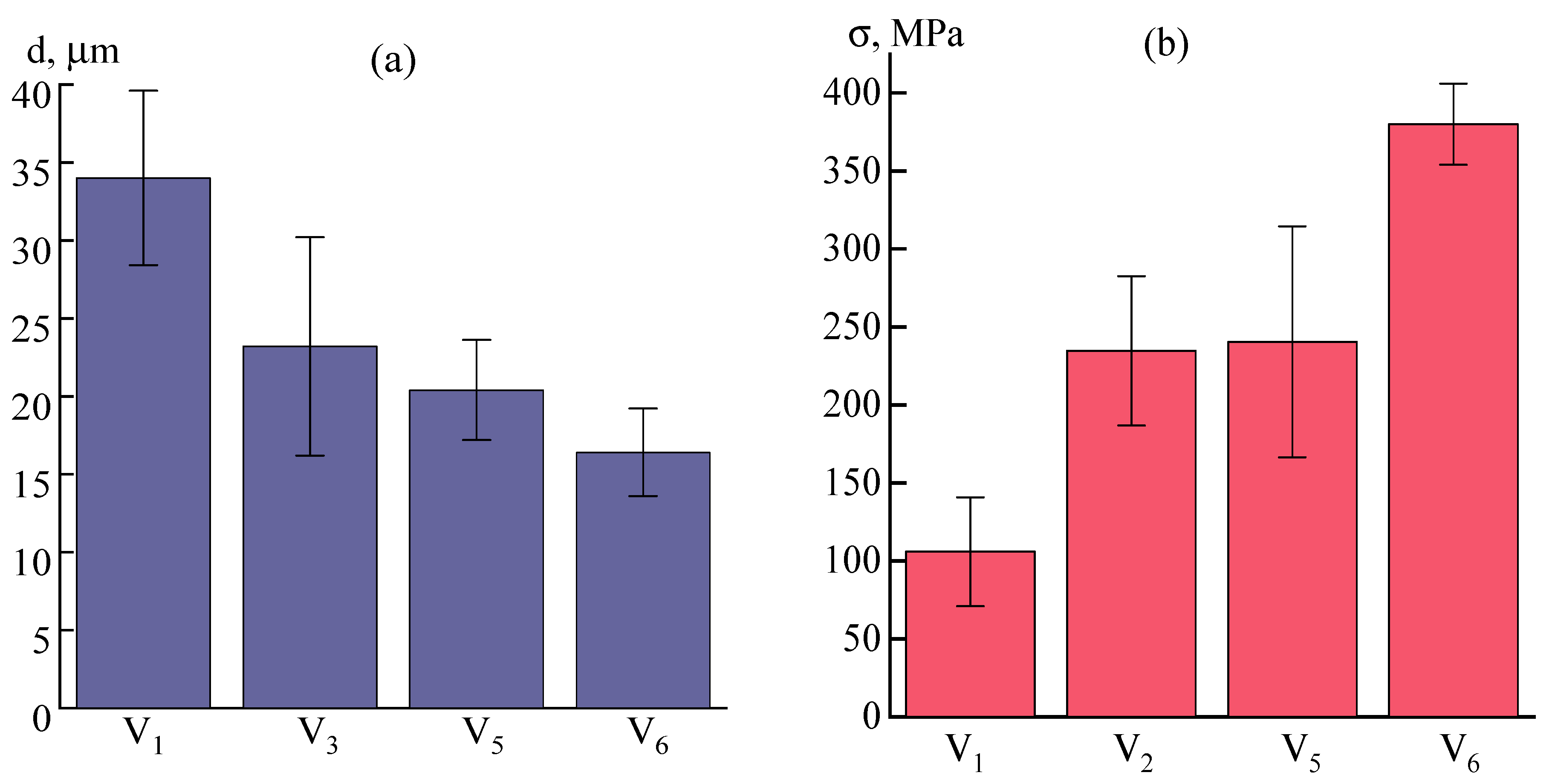
3.2.2. Influence of Orientation Drawing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khayyam, H.; Jazar, R.N.; Nunna, S.; Golkarnarenji, G.; Badii, K.; Fakhrhoseini, S.M.; Kumar, S.; Naebe, M. PAN precursor fabrication, applications and thermal stabilization process in carbon fiber production: Experimental and mathematical model-ling. Prog. Mater. Sci. 2020, 107, 100575–100614. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrolysis 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers—A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Shindo, A. Production of Carbon Fiber Reinforced Carbon Material. Japan Patent Publication JPH0551256A, 21 August 1991. [Google Scholar]
- Kasatochkin, V.I.; Smutkina, Z.S.; Kazakov, M.Y.; Radimov, N.P.; Nabatnikov, A.P.; Yaresko, T.D. O molekulyarnoy strukture anizotropnogo uglerodnogo volokna. Izv. Akad. Nauk. USSR 1972, 205, 1090–1092. [Google Scholar]
- Szepcsik, B.; Pukanszky, B. The mechanism of thermal stabilization of polyacrylonitrile. Thermochim. Acta 2019, 671, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Lian, F.; Liu, J.; Ma, Z.; Liang, J. Stretching-induced deformation of polyacrylonitrile chains both in quasicrystals and in amorphous regions during the in situ thermal modification of fibers prior to oxidative stabilization. Carbon 2012, 50, 488–499. [Google Scholar] [CrossRef]
- Morgan, P. Carbon Fibers and Their Composites, 3rd ed.; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 2005; pp. 121–148. [Google Scholar]
- Jang, D.; Lee, M.E.; Choi, J.; Cho, S.Y.; Lee, S. Strategies for the production of PAN-Based carbon fibers with high tensile strength. Carbon 2022, 186, 644–677. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Toms, R.V.; Gervald, A.Y.; Prokopov, N.I. Fiber-forming acrylonitrile copolymers: From synthesis to properties of carbon fiber precursors and prospects for industrial production. Polym. Sci. Ser. C 2020, 62, 17–50. [Google Scholar] [CrossRef]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Wangxi, Z.; Jie, L.; Gang, W. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and kinetics of the stabilization reactions of itaconic acid-modified poly-acrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Fitzer, E.M.D.J.; Müller, D.J. The influence of oxygen on the chemical reactions during stabilization of pan as carbon fiber precursor. Carbon 1975, 13, 63–69. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, C.R.; Sivadasan, P.; Katherine, B.K.; Ninan, K.N. Cyclization reaction in poly (acrylonitrile/itaconic acid) copolymer: An isothermal differential scanning calorimetry kinetic study. J. Appl. Polym. Sci. 2003, 88, 915–920. [Google Scholar] [CrossRef]
- Hao, J.; Wei, H.; Lu, C.; Liu, Y. New aspects on the cyclization mechanisms of Poly (acrylonitrile-co-itaconic acid). Eur. Polym. J. 2019, 121, 109313–109340. [Google Scholar] [CrossRef]
- Fu, Z.; Gui, Y.; Liu, S.; Wang, Z.; Liu, B.; Cao, C.; Zhang, H. Effects of an itaconic acid comonomer on the structural evolution and thermal behaviors of polyacrylonitrile used for polyacrylonitrile-based carbon fibers. J. Appl. Polym. Sci. 2014, 13, 40834–40842. [Google Scholar] [CrossRef]
- Ismar, E.; Sarac, A.S. Synthesis and characterization of poly (acrylonitrile-co-acrylic acid) as precursor of carbon nanofibers. Polym. Adv. Technol. 2016, 27, 1383–1388. [Google Scholar] [CrossRef]
- Devasia, R.; Reghunadhan Nair, C.P.; Sivadasan, P.; Ninan, K.N. High char-yielding poly [acrylonitrile-co-(itaconic ac-id)-co-(methyl acrylate)]: Synthesis and properties. Polym. Int. 2005, 54, 1110–1118. [Google Scholar] [CrossRef]
- Alcalá-Sánchez, D.; Tapia-Picazo, J.C.; Bonilla-Petriciolet, A.; Luna-Bárcenas, G.; López-Romero, J.M.; Álvarez-Castillo, A. Analysis of Terpolymerization systems for the development of carbon fiber precursors of PAN. Int. J. Polym. Sci. 2020, 2020, 8029516. [Google Scholar] [CrossRef]
- Liu, J.; He, L.; Ma, S.; Liang, J.; Zhao, Y.; Fong, H. Effects of chemical composition and post-spinning stretching process on the morphological, structural, and thermo-chemical properties of electrospun polyacrylonitrile copolymer precursor nanofibers. Polymer 2015, 61, 20–28. [Google Scholar] [CrossRef]
- Bajaj, P.; Sreekumar, T.V.; Sen, K. Structure development during dry–jet–wet spinning of acrylonitrile/vinyl acids and acry-lonitrile/methyl acrylate copolymers. J. Appl. Polym. Sci. 2002, 86, 773–787. [Google Scholar] [CrossRef]
- Liu, J.J.; Ge, H.; Wang, C.G. Modification of polyacrylonitrile precursors for carbon fiber via copolymerization of acryloni-trile with ammonium itaconate. J. Appl. Polym. Sci. 2006, 102, 2175–2179. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. Atom transfer radical polymerization (ATRP). In Fundamentals of Controlled/Living Rad-Ical Polymerization (Polymer Chemistry Series), 1st ed.; Tsarevsky, N.V., Sumerlin, B.S., Eds.; PSC Publishing: Dorchester, UK, 2013; Volume 1, pp. 287–343. [Google Scholar]
- Cai, J.Y.; McDonnell, J.; Brackley, C.; O’Brien, L.; Church, J.S.; Millington, K.; Smith, S.; Phair-Sorensen, N. Polyacrylonitrile-based precursors and carbon fibers derived from advanced RAFT technology and conventional methods–The 1st comparative study. Mater. Today Commun. 2016, 9, 22–29. [Google Scholar] [CrossRef]
- Tsai, J.S.; Lin, C.H. The effect of molecular weight on the cross section and properties of polyacrylonitrile precursor and re-sulting carbon fiber. J. Appl. Polym. Sci. 1991, 42, 3045–3050. [Google Scholar] [CrossRef]
- Xue, T.J.; McKinney, M.A.; Wilkie, C.A. The thermal degradation of polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Moskowitz, J.D.; Wiggins, J.S. Thermo-oxidative stabilization of polyacrylonitrile and its copolymers: Effect of molecular weight, dispersity, and polymerization pathway. Polym. Degrad. Stab. 2016, 125, 76–86. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Chernikova, E.V.; Kulichikhin, V.G.; Varfolomeeva, L.A.; Kuzin, M.S.; Toms, R.V.; Prokopov, N.I. The Effect of the Synthetic Procedure of Acrylonitrile–Acrylic Acid Copolymers on Rheological Properties of Solutions and Features of Fiber Spinning. Materials 2020, 13, 3454–3472. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Paliwal, D.K.; Bajaj, P. Effect of the nature and mole fraction of acidic comonomer on the stabilization of polyacrylonitrile. J. Appl. Polym. Sci. 1996, 59, 1819–1826. [Google Scholar] [CrossRef]
- Ju, A.; Guang, S.; Xu, H. Effect of comonomer structure on the stabilization and spinnability of polyacrylonitrile copolymers. Carbon 2013, 54, 323–335. [Google Scholar] [CrossRef]
- Devasia, R.; Nair, C.R.; Ninan, K.N. Comparative Rheological Properties of Dope Solutions of High Molar-Mass Poly (acry-lonitrile-co-itaconic acid) and Poly (acrylonitrile-co-methylacrylate-co-itaconic acid). Polym. Polym. Compos. 2004, 12, 667–677. [Google Scholar] [CrossRef]
- Vashchenko, A.F.; Toms, R.V.; Balashov, M.S.; Pichkunov, N.; Gervald, A.Y.; Prokopov, N.I.; Maksimov, N.M.; Plutalova, A.V.; Chernikova, E.V. Terpolymers of Acrylonitrile, Acrylic Acid, and Alkyl Acrylates: Effect of Alkyl Acrylate on the Thermal Properties of Copolymers. Polym. Sci. Ser. B 2021, 63, 802–820. [Google Scholar] [CrossRef]
- Skvortsov, I.Y.; Varfolomeeva, L.A.; Kuzin, M.S.; Vashchenko, A.F.; Chernikova, E.V.; Toms, R.V.; Kulichikhin, V.G. Effect of the comonomer addition sequence in the synthesis of an acrylonitrile terpolymer on the solution rheology and fiber properties. Mendeleev Commun. 2022, 32, 652–654. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.; Mayadunne, R.T.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- ASTM D2857-16; Standard Practice for Dilute Solution Viscosity of Polymers. ASTM International: West Conshohocken, PA, USA, 2016.
- Kulichikhin, V.G.; Skvortsov, I.Y.; Subbotin, A.V.; Kotomin, S.V.; Malkin, A.Y. A novel technique for fiber formation: Mech-anotropic spinning—Principle and realization. Polymers 2018, 10, 856–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalabin, A.L.; Pakshver, E.A. Estimation of the parametres of mechanotropic gelation in wet spinning of fibres. Fibre Chem. 2001, 33, 12–15. [Google Scholar] [CrossRef]
- Kalabin, A.L.; Pakshver, E.A.; Salakhov, M.A. Gelation in the phase space in spinning chemical fibres from polymer solutions. Fibre Chem. 2010, 42, 10–12. [Google Scholar] [CrossRef]
- Huggins, M.L. The viscosity of dilute solutions of long-chain molecules. IV. Dependence on concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Martin, A.F. Cellulose and cellulose derivatives. In Proceedings of the Memphis Meeting of the American Chemical Society, Memphis, TN, USA, 20–24 April 1942. [Google Scholar]
- Sakai, T. Huggins constant k’ for flexible chain polymers. J. Polym. Sci. Part A-2 1968, 6, 1535–1549. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of fluid and semisolid foods. In Rheology of Fluid and Semisolid Foods: Principal and Application, 2nd ed.; Chapman and Hall Food Science Book: New York, NY, USA, 1999; p. 12. [Google Scholar]
- Skvortsov, I.Y.; Maksimov, N.M.; Kuzin, M.S.; Toms, R.V.; Varfolomeeva, L.A.; Chernikova, E.V.; Kulichikhin, V.G. Influence of Alkyl Acrylate Nature on Rheological Properties of Polyacrylonitrile Terpolymers Solutions, Spinnability and Mechanical Characteristics of Fibers. Materials 2023, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Malkin, A.Y.; Isayev, A.I. Rheology: Concepts, Methods, Applications, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2017; pp. 59–60. [Google Scholar]
- Skvortsov, I.Y.; Kulichikhin, V.G.; Ponomarev, I.I.; Varfolomeeva, L.A.; Kuzin, M.S.; Razorenov, D.Y.; Skupov, K.M. Some Specifics of Defect-Free Poly-(o-aminophenylene) naphthoylenimide Fibers Preparation by Wet Spinning. Materials 2022, 15, 808–826. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Mironova, M.V.; Arkharova, N.A.; Klechkovskaya, V.V.; Kulichikhin, V.G. Morphological Transformations in the Process of Coagulation of Cellulose Solution in N-Methylmorpholine N-Oxide with Isobutanol. Polym. Sci. Ser. C 2021, 63, 161–169. [Google Scholar] [CrossRef]
- Ismail, N.; Venault, A.; Mikkola, J.P.; Bouyer, D.; Drioli, E.; Kiadeh, N.T.H. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 2020, 597, 117601. [Google Scholar] [CrossRef]
- Park, H.C.; Kim, Y.P.; Kim, H.Y.; Kang, Y.S. Membrane formation by water vapor induced phase inversion. J. Membr. Sci. 1999, 156, 169–178. [Google Scholar] [CrossRef]
- Wang, D.M.; Wu, T.T.; Lin, F.C.; Hou, J.Y.; Lai, J.Y. A novel method for controlling the surface morphology of polymeric membranes. J. Membr. Sci. 2000, 169, 39–51. [Google Scholar] [CrossRef]
- Subbotin, A.V.; Semenov, A.N. Phase separation in polymer solutions under extension. Polym. Sci. Ser. C 2018, 60, 106–117. [Google Scholar] [CrossRef]
- Bhattacharjee, S.M.; Fredrickson, G.H.; Helfand, E. Phase separation of polymer solutions in elongational flow. J. Chem. Phys. 1989, 90, 3305–3317. [Google Scholar] [CrossRef]
- Wang, P.H.; Liu, J.; Li, R.Y. Physical modification of polyacrylonitrile precursor fiber: Its effect on mechanical properties. J. Appl. Polym. Sci. 1994, 52, 1667–1674. [Google Scholar] [CrossRef]
- Gao, Q.; Jing, M.; Chen, M.; Zhao, S.; Wang, W.; Qin, J.; Wang, C. Microfibril alignment induced by stretching fields during the dry-jet wet spinning process: Reinforcement on polyacrylonitrile fiber mechanical properties. Polym. Test. 2020, 81, 106191. [Google Scholar] [CrossRef]



| Sample | Ð | Mn × 10−3 | Alkyl Acrylate | Comonomer Addition Method | fAH, mol.% | fAH, mol.% |
|---|---|---|---|---|---|---|
| MAc | 1.5 | 50 | Methyl- | Continuous | 88.4 | 72.2 |
| MAb1 | 1.4 | 76 | Batch | 89.8 | 91.3 | |
| MAb2 | 1.7 | 53 | Batch | 88.4 | 89.5 | |
| BAc | 1.6 | 85 | Butyl- | Continuous | 88.4 | 72.4 |
| BAb1 | 1.4 | 95 | Batch | 89.8 | 92.3 | |
| BAb2 | 1.7 | 61 | Batch | 88.4 | 85.5 | |
| LAc | 1.7 | 45 | Lauryl- | Continuous | 88.4 | 84.2 |
| LAb1 | 1.7 | 101 | Batch | 89.8 | 91.2 | |
| EHAb1 | 1.4 | 75 | Ethylhexyl- | Batch | 89.8 | 92.6 |
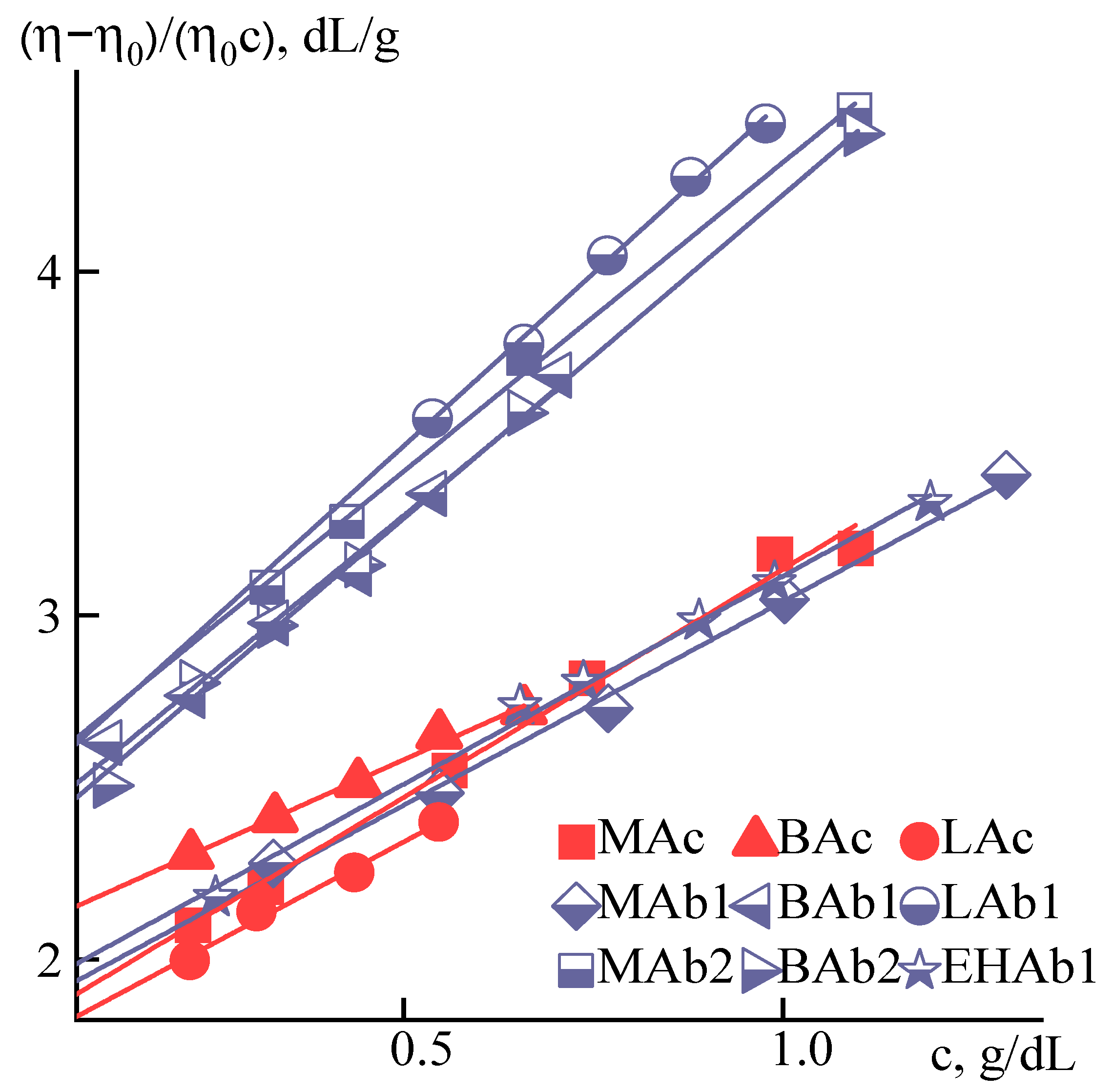
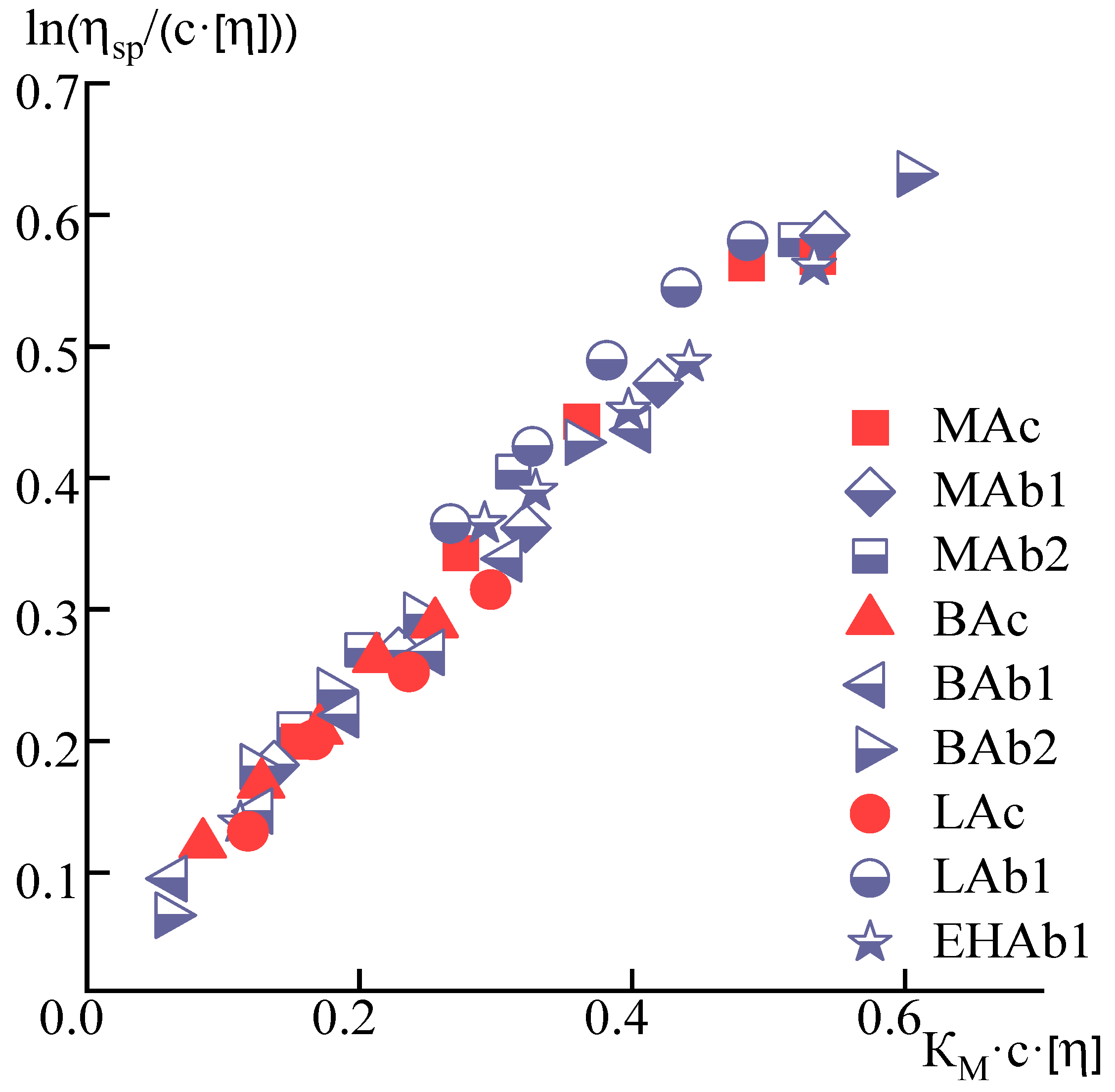
| Sample | [η], dL/g | KH | KM |
|---|---|---|---|
| MAc | 1.8 | 0.4 | 0.3 |
| MAb1 | 1.9 | 0.3 | 0.2 |
| MAb2 | 2.5 | 0.3 | 0.2 |
| BAc | 2 | 0.2 | 0.2 |
| BAb1 | 2.4 | 0.3 | 0.2 |
| BAb2 | 2.3 | 0.3 | 0.2 |
| LAc | 1.8 | 0.4 | 0.3 |
| LAb1 | 2.5 | 0.3 | 0.2 |
| EHAb1 | 1.9 | 0.3 | 0.2 |
| Title 1 | Linear Flow (V0) and Winding (V1–V6) Speed, m/min | Draw Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| V0 | V1 | V2 | V3 | V4, V5 | V6 | V6/V1 | V6/V0 | |
| MAc | 0.08 | 3.8 | 6.1 | 10 | 10 | 20 | 5.3 | 250.0 |
| MAb1 | 4.2 | 5.7 | 10.4 | 12.5 | 17.5 | 4.2 | 218.8 | |
| BAc | 4.4 | 10.4 | 12.2 | 12.3 | 21.9 | 5.0 | 273.8 | |
| BAb1 | 2.2 | 10.1 | 11.9 | 13 | 21.2 | 9.6 | 264.4 | |
| LAc | 2.2 | 6.8 | 7.1 | 7.1 | 16.3 | 7.4 | 203.8 | |
| LAb1 | 2.2 | 10 | 12.5 | 12.9 | 17.3 | 7.9 | 216.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skvortsov, I.Y.; Kuzin, M.S.; Vashchenko, A.F.; Toms, R.V.; Varfolomeeva, L.A.; Chernikova, E.V.; Shambilova, G.K.; Kulichikhin, V.G. Fiber Spinning of Polyacrylonitrile Terpolymers Containing Acrylic Acid and Alkyl Acrylates. Fibers 2023, 11, 65. https://doi.org/10.3390/fib11070065
Skvortsov IY, Kuzin MS, Vashchenko AF, Toms RV, Varfolomeeva LA, Chernikova EV, Shambilova GK, Kulichikhin VG. Fiber Spinning of Polyacrylonitrile Terpolymers Containing Acrylic Acid and Alkyl Acrylates. Fibers. 2023; 11(7):65. https://doi.org/10.3390/fib11070065
Chicago/Turabian StyleSkvortsov, Ivan Yu., Mikhail S. Kuzin, Andrey F. Vashchenko, Roman V. Toms, Lydia A. Varfolomeeva, Elena V. Chernikova, Gulbarshin K. Shambilova, and Valery G. Kulichikhin. 2023. "Fiber Spinning of Polyacrylonitrile Terpolymers Containing Acrylic Acid and Alkyl Acrylates" Fibers 11, no. 7: 65. https://doi.org/10.3390/fib11070065
APA StyleSkvortsov, I. Y., Kuzin, M. S., Vashchenko, A. F., Toms, R. V., Varfolomeeva, L. A., Chernikova, E. V., Shambilova, G. K., & Kulichikhin, V. G. (2023). Fiber Spinning of Polyacrylonitrile Terpolymers Containing Acrylic Acid and Alkyl Acrylates. Fibers, 11(7), 65. https://doi.org/10.3390/fib11070065










