Synthesis of Carbon Nanofibers from Lignin Using Nickel for Supercapacitor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Fibrous Composite Materials Lignin/PAN/Ni
2.2. Fiber Stabilization and Carbonization Process
2.3. Fabrication of Electrodes Using Carbon Nanofibers for Supercapacitors
2.4. Electrochemical Measurements
3. Results and Discussion
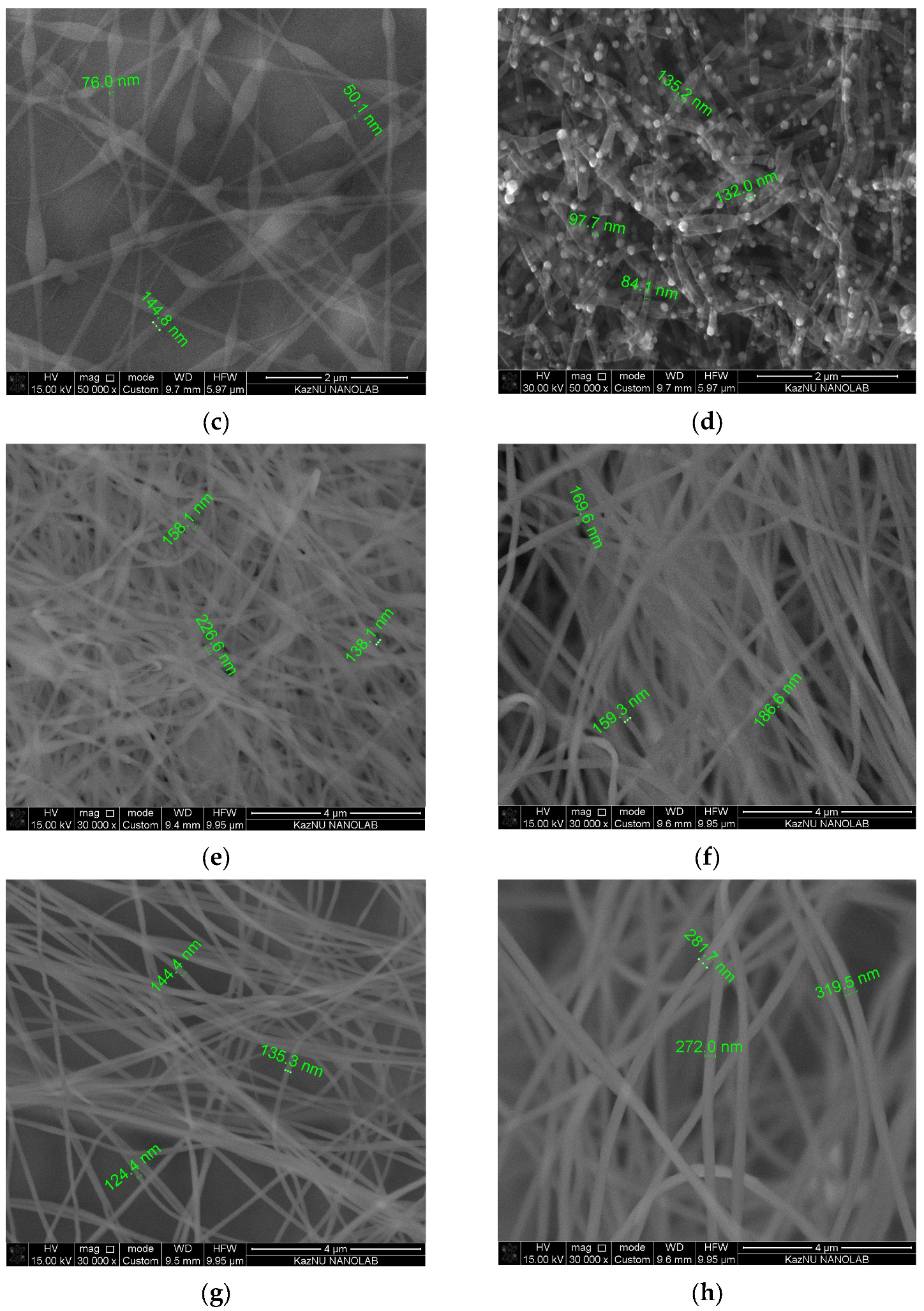
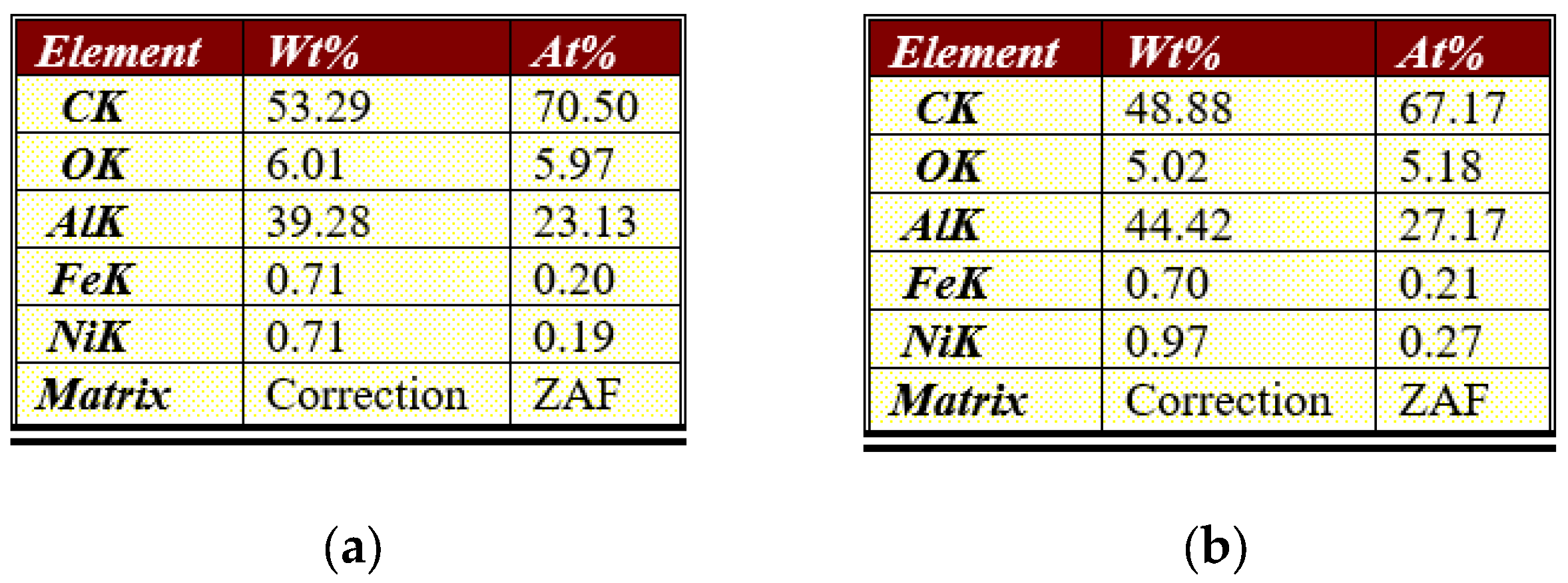
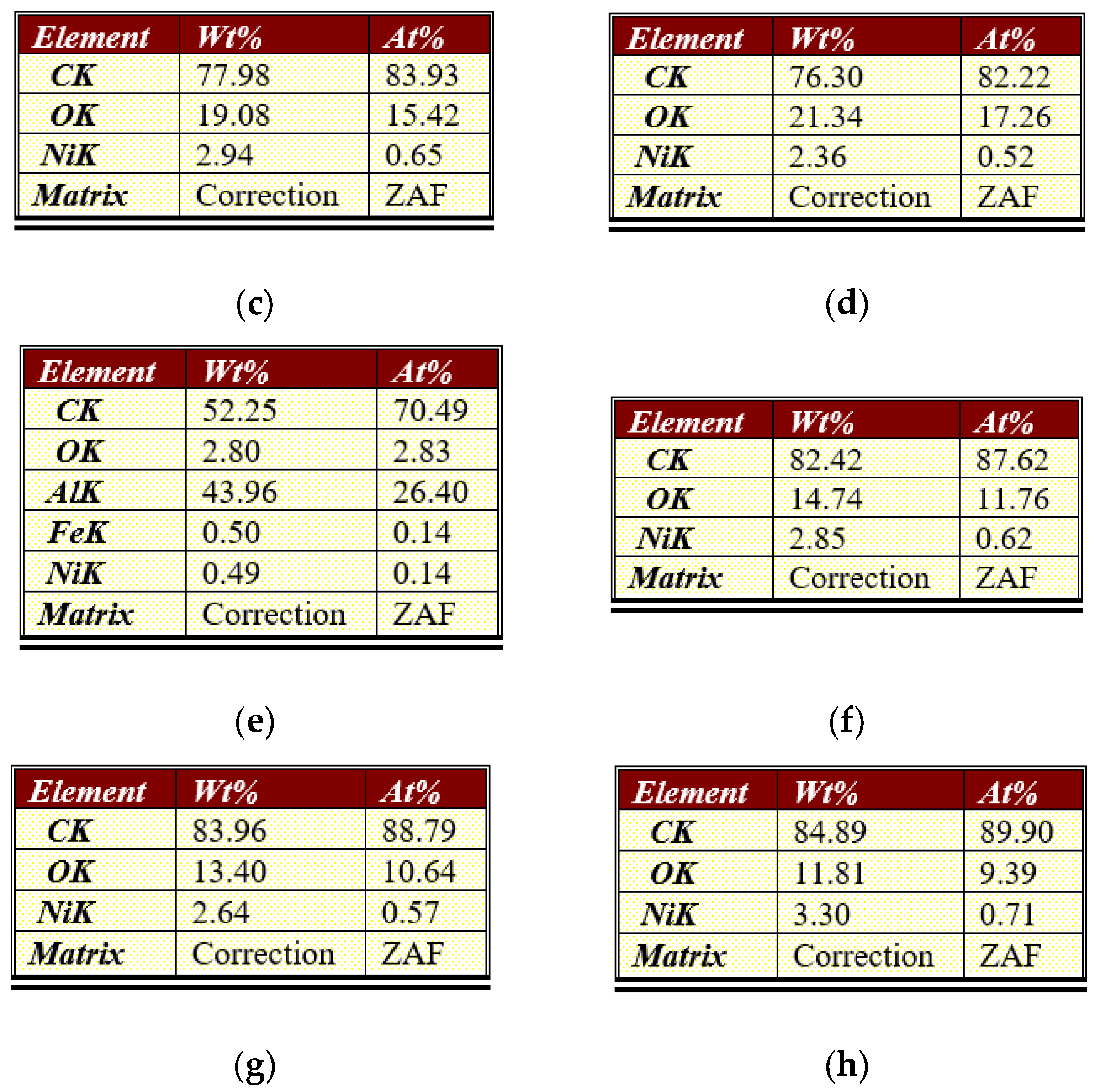
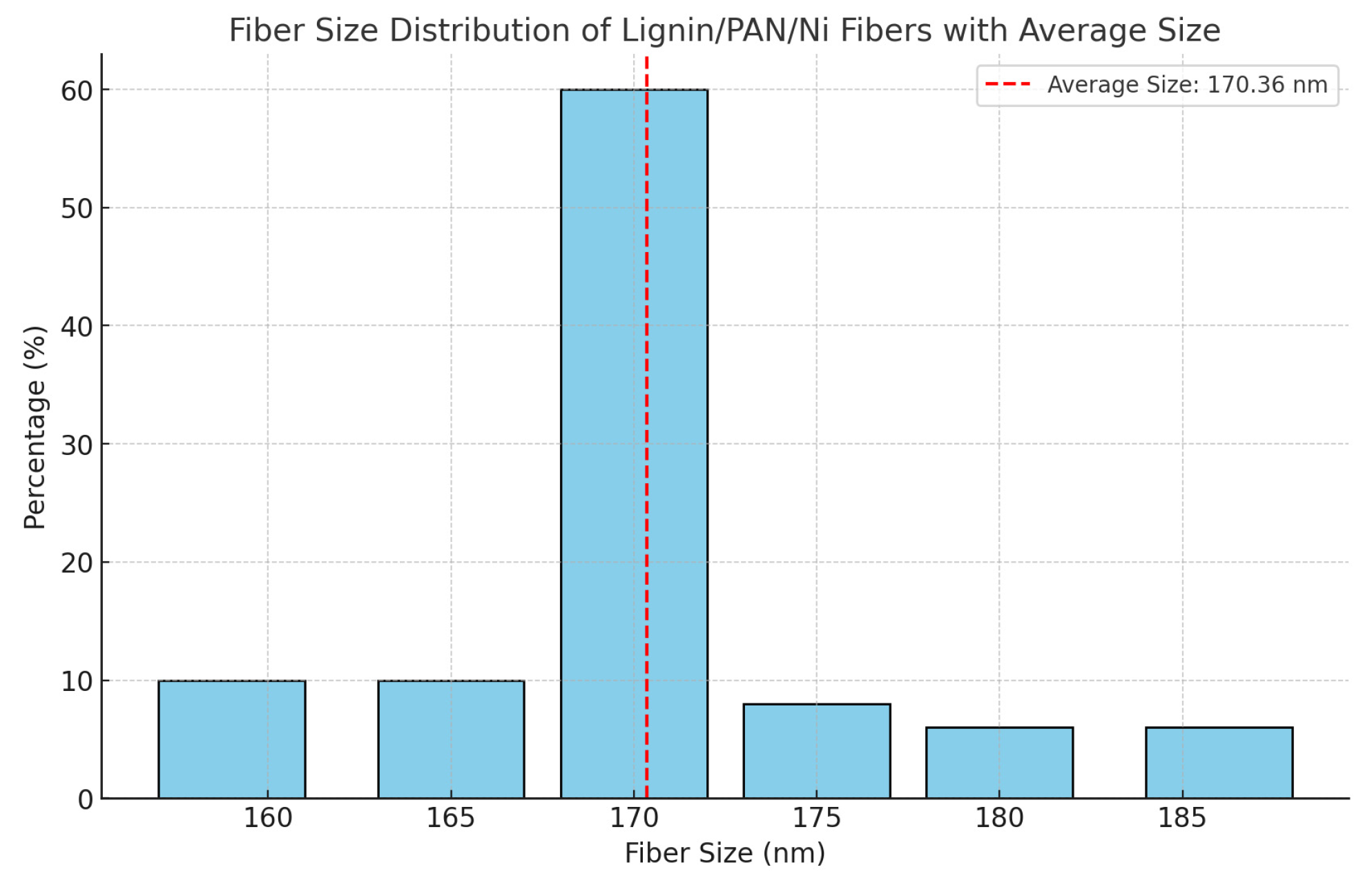
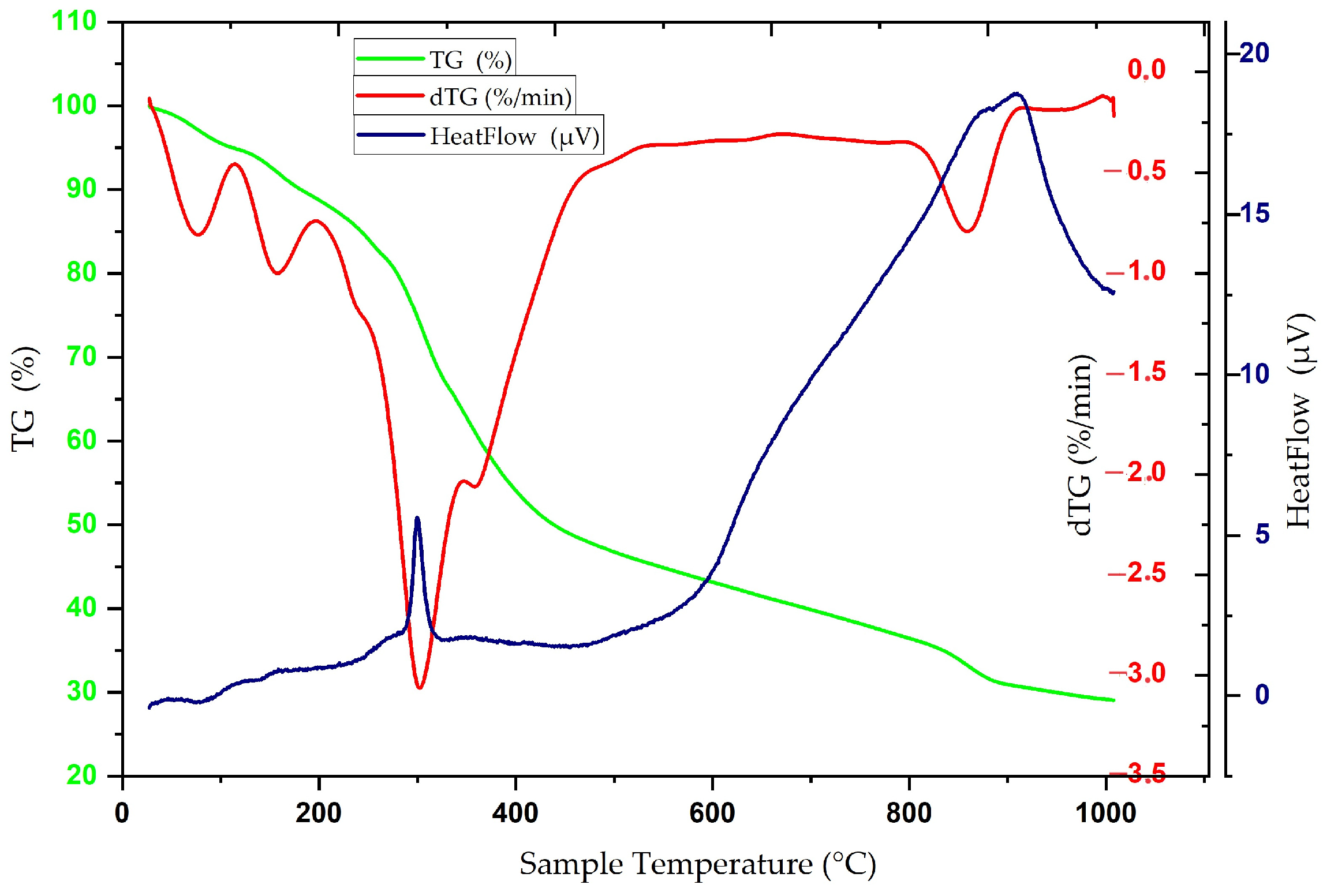
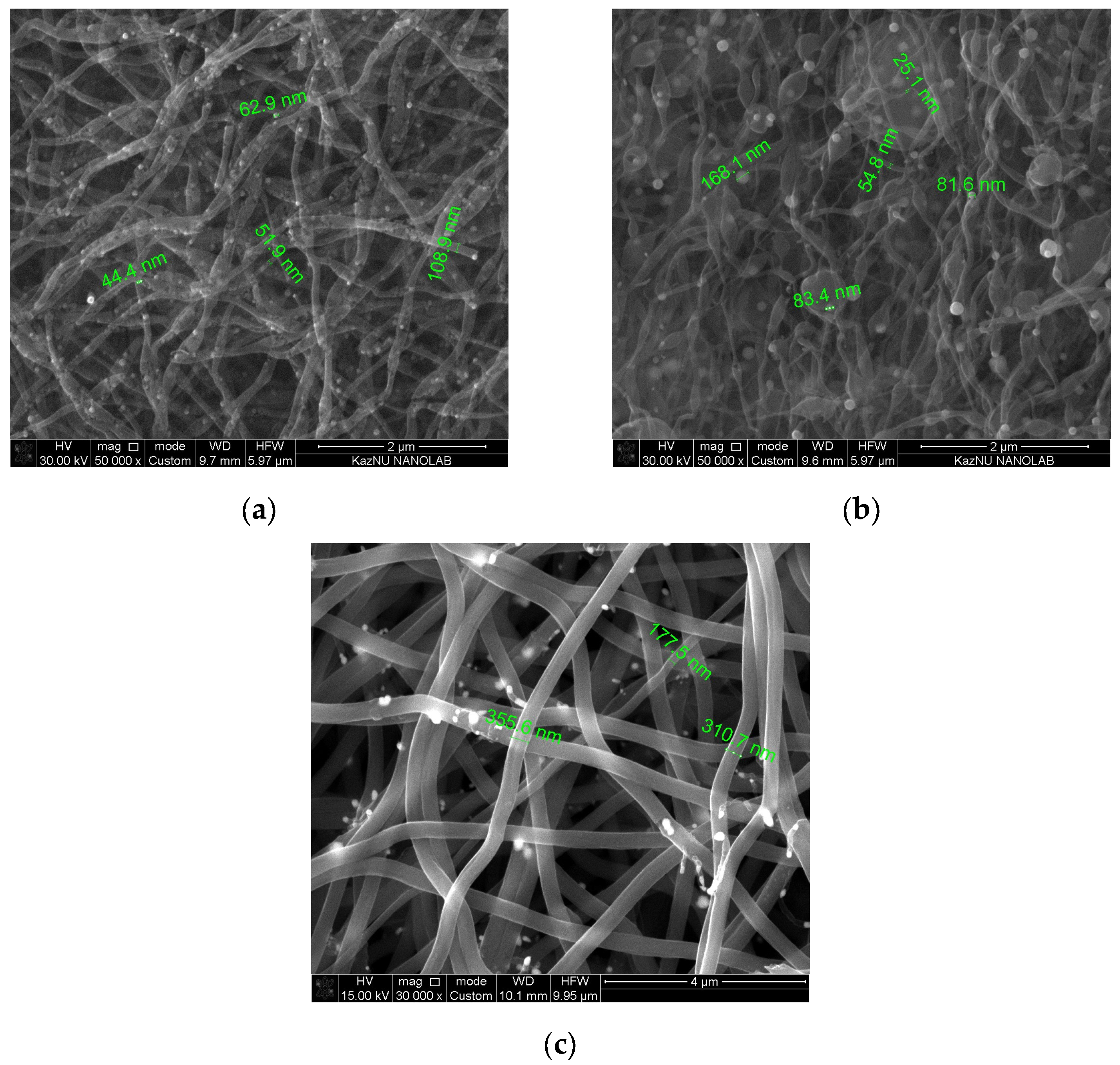
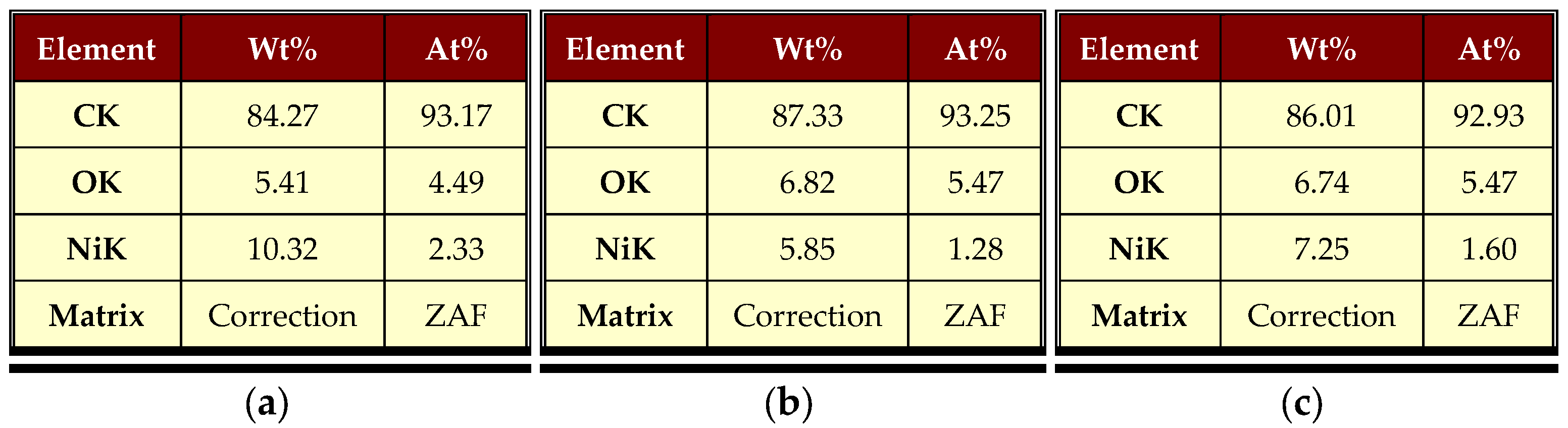
3.1. Physical and Chemical Properties of Carbon Nanofibers Derived from Lignin/PAN/Ni
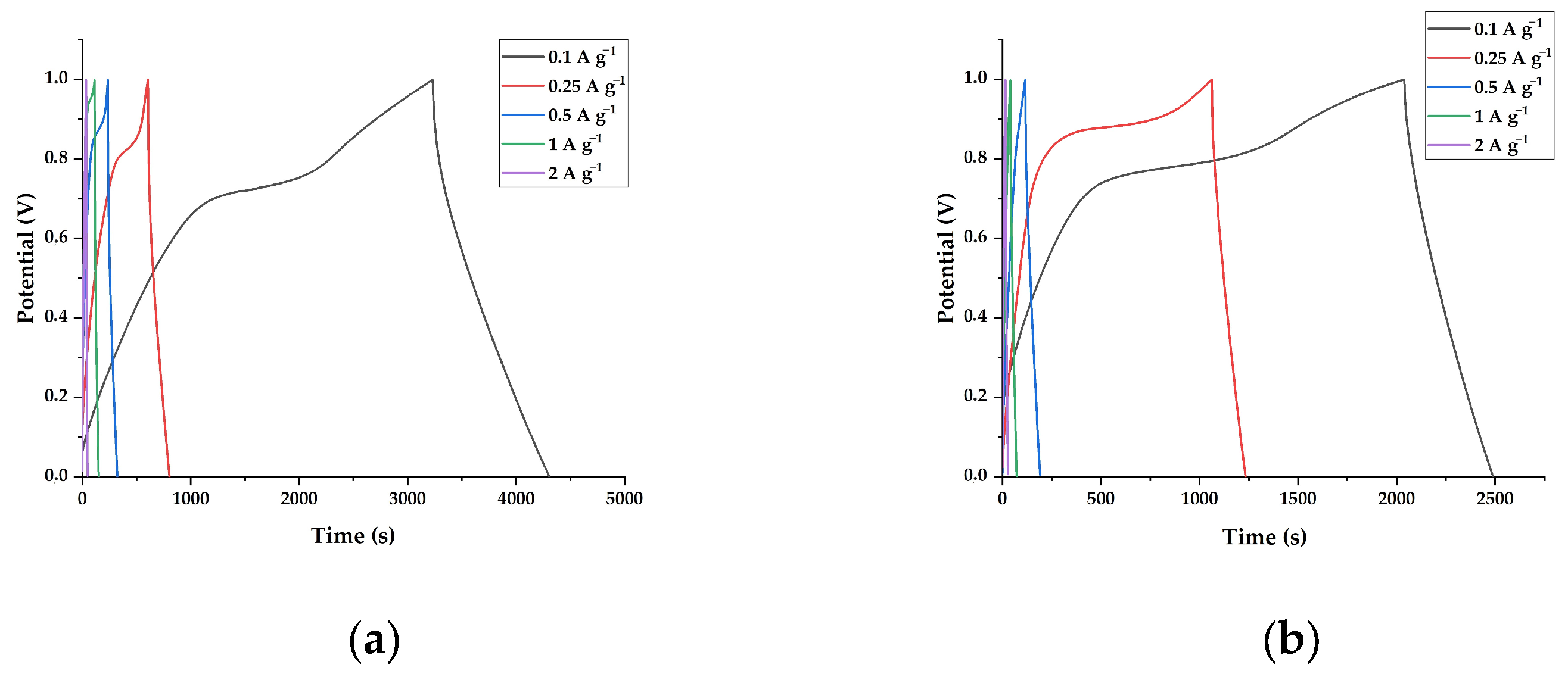
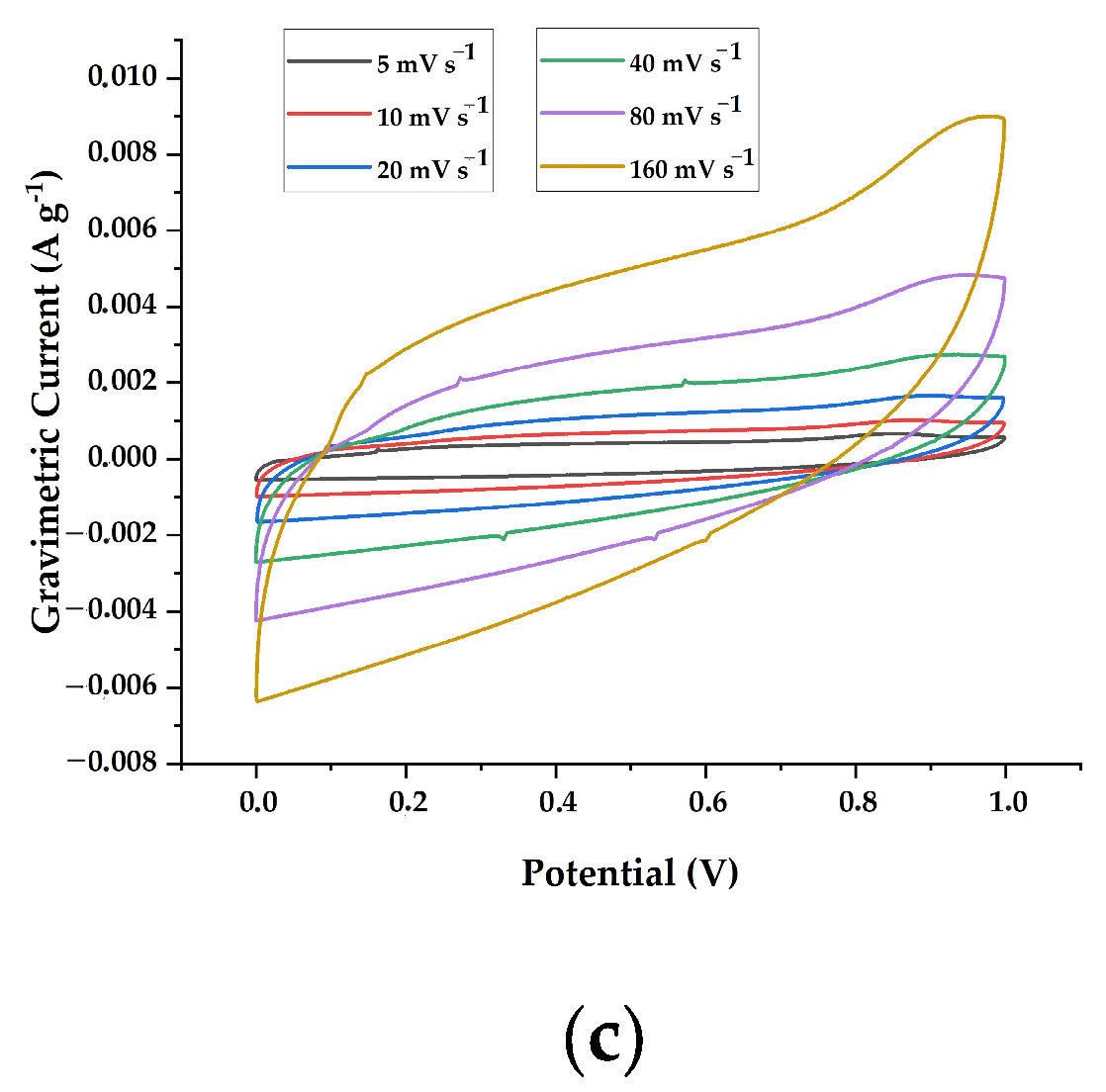
3.2. Electrochemical Properties of Electrodes Composed of Lignin/PAN/Ni Composite Materials
4. Comparative Analysis of Lignin-Based Carbon Nanofibers for Supercapacitor Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dissanayake, K.; Kularatna-Abeywardana, D. A review of supercapacitors: Materials, technology, challenges, and renewable energy applications. J. Energy Storage 2024, 96, 112563. [Google Scholar]
- Dhandapani, E.; Thangarasu, S.; Ramesh, S.; Ramesh, K.; Vasudevan, R.; Duraisamy, N. Recent development and prospective of carbonaceous material, conducting polymer and their composite electrode materials for supercapacitor—A review. J. Energy Storage 2022, 52, 104937. [Google Scholar] [CrossRef]
- Gürten, İ.I. Scalable activated carbon/graphene based supercapacitors with improved capacitance retention at high current densities. Turk. J. Chem. 2021, 45, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Liu, Y.; Feng, H.; Shi, H.; Ma, Q. A review on graphene-based electrode materials for supercapacitor. J. Ind. Eng. Chem. 2024, 137, 106–121. [Google Scholar]
- De, B.; Banerjee, S.; Verma, K.D.; Pal, T.; Manna, P.K.; Kar, K.K. Carbon Nanotube as Electrode Materials for Supercapacitors. Handbook of Nanocomposite Supercapacitor Materials II. Springer Ser. Mater. Sci. 2020, 302, 229–243. [Google Scholar]
- Dong, Y.; Zhu, J.; Li, Q.; Zhang, S.; Song, H.; Jia, D. Carbon materials for high mass-loading supercapacitors: Filling the gap between new materials and practical applications. J. Mater. Chem. A 2020, 8, 21930–21946. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Chhetri, K.; Lee, M.; Dahal, B.; Lohani, P.C.; Kim, H.-Y. A Review of Electrospun Carbon Nanofiber-Based Negative Electrode Materials for Supercapacitors. Electrochemistry 2021, 2, 17. [Google Scholar] [CrossRef]
- Cao, Q.; Zhu, H.; Xu, J.; Zhang, M.; Xiao, T.; Xu, S.; Du, B. Research progress in the preparation of lignin-based carbon nanofibers for supercapacitors using electrospinning technology: A review. Int. J. Biol. Macromol. 2024, 243, 133037. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, N.; Zhao, M.; Zhong, W.; Wu, W.-J.; Jin, Y.-C. High-performance electrode materials of heteroatom-doped lignin-based carbon materials for supercapacitor applications. Int. J. Biol. Macromol. 2024, 273, 133017. [Google Scholar] [CrossRef]
- Zhong, W.; Su, W.; Li, P.; Li, K.; Wu, W.; Jiang, B. Preparation and research progress of lignin-based supercapacitor electrode materials. Int. J. Biol. Macromol. 2024, 259, 128942. [Google Scholar]
- Zhou, Q.; Chen, Q.; Xu, W.; Wang, F.; Du, X.; Zhou, Y.; Zhan, Y.; Jiang, M. Nitrogen and sulfur co-doped carbonized lignin nanotubes for supercapacitor applications. Chem. Eng. J. 2024, 496, 154126. [Google Scholar] [CrossRef]
- Liu, L.; Solin, N.; Inganas, O. Self-discharge study of lignin/graphite hybrid material electrodes. Electrochim. Acta 2021, 371, 137836. [Google Scholar] [CrossRef]
- Ma, C.; Song, G.; Li, Z.; Wu, H.; Wang, C.; Wang, Y.; Zhang, X.; Song, Y.; Shi, J. High-areal-capacitance electrode constructed by lignin-based microporous carbon nanofibers for supercapacitors. J. Energy Storage 2024, 88, 111465. [Google Scholar] [CrossRef]
- Ehsani, A.; Moftakhar, M.K.; Karimi, F. Lignin-derived carbon as a high efficient active material for enhancing pseudocapacitance performance of p-type conductive polymer. J. Energy Storage 2021, 35, 102291. [Google Scholar] [CrossRef]
- Li, P.H.; Wei, Y.M.; Wu, C.W.; Yang, C.; Jiang, B.; Wu, W.J. Lignin-based composites for high-performance supercapacitor electrode materials. RSC Adv. 2022, 12, 19485–19494. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Jiang, C.; Xiao, X.F.; Zhang, Y.S.; Liang, L. Lignin-based Biochar/graphene Oxide Composites as Supercapacitor Electrode Materials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 3592, 012046. [Google Scholar] [CrossRef]
- Mustafov, S.D.; Mohanty, A.K.; Misra, M.; Seydibeyoglu, M.O. Fabrication of conductive Lignin/PAN carbon nanofibers with enhanced graphene for the modified electrodes. Carbon 2019, 147, 262–275. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Jiang, L.; Lubineau, G.; Payne, S.A.; Gutschmidt, D. Lignin-based carbon fibers: Carbon nanotube decoration and superior thermal stability. Carbon 2014, 80, 91–102. [Google Scholar] [CrossRef]
- Ma, X.; Kolla, P.; Zhao, Y.; Smirnova, A.L.; Fong, H. Electrospun lignin-derived carbon nanofiber mats surface-decorated with MnO2 nanowhiskers as binder-free supercapacitor electrodes with high performance. J. Power Sources 2016, 325, 541–548. [Google Scholar] [CrossRef]
- Ma, X.; Smirnova, A.L.; Fong, H. Flexible lignin-derived carbon nanofiber substrates functionalized with iron (III) oxide nanoparticles as lithium-ion battery anodes. Mater. Sci. Eng. B 2019, 241, 100–104. [Google Scholar] [CrossRef]
- Song, M.; Zhang, W.; Chen, Y.; Luo, J.; Crittenden, J.C. The preparation and performance of lignin-based activated carbon fiber adsorbents for treating gaseous streams. Front. Chem. Sci. Eng. 2017, 11, 328–337. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.; Cao, Q.; Gao, X.; He, W.; Xiao, Y.; Jin, Y.; Ren, F. Synthesis of TiO2@lignin based carbon nanofibers composite materials with highly efficient photocatalytic to methylene blue dye. J. Polym. Res. 2020, 27, 108. [Google Scholar] [CrossRef]
- Lai, C.; Kolla, P.; Zhao, Y.; Fong, H.; Smirnova, A.L. Lignin-derived electrospun carbon nanofiber mats with supercritically deposited Ag nanoparticles for oxygen reduction reaction in alkaline fuel cells. Electrochim. Acta 2014, 130, 431–438. [Google Scholar] [CrossRef]
- Yun, S.I.; Kim, S.H.; Kim, D.W.; Kim, Y.A.; Kim, B.H. Facile preparation and capacitive properties of low-cost carbon nanofibers with ZnO derived from lignin and pitch as supercapacitor electrodes. Carbon 2019, 149, 637–645. [Google Scholar] [CrossRef]
- Ma, C.; Wu, L.; Dirican, M.; Cheng, H.; Li, J.; Song, Y.; Shi, J.; Zhang, X. ZnO-assisted synthesis of lignin-based ultra-fine microporous carbon nanofibers for supercapacitors. J. Colloid Interface Sci. 2021, 586, 412–422. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, S.; Pan, N.; Hsieh, Y.L. High energy density supercapacitors from lignin derived submicron activated carbon fibers in aqueous electrolytes. J. Power Sources 2014, 270, 106–112. [Google Scholar] [CrossRef]
- Ma, C.; Li, Z.; Li, J.; Fan, Q.; Wu, L.; Shi, J.; Song, Y. Lignin-based hierarchical porous carbon nanofiber films with superior performance in supercapacitors. Appl. Surf. Sci. 2018, 456, 568–576. [Google Scholar] [CrossRef]
- Gu, C.; Ma, H.; Zhang, Q.; Li, M.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Nano MnO2 Radially Grown on Lignin-Based Carbon Fiber by One-Step Solution Reaction for Supercapacitors with High Performance. Nanomaterials 2020, 10, 594. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, D.; Liu, T.; Jaroniec, M.; Yu, J. Nickel-based materials for supercapacitors. Mater. Today 2019, 25, 35–65. [Google Scholar] [CrossRef]
- Xiao, H.; Qu, F.; Wu, X. Ultrathin NiO nanoflakes electrode materials for supercapacitors. Appl. Surf. Sci. 2016, 360, 8–13. [Google Scholar] [CrossRef]
- Brisse, A.-L.; Stevens, P.; Toussain, G.; Crosnier, O.; Brousse, T. Ni(OH)2 and NiO Based Composites: Battery Type Electrode Materials for Hybrid Supercapacitor Devices. Materials 2018, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Wang, Y.; Liu, L.; Hu, C. Effect of Ni(NO3)2 Pretreatment on the Pyrolysis of Organsolv Lignin Derived from Corncob Residue. Processes 2021, 9, 23. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; O’ Keefe, C.A.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Tomani, P.; Grey, C.P.; Titirici, M.-M. Hardwood versus softwood Kraft lignin—precursor-product relationships in the manufacture of porous carbon nanofibers for supercapacitors. J. Mater. Chem. A 2020, 8, 23543–23554. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Maltay, A.B.; Lesbayev, B.T.; Assylkhanova, D.D. Synthesis of Lignin/PAN Fibers from Sawdust. Fibers 2024, 12, 27. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The production of submicron diameter carbon fibers by the electrospinning of lignin. Carbon 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Rodríguez-Mirasol, J.; Cordero, T.; Rodriguez, J.J. High-temperature carbons from kraft lignin. Carbon 1996, 34, 43–52. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ahvazi, B.; Boluk, Y.; Ayranci, C. Carbon fiber production from electrospun sulfur free softwood lignin precursorsc. J. Eng. Fibers Fabr. 2017, 12, 33–43. [Google Scholar]
- Saha, D.; Li, Y.; Bi, Z.; Chen, J.; Keum, J.K.; Hensley, D.K.; Grappe, H.A.; Meyer, H.M.; Dai, S.; Paranthaman, M.P.; et al. Studies on Supercapacitor Electrode Material from Activated Lignin-Derived Mesoporous Carbon. Langmuir 2014, 30, 900–910. [Google Scholar] [CrossRef]




| Current Density, A g−1 | |||||
|---|---|---|---|---|---|
| Samples | 0.1 | 0.25 | 0.5 | 1 | 2 |
| Specific Capacitance, F g−1 | |||||
| lignin/PAN/Ni 30/70/10 at 800 °C | 108 ± 0.3 | 102 ± 0.4 | 89 ± 0.4 | 78 ± 0.2 | 53 ± 0.3 |
| lignin/PAN/Ni 30/70/10 at 900 °C | 96 ± 0.4 | 87 ± 0.3 | 73 ± 0.2 | 66 ± 0.3 | 48 ± 0.2 |
| lignin/PAN/Ni 30/70/10 at 1000 °C | 81 ± 0.2 | 69 ± 0.2 | 59 ± 0.2 | 35 ± 0.2 | 21 ± 0.2 |
| Scan Rate, mV s−1 | ||||||
|---|---|---|---|---|---|---|
| Samples | 5 | 10 | 20 | 40 | 80 | 160 |
| Specific Capacitance, F g−1 | ||||||
| lignin/PAN/Ni 30/70/10 at 800 °C | 91 ± 0.4 | 78± 0.4 | 65 ± 0.5 | 57 ± 0.3 | 45 ± 0.4 | 40 ± 0.4 |
| lignin/PAN/Ni 30/70/10 at 900 °C | 78 ± 0.3 | 63 ± 0.2 | 49 ± 0.4 | 37 ± 0.2 | 28 ± 0.3 | 23 ± 0.3 |
| lignin/PAN/Ni 30/70/10 at 1000 °C | 66 ± 0.3 | 51 ± 0.3 | 41 ± 0.3 | 32 ± 0.3 | 27 ± 0.5 | 21 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazhipkyzy, M.; Maltay, A.B.; Seilkhanov, T.M. Synthesis of Carbon Nanofibers from Lignin Using Nickel for Supercapacitor Applications. Fibers 2024, 12, 87. https://doi.org/10.3390/fib12100087
Nazhipkyzy M, Maltay AB, Seilkhanov TM. Synthesis of Carbon Nanofibers from Lignin Using Nickel for Supercapacitor Applications. Fibers. 2024; 12(10):87. https://doi.org/10.3390/fib12100087
Chicago/Turabian StyleNazhipkyzy, Meruyert, Anar B. Maltay, and Tulegen M. Seilkhanov. 2024. "Synthesis of Carbon Nanofibers from Lignin Using Nickel for Supercapacitor Applications" Fibers 12, no. 10: 87. https://doi.org/10.3390/fib12100087
APA StyleNazhipkyzy, M., Maltay, A. B., & Seilkhanov, T. M. (2024). Synthesis of Carbon Nanofibers from Lignin Using Nickel for Supercapacitor Applications. Fibers, 12(10), 87. https://doi.org/10.3390/fib12100087







