Polybenzoxazine/Epoxy Copolymer Reinforced with Phosphorylated Microcrystalline Cellulose: Curing Behavior, Thermal, and Flame Retardancy Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.2.1. Phosphorylation Procedure
2.2.2. Composite Preparation
2.3. Characterization Methods
2.3.1. Fourier Transform Infra-Red Spectroscopy (FTIR)
2.3.2. X-ray Diffraction (XRD) Analysis
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Differential Scanning Calorimetry
2.3.6. Calorimetry ISO 1716 Test
2.3.7. Vertical Burning Test
3. Results and Discussions
3.1. Characterization of MCC and MCC-P
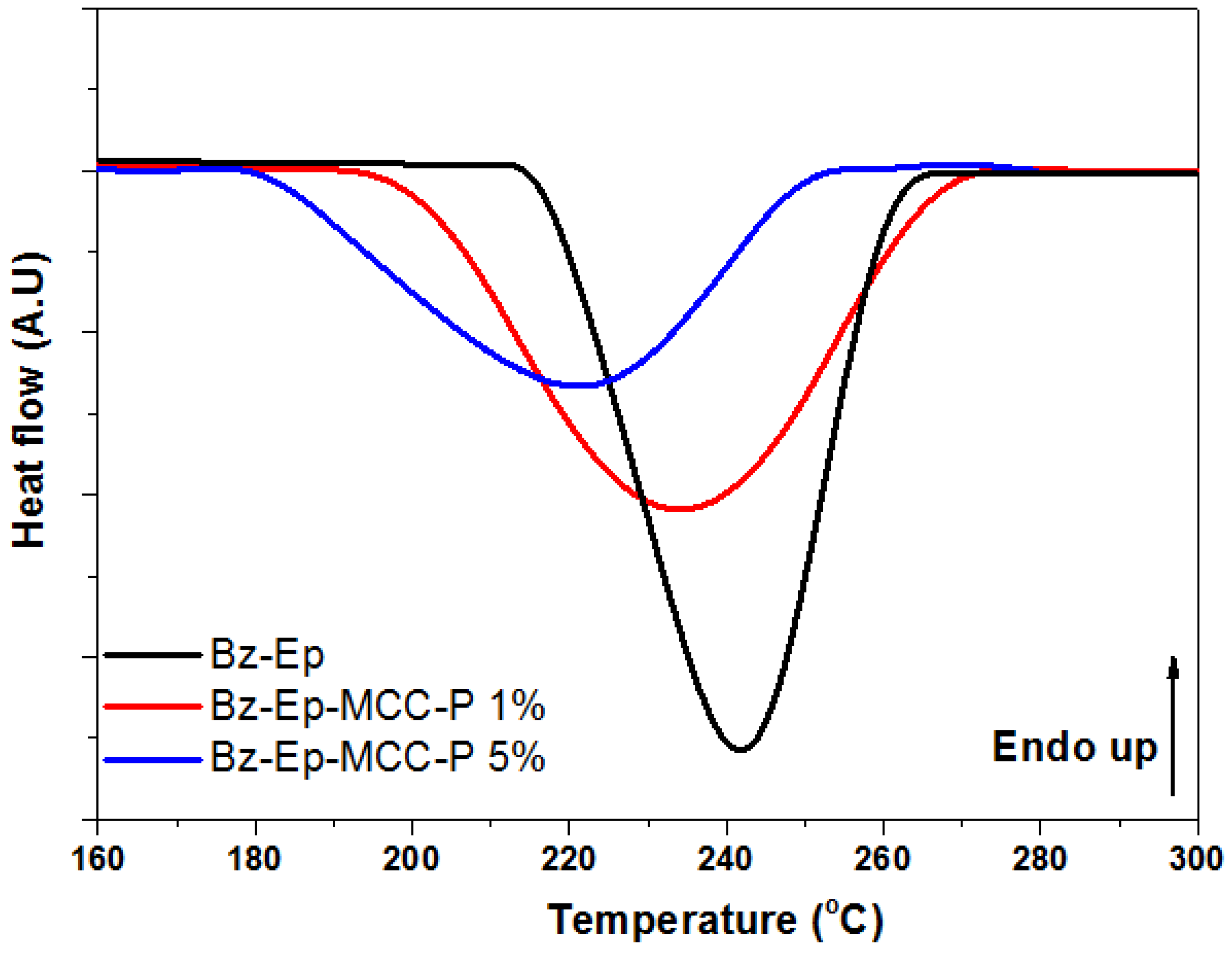
3.2. Curing Behavior Investigation
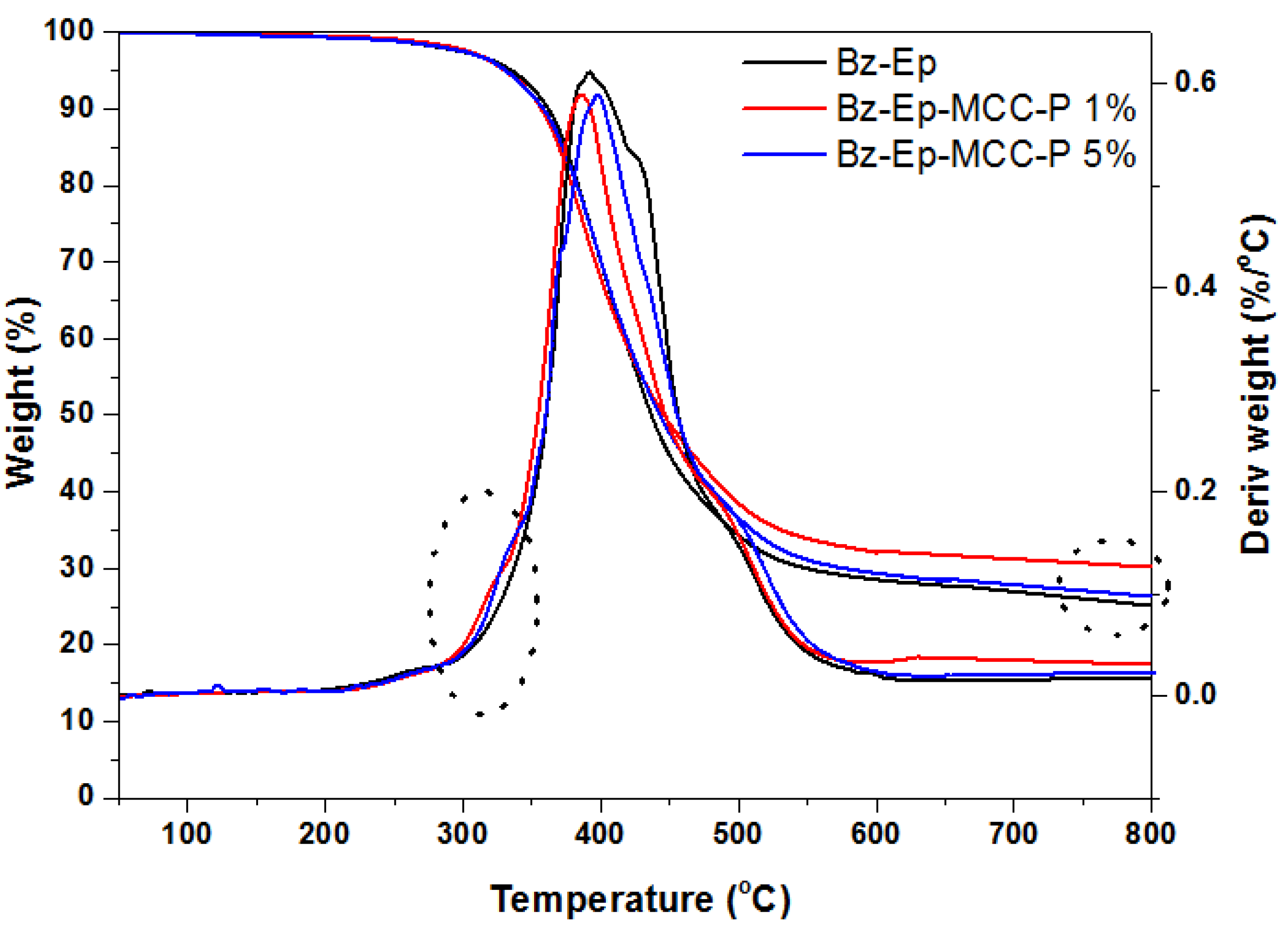
3.3. Thermal Decomposition
3.4. Flame Retardancy Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, D.; Lee, Y.H.; Chen, W.; Lee, S.Y. Nanocellulose for energy storage systems: Beyond the limits of synthetic materials. Adv. Mater. 2019, 31, 1804826. [Google Scholar] [CrossRef] [PubMed]
- Trache, D. Microcrystalline cellulose and related polymer composites: Synthesis, characterization and properties. In Handbook of Composites from Renewable Materials, Structure and Chemistry; John Wiley & Sons: New York, NY, USA, 2016; Volume 1, pp. 61–92. [Google Scholar]
- Fernandes, E.M.; Pires, R.A.; Mano, J.F.; Reis, R.L. Bionanocomposites from lignocellulosic resources: Properties, applications and future trends for their use in the biomedical field. Prog. Polym. Sci. 2013, 38, 1415–1441. [Google Scholar] [CrossRef]
- Singh, T.; Gangil, B.; Patnaik, A.; Biswas, D.; Fekete, G. Agriculture waste reinforced corn starch-based biocomposites: Effect of rice husk/walnut shell on physicomechanical, biodegradable and thermal properties. Mater. Res. Express 2019, 6, 045702. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Mei, C.; Sun, J.; Lee, J.; Wu, Q.; Hubbe, M.A.; Li, M.-C. Recent advances in metal organic framework and cellulose nanomaterial composites. Coord. Chem. Rev. 2022, 461, 214496. [Google Scholar] [CrossRef]
- Afrin, S.; Karim, Z. Isolation and surface modification of nanocellulose: Necessity of enzymes over chemicals. ChemBioEng Rev. 2017, 4, 289–303. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Lai, T.K.; Tye, Y.Y.; Paridah, M.; Fazita, M.N.; Azniwati, A.; Dungani, R.; Rizal, S. Preparation and characterization of microcrystalline cellulose from sacred bali bamboo as reinforcing filler in seaweed-based composite film. Fibers Polym. 2018, 19, 423–434. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly(vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef]
- Jubsilp, C.; Punson, K.; Takeichi, T.; Rimdusit, S. Curing kinetics of benzoxazine–epoxy copolymer investigated by non-isothermal differential scanning calorimetry. Polym. Degrad. Stab. 2010, 95, 918–924. [Google Scholar] [CrossRef]
- Yang, W.; Wu, Q.; Zhou, Y.; Zhu, S.; Wei, C.; Lu, H.; Yang, W.; Yuen, R.K. Multifunctional phosphorus-containing porphyrin dye for efficiently improving the thermal, toughness, flame retardant and dielectric properties of epoxy resins. Prog. Org. Coat. 2024, 186, 107967. [Google Scholar] [CrossRef]
- Shi, J.-C.; Bai, W.-B.; Lin, Y.-C.; Ding, F.-C.; Jian, R.-K. Synthesis of a novel dimethylglyoxime-bridged phosphinate and its application in flame-retardant epoxy resins. Polym. Degrad. Stab. 2024, 220, 110662. [Google Scholar] [CrossRef]
- Musa, A.; Alamry, K.; Hussein, M. Polybenzoxazine-modified epoxy resin: Thermal properties and coating performance. Int. J. Polym. Anal. Charact. 2021, 26, 189–203. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Zhang, J.; Zhang, H.; Fan, H.; Run, M. Polymerization behavior and thermal properties of benzoxazine based on 4, 4′-diaminodiphenyl ether. J. Therm. Anal. Calorim. 2013, 111, 1523–1530. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Mechanical characterization of copolymers based on benzoxazine and epoxy. Polymer 1996, 37, 4487–4495. [Google Scholar] [CrossRef]
- Mora, P.; Rimdusit, S.; Karagiannidis, P.; Srisorrachatr, U.; Jubsilp, C. Mechanical properties and curing kinetics of bio-based benzoxazine–epoxy copolymer for dental fiber post. Bioresour. Bioprocess. 2023, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.M.; Deanin, R.D.; Pope, C.J. Man-made fibers: Flame retardance and flame retardants. Polym.-Plast. Technol. Eng. 1981, 16, 1–39. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Cruz-Izquierdo, A.; Castaing, R.; Kandola, B.; Scott, J.L. Facile preparation of flame-retardant cellulose composite with biodegradable and water resistant properties for electronic device applications. Sci. Rep. 2023, 13, 3168. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Zhao, Z.; Xue, Z.; Xu, P.; Xia, Y. Preparation of ion-exchanged tempo-oxidized celluloses as flame retardant products. Molecules 2019, 24, 1947. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, P.S.; Bhakare, M.A.; Lokhande, K.D.; Bondarde, M.P.; Some, S. Bio-waste derived, phosphorus decorated composite for highly efficient flame retardant for cotton fabric. Cellulose 2022, 29, 8879–8888. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef]
- Ning, X.; Ishida, H. Phenolic materials via ring-opening polymerization: Synthesis and characterization of bisphenol—A based benzoxazines and their polymers. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1121–1129. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wågberg, L. Phosphorylated cellulose nanofibrils: A renewable nanomaterial for the preparation of intrinsically flame-retardant materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Kazanci, B.; Cellat, K.; Paksoy, H. Preparation, characterization, and thermal properties of novel fire-resistant microencapsulated phase change materials based on paraffin and a polystyrene shell. RSC Adv. 2020, 10, 24134–24144. [Google Scholar] [CrossRef] [PubMed]
- ISO 1716:2018; Reaction to Fire Tests for Products–Determination of the Gross Heat of Combustion (Calorific Value). European Committee for Standardization (CEN): Brussels, Belgium, 2018.
- Suflet, D.M.; Chitanu, G.C.; Popa, V.I. Phosphorylation of polysaccharides: New results on synthesis and characterisation of phosphorylated cellulose. React. Funct. Polym. 2006, 66, 1240–1249. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Khelifa, F.; Rose, G.; Brohez, S.; Delvosalle, C.; Dubois, P. Cellulose/phosphorus combinations for sustainable fire retarded polylactide. Eur. Polym. J. 2016, 74, 218–228. [Google Scholar] [CrossRef]
- Ait Benhamou, A.; Kassab, Z.; Nadifiyine, M.; Salim, M.H.; Sehaqui, H.; Moubarik, A.; El Achaby, M. Extraction, characterization and chemical functionalization of phosphorylated cellulose derivatives from giant reed plant. Cellulose 2021, 28, 4625–4642. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, N.; Meyer, V.; Petit-Conil, M.; Bras, J. Production of fire-retardant phosphorylated cellulose fibrils by twin-screw extrusion with low energy consumption. Cellulose 2019, 26, 5635–5651. [Google Scholar] [CrossRef]
- Bessa, W.; Trache, D.; Tarchoun, A.F.; Abdelaziz, A.; Hussin, M.H.; Brosse, N. Insight into the physicochemical properties and thermal behavior of cellulose microcrystals isolated from aleppo pine using different delignification approaches. Biomass Convers. Biorefin. 2023, 1–13. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A., Jr.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Kokol, V.; Božič, M.; Vogrinčič, R.; Mathew, A.P. Characterisation and properties of homo-and heterogenously phosphorylated nanocellulose. Carbohydr. Polym. 2015, 125, 301–313. [Google Scholar] [CrossRef]
- Rimdusit, S.; Ishida, H. Synergism and multiple mechanical relaxations observed in ternary systems based on benzoxazine, epoxy, and phenolic resins. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1687–1698. [Google Scholar] [CrossRef]
- Ishida, H.; Ohba, S. Thermal analysis and mechanical characterization of maleimide-functionalized benzoxazine/epoxy copolymers. J. Appl. Polym. Sci. 2006, 101, 1670–1677. [Google Scholar] [CrossRef]
- Chutayothin, P.; Ishida, H. Cationic ring-opening polymerization of 1,3-benzoxazines: Mechanistic study using model compounds. Macromolecules 2010, 43, 4562–4572. [Google Scholar] [CrossRef]
- Bessa, W.; Trache, D.; Ghalmi, C.; Aidi, S.; Tarchoun, A.F.; Gahfif, F.; Abdelaziz, A.; Thakur, S.; Hussin, M.H. Curing kinetics of composites based on benzoxazine resin and microcrystalline cellulose modified by phosphorus salt. Thermochim. Acta 2024, 735, 179718. [Google Scholar] [CrossRef]
- Gouni, S.R. Cure Kinetics of Benzoxazine/Cycloaliphatic Epoxy Resin by Differential Scanning Calorimetry; California State University: Long BeacH, CA, USA, 2018. [Google Scholar]
- Casarino, A.F.; Bortolato, S.A.; Casis, N.; Estenoz, D.A.; Spontón, M.E. Novel polybenzoxazine and polybenzoxazine/epoxy thermosetting copolymers containing polysilsesquioxane nanostructures for high-performance thermal protection systems. Eur. Polym. J. 2023, 182, 111722. [Google Scholar] [CrossRef]
- Shutov, V.V.; Bornosuz, N.V.; Korotkov, R.F.; Gorbunova, I.Y.; Sirotin, I.S. Kinetics of benzoxazine and epoxy oligomer copolymerization. Thermochim. Acta 2022, 714, 179254. [Google Scholar] [CrossRef]
- Spontón, M.; Lligadas, G.; Ronda, J.; Galia, M.; Cádiz, V. Development of a dopo-containing benzoxazine and its high-performance flame retardant copolybenzoxazines. Polym. Degrad. Stab. 2009, 94, 1693–1699. [Google Scholar] [CrossRef]
- Zhou, C.; Fu, M.; Xie, H.; Gong, Y.; Chen, J.; Liu, J.; Xin, Z. Polybenzoxazine/epoxy composite coatings: Effect of crosslinking on corrosion resistance. Ind. Eng. Chem. Res. 2021, 60, 1675–1683. [Google Scholar] [CrossRef]
- Liu, C.; Sun, M.; Zhang, B.; Zhang, X.; Li, J.; Wang, L.; Xue, G.; Zhao, M.; Song, C.; Li, Q. Preparation and properties of acetylene-terminated benzoxazine/epoxy copolymers. React. Funct. Polym. 2017, 120, 98–103. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Zhao, C.; Li, Y.; Xiang, D.; Wu, Y.; Wei, J.; Que, Y. Polybenzoxazine resins with cellulose phosphide: Preparation, flame retardancy and mechanisms. Polymers 2021, 13, 4288. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, Z.; Wei, J.; Li, Y.; Xiang, D.; Wu, Y.; Que, Y. A phosphorous-containing bio-based furfurylamine type benzoxazine and its application in bisphenol-a type benzoxazine resins: Preparation, thermal properties and flammability. Polymers 2022, 14, 1597. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.-C.; Zhang, D.-X.; Zhu, R.-Q.; Gu, Y. The structural transformation during polymerization of benzoxazine/fecl3 and the effect on the thermal stability. Polymer 2012, 53, 4119–4127. [Google Scholar] [CrossRef]
- Jedvert, K.; Heinze, T. Cellulose modification and shaping—A review. J. Polym. Eng. 2017, 37, 845–860. [Google Scholar] [CrossRef]
- Van Krevelen, D. Some basic aspects of flame resistance of polymeric materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- John, M.J. Flammability performance of biocomposites. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–58. [Google Scholar]
- Guo, Y.; He, S.; Zuo, X.; Xue, Y.; Chen, Z.; Chang, C.-C.; Weil, E.; Rafailovich, M. Incorporation of cellulose with adsorbed phosphates into poly(lactic acid) for enhanced mechanical and flame retardant properties. Polym. Degrad. Stab. 2017, 144, 24–32. [Google Scholar] [CrossRef]
- Tietze, R.; Chaudhari, M. Advanced benzoxazine chemistries provide improved performance in a broad range of applications. In Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011; pp. 595–604. [Google Scholar]
- Fox, D.M.; Novy, M.; Brown, K.; Zammarano, M.; Harris, R.H., Jr.; Murariu, M.; McCarthy, E.D.; Seppala, J.E.; Gilman, J.W. Flame retarded poly (lactic acid) using poss-modified cellulose. 2. Effects of intumescing flame retardant formulations on polymer degradation and composite physical properties. Polym. Degrad. Stab. 2014, 106, 54–62. [Google Scholar] [CrossRef]
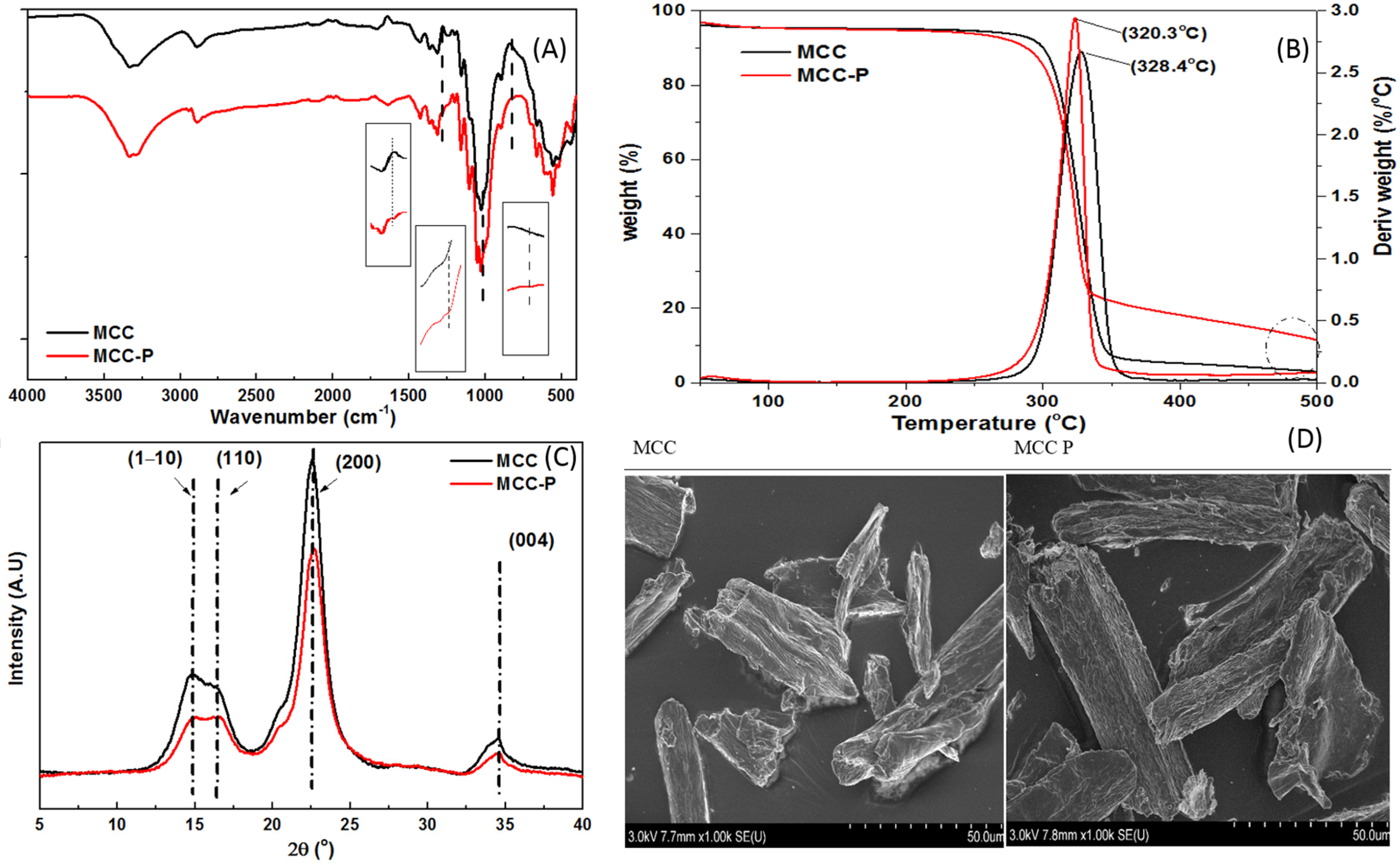
| Sample | Crystallinity % |
|---|---|
| MCC | 82.85 |
| MCC P | 78.53 |
| Heating rate | Composites | Tonset (°C) | Tpic (°C) | (J/g) |
|---|---|---|---|---|
| β = 15 °C/min | Bz-Ep | 209.3 | 241.6 | −337.6 |
| Bz-Ep-MCC-P-1% | 188.3 | 234.8 | −316.5 | |
| Bz-Ep-MCC-P-5% | 177.9 | 221.2 | −312.4 |
| Sample | Degradation Temperature (°C) | Pics of Degradation DTG | Residual Weight TGA % at 800 °C | LOI | ||
|---|---|---|---|---|---|---|
| Tonset | T10% | T50% | T max | |||
| Bz-Ep | 300 | 360 | 434 | 399 | 24.1 | 27.14 |
| Bz-Ep-MCC-P 1% | 280 | 353 | 449 | 387 | 30.8 | 29.82 |
| Bz-Ep-MCC-P-5% | 278 | 349 | 443 | 397 | 26.5 | 28.12 |
| Composites | Time (s) | Drops | Classification | |
|---|---|---|---|---|
| Bz-Ep | 1st test | 55 | No drops | FAILED |
| 2nd test | 0 | No drops | ||
| Bz-Ep-MCC-P 1% | 1st test | 28 | No drops | V-2 |
| 2nd test | 0 | No drops | ||
| Bz-Ep-MCC-P 5% | 1st test | 24 | No drops | V-2 |
| 2nd test | 0 | No drops | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessa, W.; Trache, D.; Moulai, S.-A.; Tarchoun, A.F.; Abdelaziz, A.; Hamidon, T.S.; Hussin, M.H. Polybenzoxazine/Epoxy Copolymer Reinforced with Phosphorylated Microcrystalline Cellulose: Curing Behavior, Thermal, and Flame Retardancy Properties. Fibers 2024, 12, 61. https://doi.org/10.3390/fib12080061
Bessa W, Trache D, Moulai S-A, Tarchoun AF, Abdelaziz A, Hamidon TS, Hussin MH. Polybenzoxazine/Epoxy Copolymer Reinforced with Phosphorylated Microcrystalline Cellulose: Curing Behavior, Thermal, and Flame Retardancy Properties. Fibers. 2024; 12(8):61. https://doi.org/10.3390/fib12080061
Chicago/Turabian StyleBessa, Wissam, Djalal Trache, Sid-Ali Moulai, Ahmed Fouzi Tarchoun, Amir Abdelaziz, Tuan Sherwyn Hamidon, and Mohd Hazwan Hussin. 2024. "Polybenzoxazine/Epoxy Copolymer Reinforced with Phosphorylated Microcrystalline Cellulose: Curing Behavior, Thermal, and Flame Retardancy Properties" Fibers 12, no. 8: 61. https://doi.org/10.3390/fib12080061
APA StyleBessa, W., Trache, D., Moulai, S. -A., Tarchoun, A. F., Abdelaziz, A., Hamidon, T. S., & Hussin, M. H. (2024). Polybenzoxazine/Epoxy Copolymer Reinforced with Phosphorylated Microcrystalline Cellulose: Curing Behavior, Thermal, and Flame Retardancy Properties. Fibers, 12(8), 61. https://doi.org/10.3390/fib12080061









