Recovery of N-Methylmorpholine N-Oxide (NMMO) in Lyocell Fibre Manufacturing Process
Highlights
- We developed a calibration curve for the determination of N-methyl-morpholine-N-oxide (NMMO) content in aqueous solutions using Fourier Transform Infrared Spectroscopy (FTIR).
- We successfully recovered used NMMO from lab-scale lyocell manufacturing wastewater through evaporation using a rotary evaporator without degrading the NMMO.
- This first comprehensive survey of the NMMO recovery processes and characterization techniques will guide the industry’s advances in L-MMCF manufacturing.
- The FTIR NMMO calibration curve provides an easy strategy to monitor the NMMO content in wastewater and optimize the L-MMCF manufacturing process.
Abstract
:1. Introduction
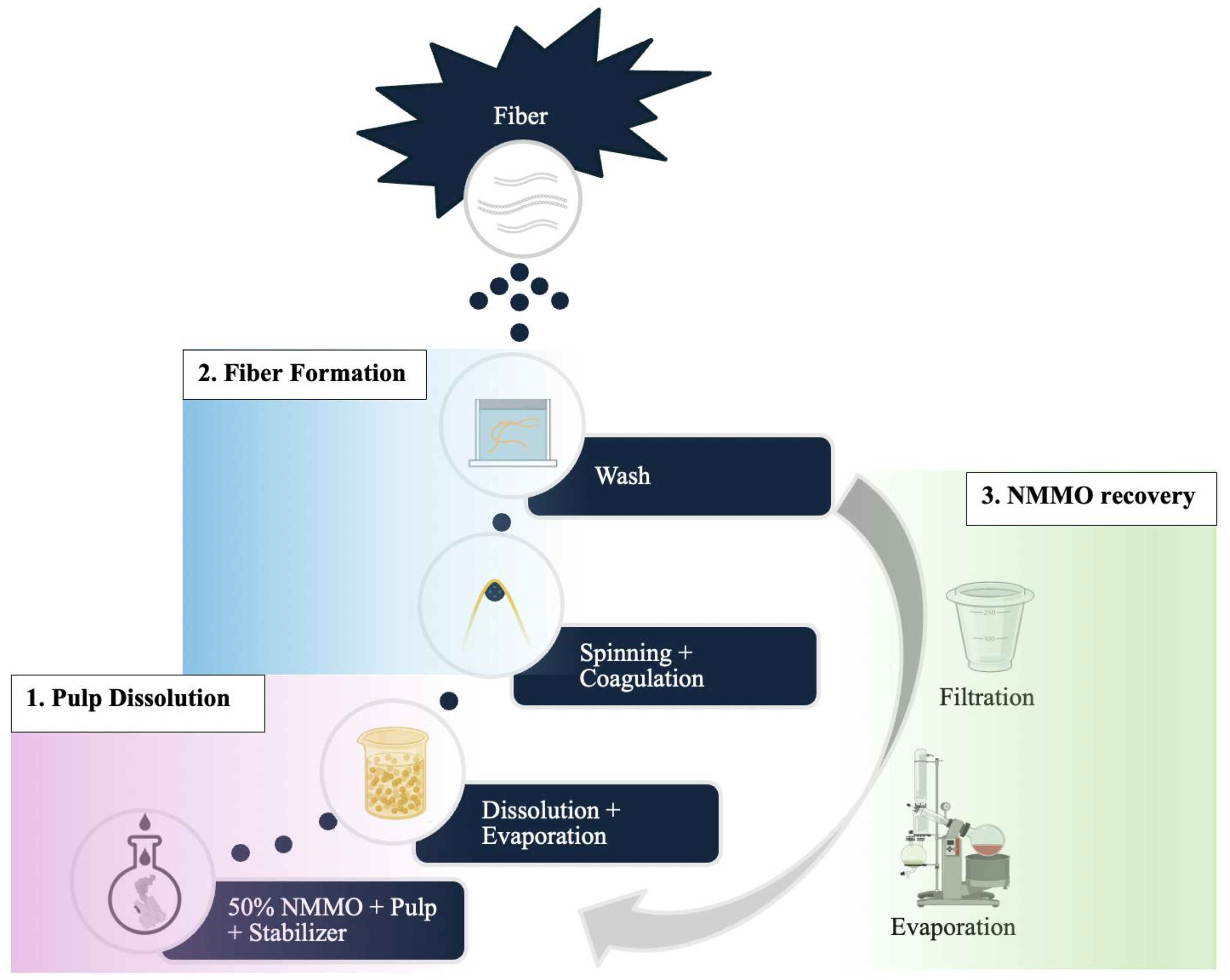
2. L-MMCF Manufacturing Process
2.1. Pulp Dissolution
2.2. Fibre Formation
2.3. Solvent Recovery
2.4. Reuse of Recovered NMMO
3. NMMO Degradation
4. NMMO Characterization
4.1. NMMO Content Measurement Techniques
4.2. NMMO Degradation Monitoring Techniques
5. Pilot Study of NMMO Residual Content Characterization and Recovery
5.1. Materials
5.2. Methods
5.2.1. NMMO Content Characterization
5.2.2. NMMO Recycling Process
5.3. Results
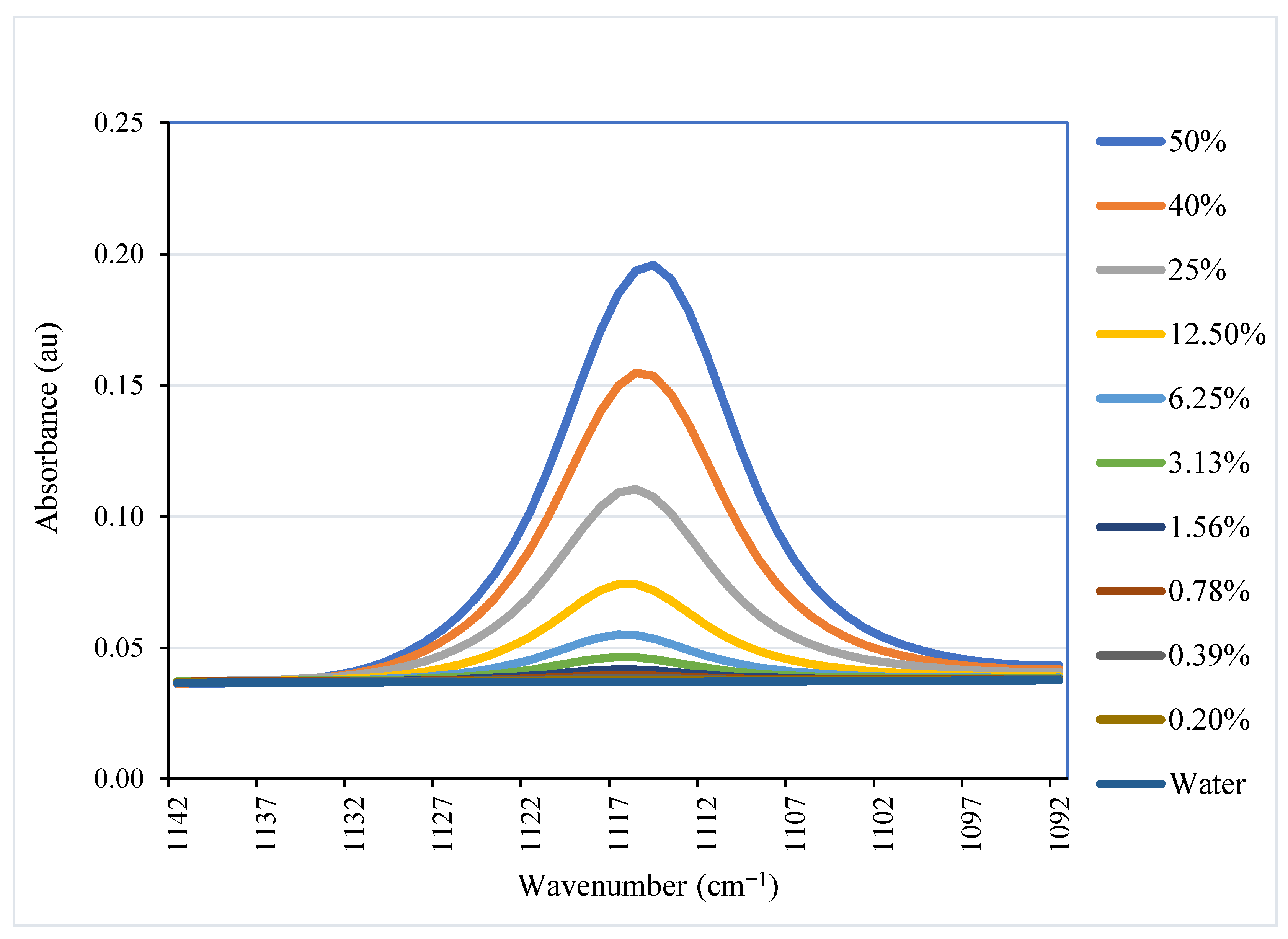
5.3.1. Peak Selection
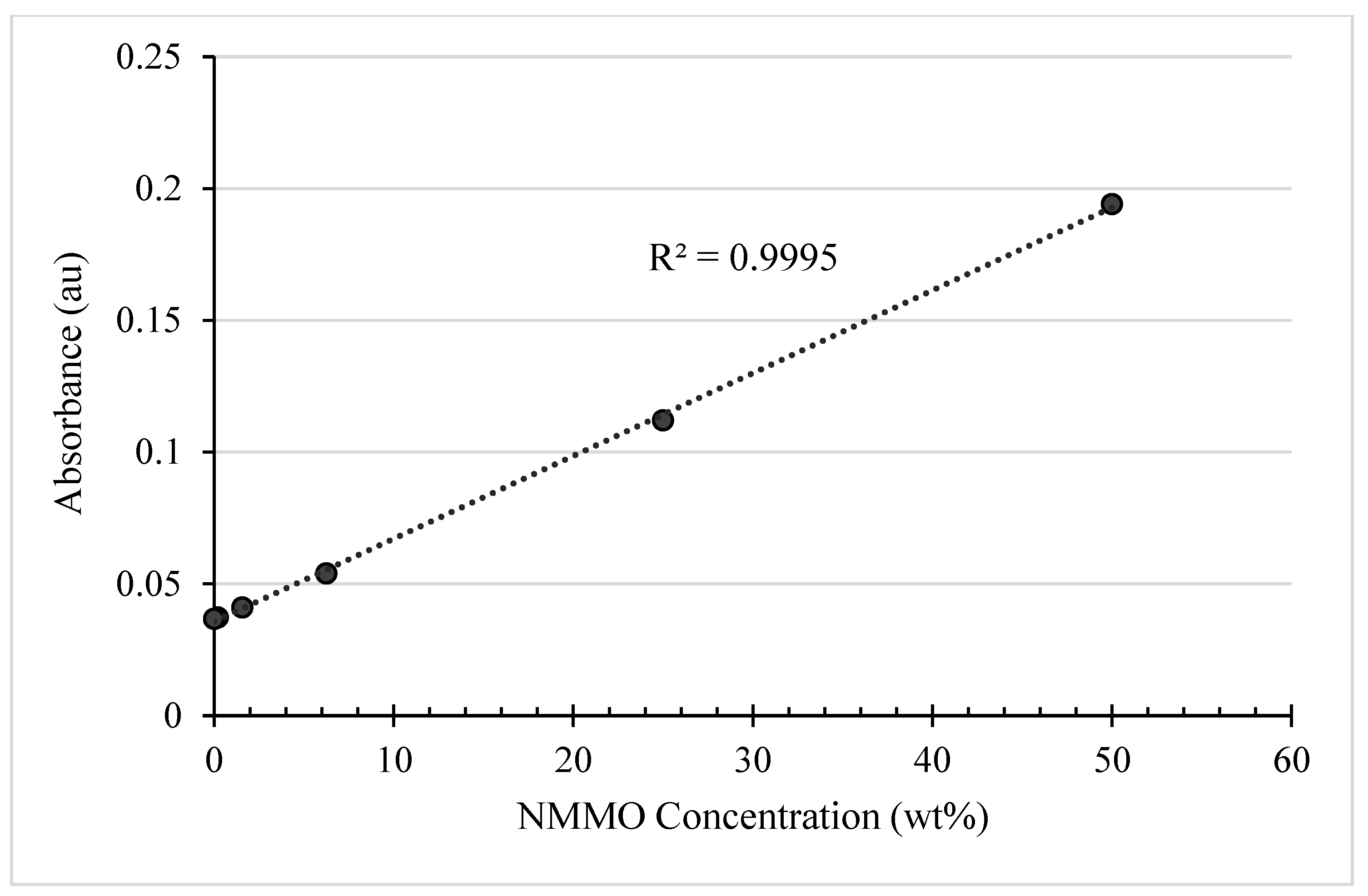
5.3.2. Calibration Curve for NMMO Content Determination
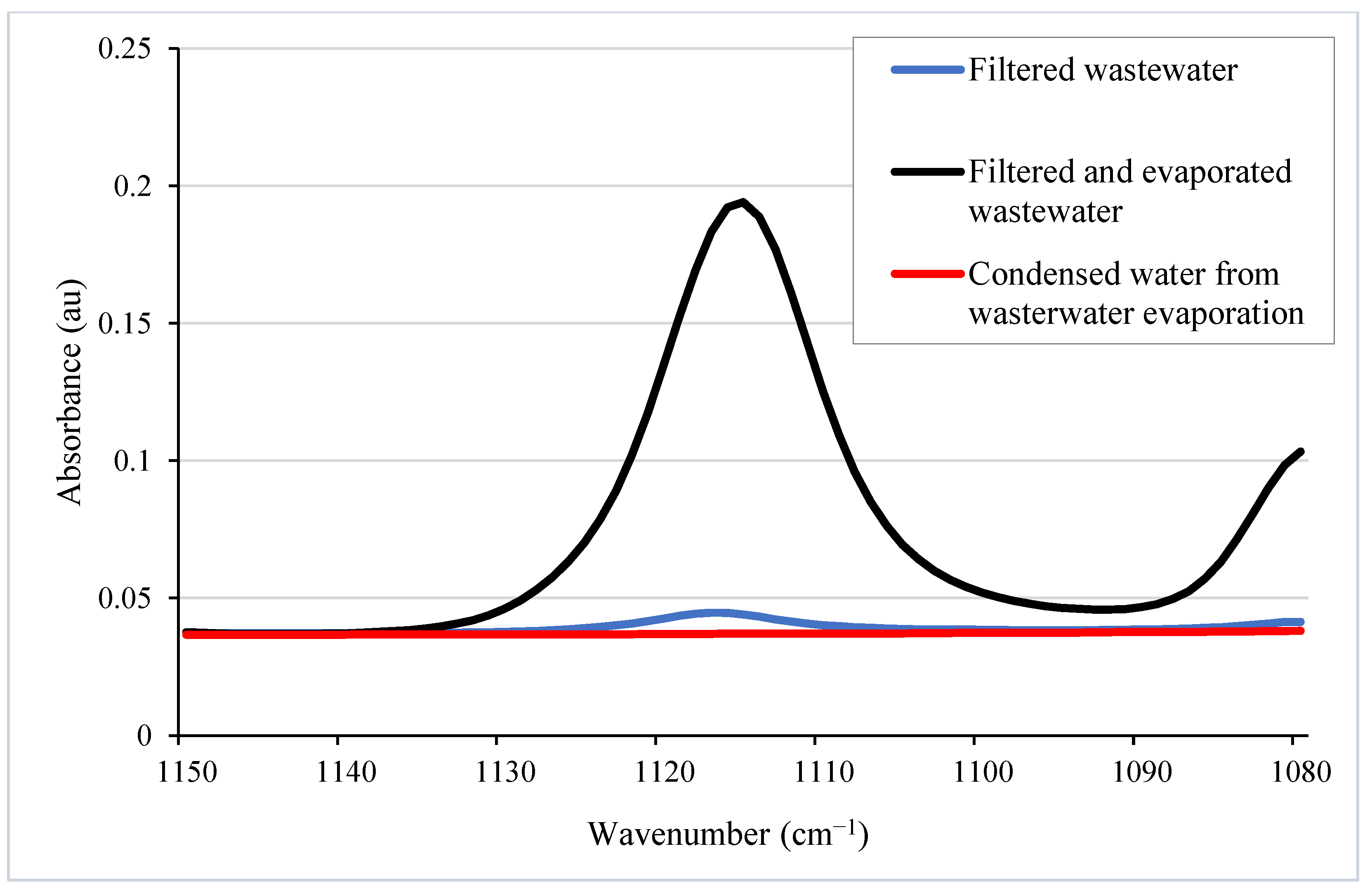
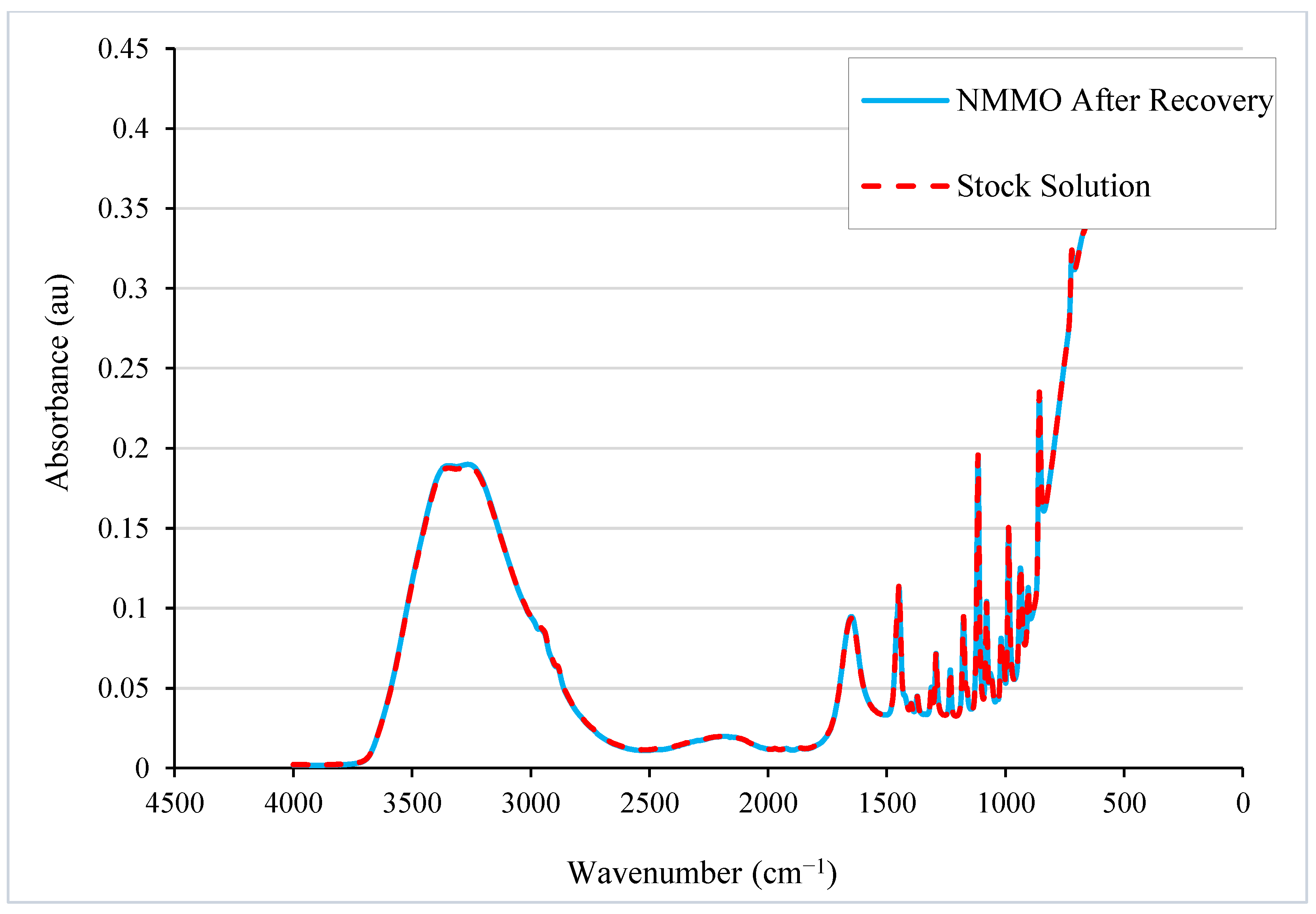
5.3.3. NMMO Recycling from L-MMCF Production Wastewater
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sayyed, A.J.; Mohite, L.V.; Deshmukh, N.A.; Pinjari, D.V. Structural characterization of cellulose pulp in aqueous NMMO solution under the process conditions of lyocell slurry. Carbohydr. Polym. 2019, 206, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.-P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Lawson, L.; Ford, M.; Hoque, M.S.; Chute, W.; Bressler, D.C.; Dolez, P.I. Processes and Challenges for the Manufacturing of Lyocell Fibres with Alternative Agricultural Feedstocks. Appl. Sci. 2023, 13, 12759. [Google Scholar] [CrossRef]
- Bagherjeri, M.; Monhemi, H.; Haque, A.N.M.A.; Naebe, M. Molecular mechanism of cellulose dissolution in N-methyl morpholine-N-oxide: A molecular dynamics simulation study. Carbohydr. Polym. 2024, 323, 121433. [Google Scholar] [CrossRef] [PubMed]
- Granacher, C.; Sallmann, R. Cellulose Solutions and Process of Making Same. US Patent 2,179,181, 7 November 1939. [Google Scholar]
- Jiang, X.; Bai, Y.; Chen, X.; Liu, W. A review on raw materials, commercial production and properties of lyocell fiber. J. Bioresour. Bioprod. 2020, 5, 16–25. [Google Scholar] [CrossRef]
- Shen, L.; Patel, M.K. Life cycle assessment of man-made cellulose fibres. Lenzing. Berichte 2010, 88, 1–59. [Google Scholar]
- Lawson, L.; Sayed, M.A.; Noor, K.M.A.; Haddis, D.; Araujo Souto, B.; Chute, W.; Batcheller, J.; Bressler, D.C.; Dolez, P.I. A Tiered Approach Towards Optimization of Lyocell Prototype Development. In Proceedings of the 35th Canadian Materials Science Conference—CMSC 2024, Edmonton, AB, Canada, 22–24 May 2024. [Google Scholar]
- Linton, E.P. The dipole moments of amine oxides. J. Am. Chem. Soc. 1940, 62, 1945–1948. [Google Scholar] [CrossRef]
- Hytönen, E.; Sorsamäki, L.; Kolehmainen, E.; Sturm, M.; Weymarn, N. Lyocell fibre production using NMMO—A simulation-based techno-economic analysis. BioResources 2023, 18, 6384–6411. [Google Scholar] [CrossRef]
- Ri, C.J.; Kim, Y.C.; Kim, S.W.; Chloe, J.H. A simulation of evaporation dissolution process for a lyocell solution in a vertical wiped film evaporator (1: Simulation of flow process for solution). Cellul. Chem. Technol. 2023, 57, 741–748. [Google Scholar] [CrossRef]
- Perepelkin, K.E. Lyocell fibres based on direct dissolution of cellulose in N-methylmorpholine N-oxide: Development and prospects. Fibre Chem. 2007, 39, 163–172. [Google Scholar] [CrossRef]
- Gao, D.C.; Lu, X.Y.; Wang, H.P. Technological development of industrial process of lyocell fiber. Artif. Fiber 2019, 49, 2–16. [Google Scholar]
- Research and Markets. Lyocell Fiber Market Report: Trends, Forecast and Competitive Analysis to 2030; Research and Markets: Dublin, UK, 2024; 150p. [Google Scholar]
- Quintana, E.; Valls, C.; Roncero, M.B. Dissolving-grade pulp: A sustainable source for fiber production. Wood Sci. Technol. 2024, 58, 23–85. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated cellulose by the Lyocell process, a brief review of the process and properties. BioResources 2018, 13, 4577–4592. [Google Scholar] [CrossRef]
- Wendler, F.; Graneß, G.; Heinze, T. Characterization of Autocatalytic Reactions in Modified Cellulose/NMMO Solutions by Thermal Analysis and UV/VIS Spectroscopy. Cellulose 2005, 12, 411–422. [Google Scholar] [CrossRef]
- Lang, H.; Laskowski, I.; Lukanoff, B.; Schleicher, H.; Mertel, H.; Franz, H.; Taeger, E. Untersuchungen an Lösungen von Cellulose in N-Methylmorpholin-N-Oxid (MMNO). Cellul. Chem. Technol. 1986, 20, 289–301. [Google Scholar]
- Melikhov, I.; Bacher, M.; Hosoya, T.; Hettegger, H.; Potthast, A.; Rosenau, T. On the chemical fate of propyl gallate as stabilizer in Lyocell spinning dopes. Cellulose 2023, 30, 5373–5390. [Google Scholar] [CrossRef]
- Oliva, A.; Tan, L.C.; Papirio, S.; Esposito, G.; Lens, P.N.L. Use of N-Methylmorpholine N-oxide (NMMO) pretreatment to enhance the bioconversion of lignocellulosic residues to methane. Biomass Convers. Biorefinery 2024, 14, 11113–11130. [Google Scholar] [CrossRef]
- Le Moigne, N.; Jardeby, K.; Navard, P. Structural changes and alkaline solubility of wood cellulose fibers after enzymatic peeling treatment. Carbohydr. Polym. 2010, 79, 325–332. [Google Scholar] [CrossRef]
- Konkin, A.; Wendler, F.; Meister, F.; Roth, H.-K.; Aganov, A.; Ambacher, O. Degradation processes in the cellulose/N-methylmorpholine-N-oxide system studied by HPLC and ESR. Radical formation/recombination kinetics under UV photolysis at 77 K. Cellulose 2007, 14, 457–468. [Google Scholar] [CrossRef]
- Yeoh, S.J. Electrospun Cellulose Fibres from Kraft Pulp. Ph.D. Dissertation, University of British Columbia, Vancouver, BC, Canada, 2009. [Google Scholar]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Deng, Z.; Zhou, G.; Fang, T.; Fang, K.; Liu, X. The bidirectional regulation mechanism of NMMO concentration change on cellulose dissolution and regeneration. Cellulose 2024, 31, 1205–1222. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, J.; Sun, T.; Xing, L.; Cheng, C.; Chi, K.; Xu, J.; Li, T. The purification process and side reactions in the N-methylmorpholine-N-oxide (NMMO) recovery system. Cellulose 2021, 28, 7609–7617. [Google Scholar] [CrossRef]
- Lukanoff, B.; Philipp, B.; Schleicher, H. Untersuchungen zum thermischen Abbau von cellulose unter homogenen Reaktionsbedingungen. Acta Polym. 1984, 35, 339–343. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Hofinger, A.; Kosma, P. Isolation and Identification of Residual Chromophores in Cellulosic Materials. Macromol. Symp. 2005, 223, 239–252. [Google Scholar] [CrossRef]
- Bou-Diab, L.; Fierz, H. 2002. Autocatalytic decomposition reactions, hazards and detection. J. Hazard. Mater. 2002, 93, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Rosenau, T.; Potthast, A.; Kosma, P.; Chen, G.; Josef, S. Autocatalytic Decomposition of N-Methylmorpholine N-Oxide Induced by Mannich Intermediates. J. Org. Chem. 1999, 64, 2166–2167. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Hettegger, H.; Bacher, M.; Opietnik, M.; Röder, T.; Adorjan, I. On the role of N-methylmorpholine-N-oxide (NMMO) in the generation of elemental transition metal precipitates in cellulosic materials. Cellulose 2021, 28, 10143–10161. [Google Scholar] [CrossRef]
- Wendler, F.; Konkin, A.; Heinze, T. Studies on the Stabilization of Modified Lyocell Solutions. Macromol. Symp. 2008, 262, 72–84. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Adorjan, I.; Hofinger, A.; Sixta, H.; Firgo, H.; Kosma, P. Cellulose solutions in N-methylmorpholine-N-oxide (NMMO)–degradation processes and stabilizers. Cellulose 2002, 9, 283–291. [Google Scholar] [CrossRef]
- McCorsely, C.C.; Varga, J.K. Process for Making a Precursor of a Solution of Cellulose. US Patent 4, 142,913, 6 March 1979. [Google Scholar]
- Kabir, M.; Niklasson, C.; Taherzadeh, M.; Horváth, I. Biogas production from lignocelluloses by N-methylmorpholine-N-oxide (NMMO) pretreatment: Effects of recovery and reuse of NMMO. Bioresour. Technol. 2014, 161, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Koo, W.; Kim, H. 2003. Preparation and Properties of New Regenerated Cellulose Fibers. Text. Res. J. 2003, 73, 998–1004. [Google Scholar] [CrossRef]
- Gregor, W.; Grabner, G.; Adelwöhrer, C.; Rosenau, T.; Gille, L. Antioxidant properties of natural and synthetic chromanol derivatives: Study by fast kinetics and electron spin resonance spectroscopy. J. Org. Chem. 2005, 70, 3472–3483. [Google Scholar] [CrossRef]
- Li, C.; Fang, T.; Zhou, G.; Ge, M.; Li, Y.; Liu, X. Mechanism and conformation changes for the whole regeneration process of cellulose in pyridinium-based ionic liquids. Cellulose 2022, 29, 5479–5492. [Google Scholar] [CrossRef]
- Periyasamy, A.; Khanum, R. Effect of fibrillation on pilling tendency of Lyocell fiber. Bangladesh Text. Today 2012, 4, 31–39. [Google Scholar]
- Teghammar, A.; Forgács, G.; Sárvári Horváth, I.; Taherzadeh, M. Techno-economic study of NMMO pretreatment and biogas production from forest residues. Appl. Energy 2014, 116, 125–133. [Google Scholar] [CrossRef]
- Duan, J.; Shao, H.; Zhang, T.; Hu, X. Influence of the coagulation bath temperature on the structure and properties of lyocell fiber. J. Text. Res. 2001, 22, 13–15. [Google Scholar]
- Mo, D. Review of spinning process of lyocell fiber. Guangxi Chem. Fiber Commun. 2002, 1, 25–29. [Google Scholar]
- Chen, Y.; Wang, W.; Zhang, Q. Investigation of Factors Affecting the Rate of High-Boiling-Point Solvent Removal Using a Rotary Evaporator. Synlett 2024, 35, 598–602. [Google Scholar]
- Maron, R.; Michels, C.; Taeger, E. Investigations for preparation of cellulose solutions in NMMO and the following forming. Lenzing. Berichte 1994, 74, 27–29. [Google Scholar]
- Jeihanipour, A.; Karimi, K.; Niklasson, C.; Taherzadeh, M. A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles. Waste Manag. 2010, 30, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.; Peguy, A.; Ball, R. Influence of the physical process parameters on the structure formation of lyocell fibres. Cell. Chem. Technol. 1996, 30, 251–266. [Google Scholar]
- Nomura, S.; Sato, S.; Erata, T. DFT approach to the pathway of conformational changes of cellulose C6-hydroxymethyl group with simple cellotetraose model involving the mechanism of mercerization process. Chem. Phys. Lett. 2020, 742, 137154. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Bokhary, A.; Leitch, M.; Liao, B.-Q. Liquid-liquid extraction technology for resource recovery: Applications, potential, and perspectives. J. Water Process Eng. 2021, 40, 101762. [Google Scholar] [CrossRef]
- Patel, J.; Desai, H. Removal of phenol by liquid-liquid extraction from pharmaceutical wastewater. Mater. Today Proc. 2022, 57, 2396–2399. [Google Scholar] [CrossRef]
- Sanchis, J.; Llorca, M.; Barceló, D.; Farré, M. Sample treatment procedures for environmental sensing and biosensing. Curr. Opin. Biotechnol. 2017, 45, 170–174. [Google Scholar] [CrossRef]
- Shafiei, M.; Karimi, K.; Zilouei, H.; Taherzadeh, M. Enhanced ethanol and biogas production from pinewood by NMMO pretreatment and detailed biomass analysis. BioMed Res. Int. 2014, 2014, 469378. [Google Scholar] [CrossRef]
- Agarwala, P.; Kanthale, P.; Thakre, S. Flocculation of spin bath NMMO solution for removal of colloidal impurities in lyocell process. Sep. Sci. Technol. 2019, 54, 2516–2527. [Google Scholar] [CrossRef]
- Uzunsakala, S.; Zeytincia, S.; Uyanık, Ö.; Uyanık, N. Adsorption Kinetics Studies of Polymeric Nanocomposite Coagulants. J. Appl. Polym. Sci. 2013, 130, 481–486. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustain. Energy Fuels 2018, 3, 11–62. [Google Scholar] [CrossRef]
- Kalt, H.; Rosenau, T. NMM can be recombined with H2O2 to regenerate NMMO. Cellul. Chem. Technol. 1999, 33, 481–486. [Google Scholar]
- Marini, A.; Brauneis, W. Homolytic and heterolytic reactions by neutralizing transition metals. J. Inorg. Chem. 1996, 35, 481–486. [Google Scholar]
- Zeng, T.; Ke, X.; Li, L.; Cheng, X.; Ni, Y.; Ouyang, X.; Zhang, X.; Chen, L.; Huang, L.; Hu, H. Quantification of N-methyl morpholine N-oxide in biorefinery process solution by headspace gas chromatography. Cellulose 2020, 27, 6861–6870. [Google Scholar] [CrossRef]
- Dien, J.; Fierz, H.; Stoessel, F.; Killé, G. The Thermal Risk of Autocatalytic Decompositions: A Kinetic Study. Chimia 1994, 48, 542–550. [Google Scholar] [CrossRef]
- Singh, M.; Singh, A. Chapter 9—Thermal characterization of materials using differential scanning calorimeter. In Characterization of Polymers and Fibres; Singh, M.K., Singh, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–222. [Google Scholar]
- Corradini, D. Handbook of HPLC; Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Stockinger, H.; Brauneis, W. High-performance liquid chromatography (HPLC) method for the determination of N-methylmorpholine N-oxide (NMMO) in cellulose solutions. J. Chromatogr. A 1996, 740, 123–130. [Google Scholar]
- Sohn, J.; Kim, S. High-performance liquid chromatography (HPLC) method for the analysis of N-methylmorpholine N-oxide (NMMO) in cellulose solutions. J. Chromatogr. A 1982, 234, 123–130. [Google Scholar]
- Gómez-Hens, A.; Godoy-Navajas, J. Determination of propyl, octyl, and dodecyl gallates. In Flow Injection Analysis of Food Additives, 1st ed.; CRC Press: Oxford, UK, 2015; pp. 241–260. [Google Scholar]
- Singh, M.; Singh, A. Chapter 14—Nuclear magnetic resonance spectroscopy. In Characterization of Polymers and Fibres; Singh, M.K., Singh, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 321–339. [Google Scholar]
- Schenzel, K.; Fischer, S. NIR FT Raman Spectroscopy—A Rapid Analytical Tool for Detecting the Transformation of Cellulose Polymorphs. Cellulose 2001, 8, 49–57. [Google Scholar] [CrossRef]
- Fadavi, R.; Karimi, M. NMR spectroscopy of N-methylmorpholine N-oxide (NMMO) in cellulose solutions. J. Polym. Sci. 2022, 60, 1234–1245. [Google Scholar]
- Zieher, J.; Melikhov, I.; Bacher, M.; Hettegger, H.; Potthast, A.; Rosenau, T. Gallic acid derivatives as stabilizers in cellulose solutions: Analysis by 31P NMR spectroscopy. Cellulose 2023, 30, 6109–6118. [Google Scholar] [CrossRef]
- Fink, H.; Weigel, P.; Ganster, J.; Rihm, R.; Puls, J.; Sixta, H.; Parajo, J. Evaluation of new organosolv dissolving pulps. Part II: Structure and NMMO processability of the pulps. Cellulose 2004, 11, 85–98. [Google Scholar] [CrossRef]
- Konkin, A.; Wendler, F.; Meister, F.; Roth, H.; Aganov, A.; Ambacher, O. Degradation processes in the cellulose/N-methylmorpholine-N-oxide system studied by HPLC and ESR. Cellulose 2008, 15, 457–468. [Google Scholar]
- Singh, M.K.; Singh, A. (Eds.) Chapter 13—Fourier transform infrared (FTIR) analysis. In Characterization of Polymers and Fibres; Elsevier: Amsterdam, The Netherlands, 2022; pp. 295–320. [Google Scholar]
- Wu, C.; Qiu, G.; Shentu, B.; Jin, Y. Effect of N-methylmorpholine-N-oxide (NMMO) pretreatment on liquefaction behavior of cornstalk. Biomass Convers. Biorefinery 2024, 14, 27407–27417. [Google Scholar] [CrossRef]
- Fang, H.; Sun, J.; Zhang, L. Characterization of bamboo cellulose-based green composite film by NMMO technology. Front. For. China 2008, 3, 514–517. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.; Wang, Y.; Franz, J.; White, J.; Holladay, J.E. Interactions between cellulose and N-methylmorpholine-N-oxide. Carbohydr. Polym. 2007, 67, 97–103. [Google Scholar] [CrossRef]
- Dai, J.; Chae, M.; Beyene, D.; Danumah, C.; Tosto, F.; Bressler, D. Co-Production of Cellulose Nanocrystals and Fermentable Sugars Assisted by Endoglucanase Treatment of Wood Pulp. Materials 2018, 11, 1645. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, H.; Song, C.; Meng, Y.; Li, X. Determination of Cellulose, Water, and N-Methylmorpholine N-Oxide during the Production of Lyocell by Attenuated Total Reflection (ATR)—Fourier Transform Infrared (FTIR) Spectroscopy. Anal. Lett. 2021, 55, 388–398. [Google Scholar] [CrossRef]
- Textmap, W. Organic Chemistry; LibreTexts: Davis, CA, USA, 2024. [Google Scholar]
- Sun, Z.; Qi, S.; Lees, R.; Xu, L. Lamb-dip spectroscopy of the C− N stretching band of methylamine by using frequency-tunable microwave sidebands of CO2 laser lines. Sci. Rep. 2016, 6, 34270. [Google Scholar] [CrossRef]
- FTIR Functional Group Database Table with Search—InstaNANO. 2024. Available online: https://instanano.com/all/characterization/ftir/ftir-functional-group-search/ (accessed on 21 October 2024).
- Spectrabase. Spectral Database for Organic Compounds (SDBS); John Wiley Sons, Inc.: Hoboken, NJ, USA, 2024. [Google Scholar]
- Eunice, E.; Prasana, J.C.; Muthu, S.; Anuradha, A. Molecular Structure, Spectroscopic, Quantum Computational, and Molecular Docking Investigations on Propyl Gallate. Polycycl. Aromat. Compd. 2023, 43, 5747–5767. [Google Scholar]

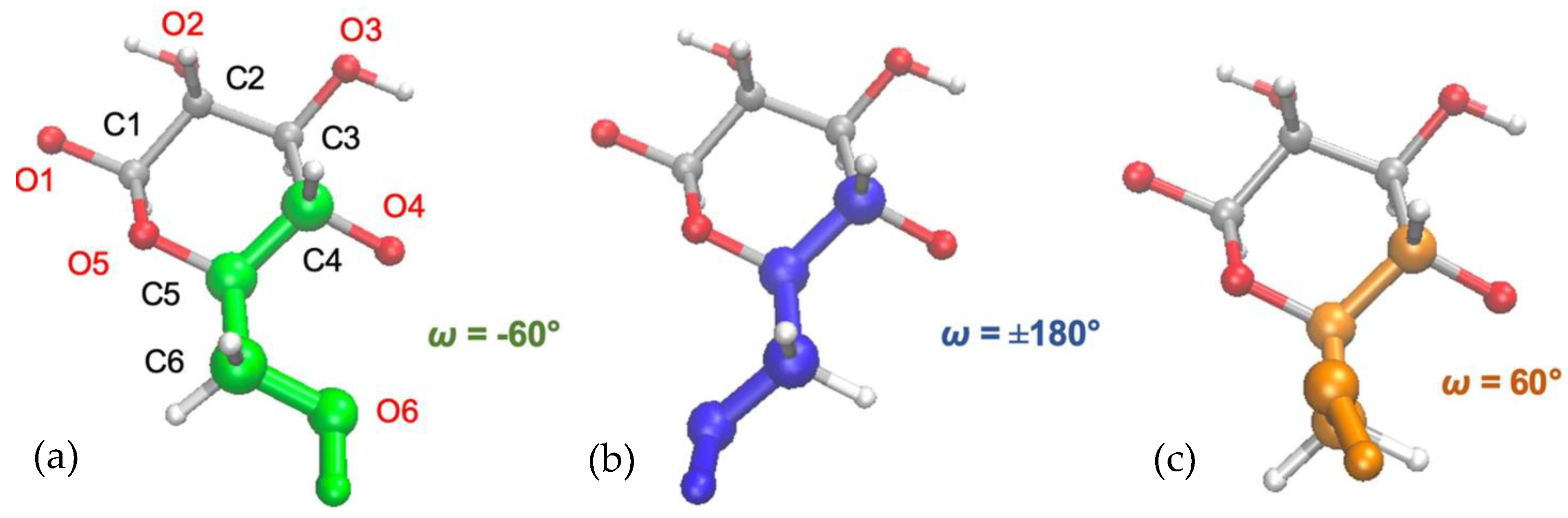
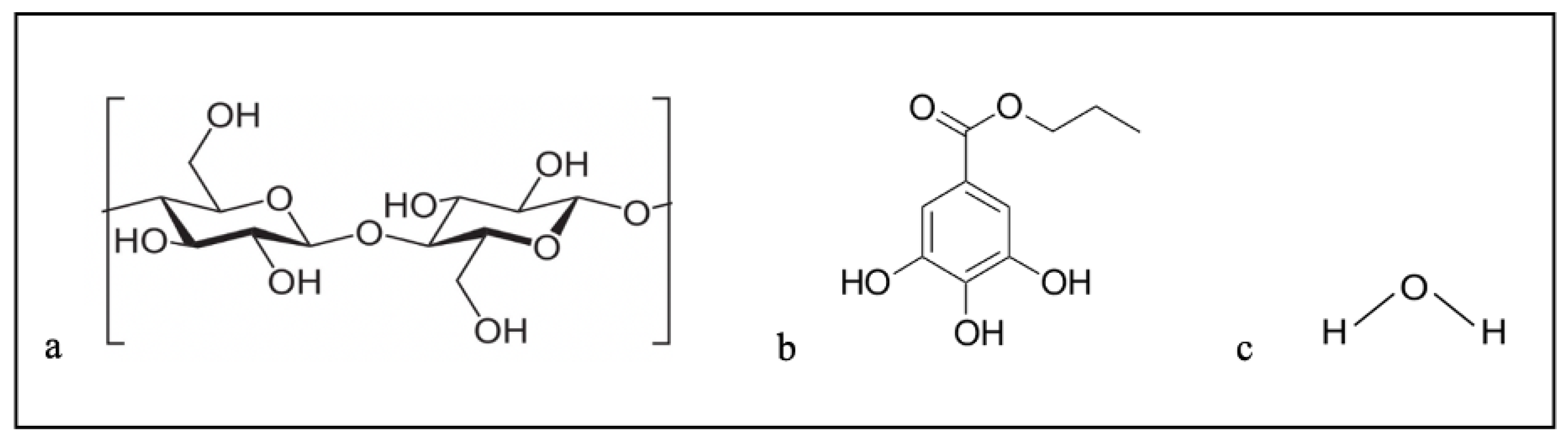
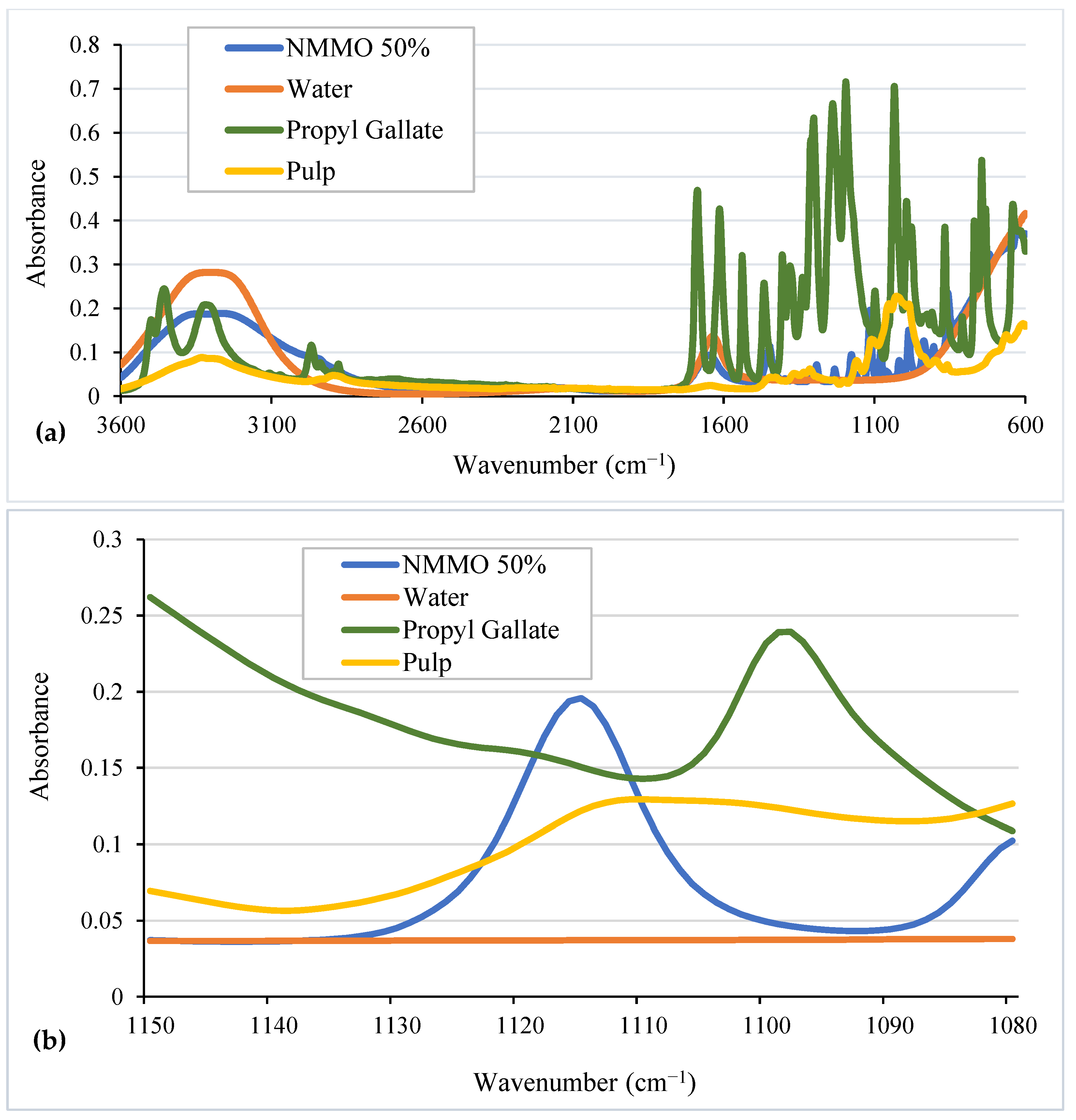
| Characterization Technique | Findings | References |
|---|---|---|
| Density |
| [24,58] |
| Differential scanning calorimetry |
| [17,22,29,59,60] |
| High-performance liquid chromatography |
| [22,61,62,63,64] |
| UV Detection |
| [17] |
| Headspace gas chromatography |
| [16,19,58] |
| Nuclear magnetic resonance |
| [19,65,66,67,68,69] |
| Electron spin resonance |
| [22,70] |
| X-ray diffractometer |
| [20,71,72,73,74,75] |
| Fourier Transform Infrared Spectroscopy |
| [67,71,72,73,74,75] |
| Concentration (wt%) | |
|---|---|
| Limit of detection (LOD) | 0.04 |
| Limit of quantification (LOQ) | 0.1 |
| Sample | NMMO Concentration (wt%) |
| Filtered wastewater | 2.7 |
| Filtered and evaporated wastewater | 51.4 |
| Condensed water from wastewater evaporation | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawiak, M.; Souto, B.A.; Lawson, L.; Lo, J.; Dolez, P.I. Recovery of N-Methylmorpholine N-Oxide (NMMO) in Lyocell Fibre Manufacturing Process. Fibers 2025, 13, 3. https://doi.org/10.3390/fib13010003
Sawiak M, Souto BA, Lawson L, Lo J, Dolez PI. Recovery of N-Methylmorpholine N-Oxide (NMMO) in Lyocell Fibre Manufacturing Process. Fibers. 2025; 13(1):3. https://doi.org/10.3390/fib13010003
Chicago/Turabian StyleSawiak, Maria, Bernardo A. Souto, Lelia Lawson, Joy Lo, and Patricia I. Dolez. 2025. "Recovery of N-Methylmorpholine N-Oxide (NMMO) in Lyocell Fibre Manufacturing Process" Fibers 13, no. 1: 3. https://doi.org/10.3390/fib13010003
APA StyleSawiak, M., Souto, B. A., Lawson, L., Lo, J., & Dolez, P. I. (2025). Recovery of N-Methylmorpholine N-Oxide (NMMO) in Lyocell Fibre Manufacturing Process. Fibers, 13(1), 3. https://doi.org/10.3390/fib13010003






