Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Ginger Nanofiber (GNF)
2.2. Preparation of Bionanocomposites
2.3. Characterization and Measurement of the GNF and Bionanocomposites
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR-ATR)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. X-ray Diffraction (XRD)
2.3.4. Mechanical Test
2.3.5. Differential Scanning Calorimetry (DSC)
2.4. Antimicrobial Activity
3. Results and Discussions
3.1. Fourier Transform Infrared Spectroscopy (FTIR-ATR)
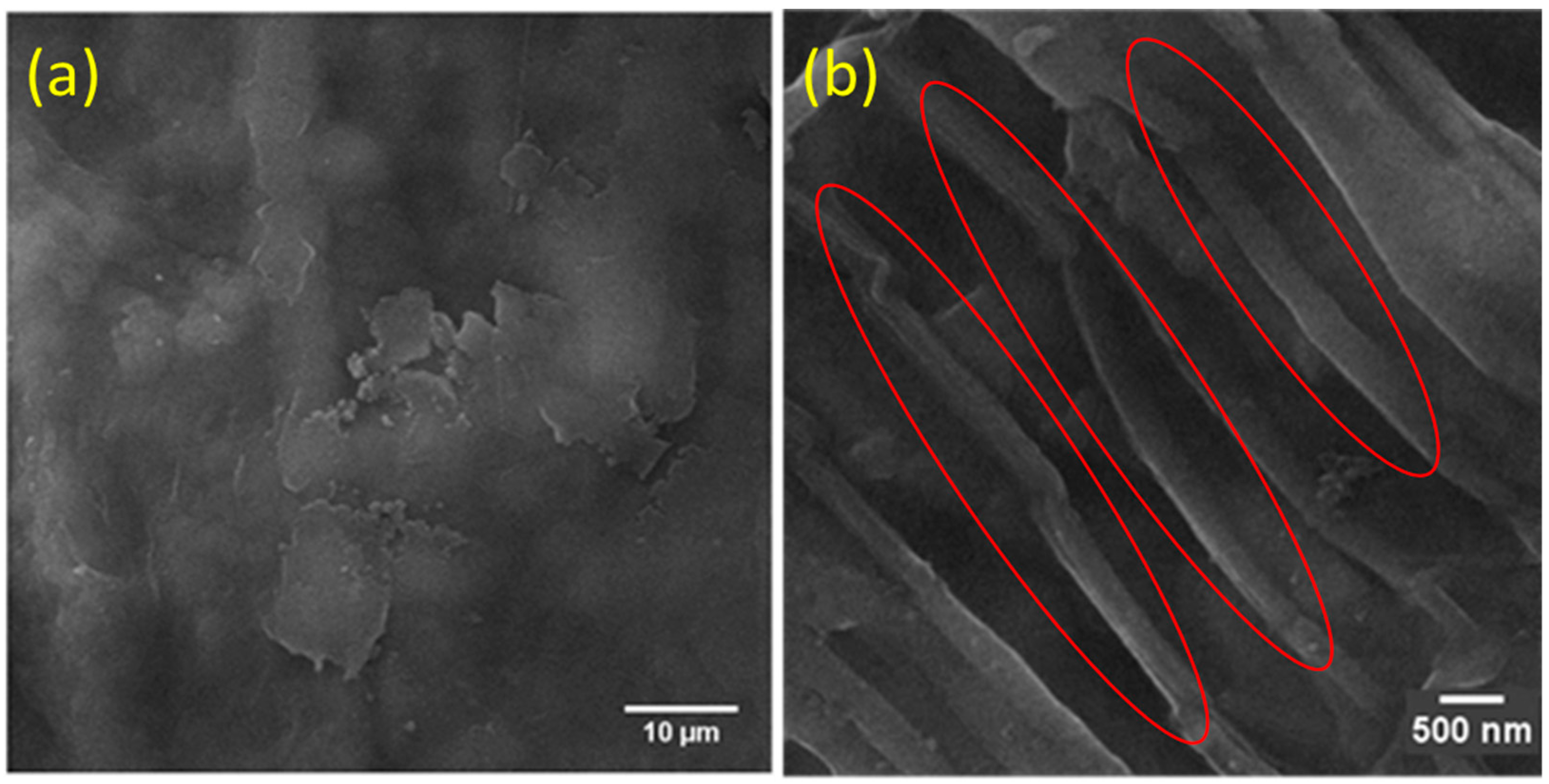
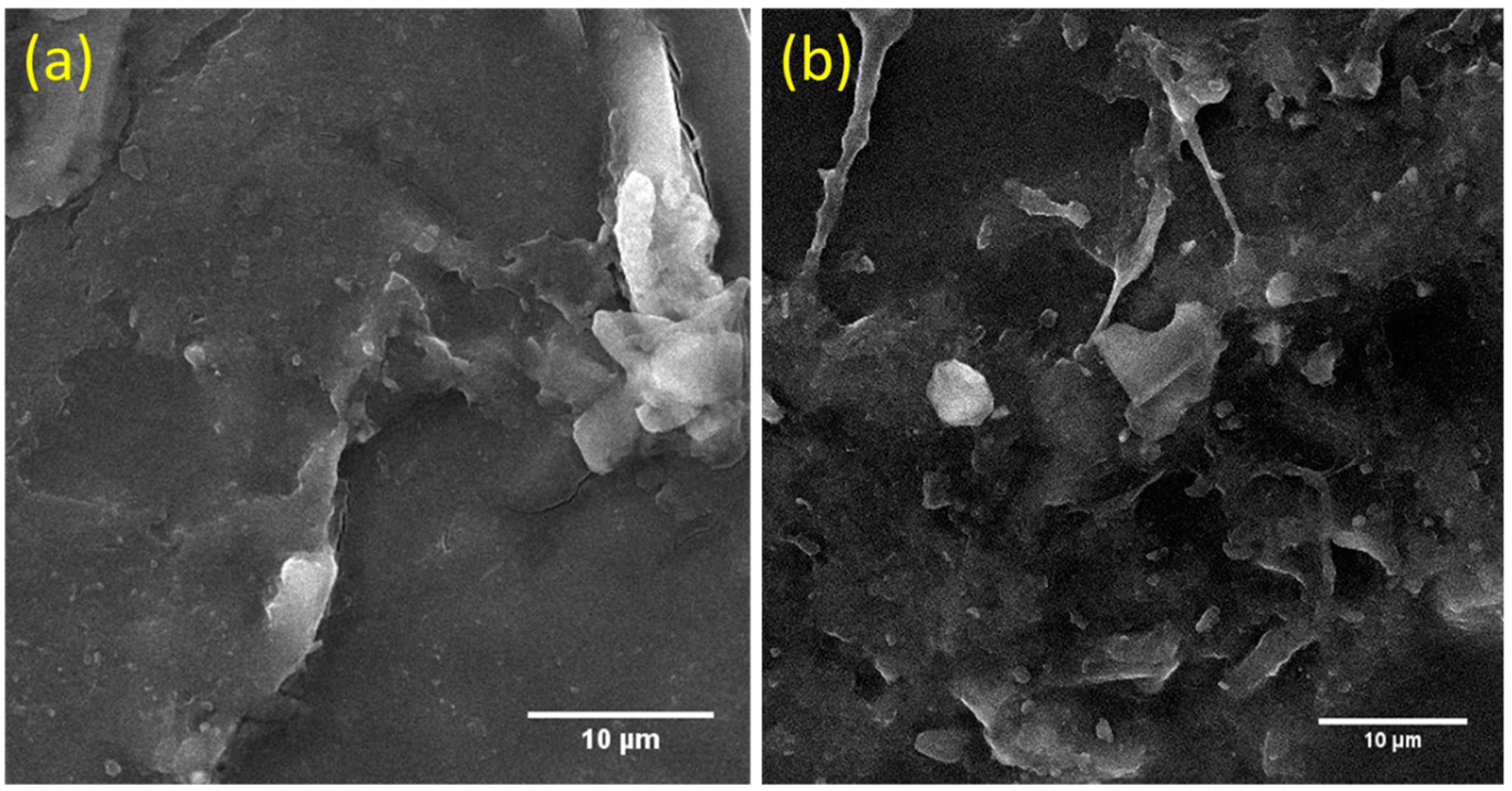
3.2. Scanning Electron Micrograph (SEM)
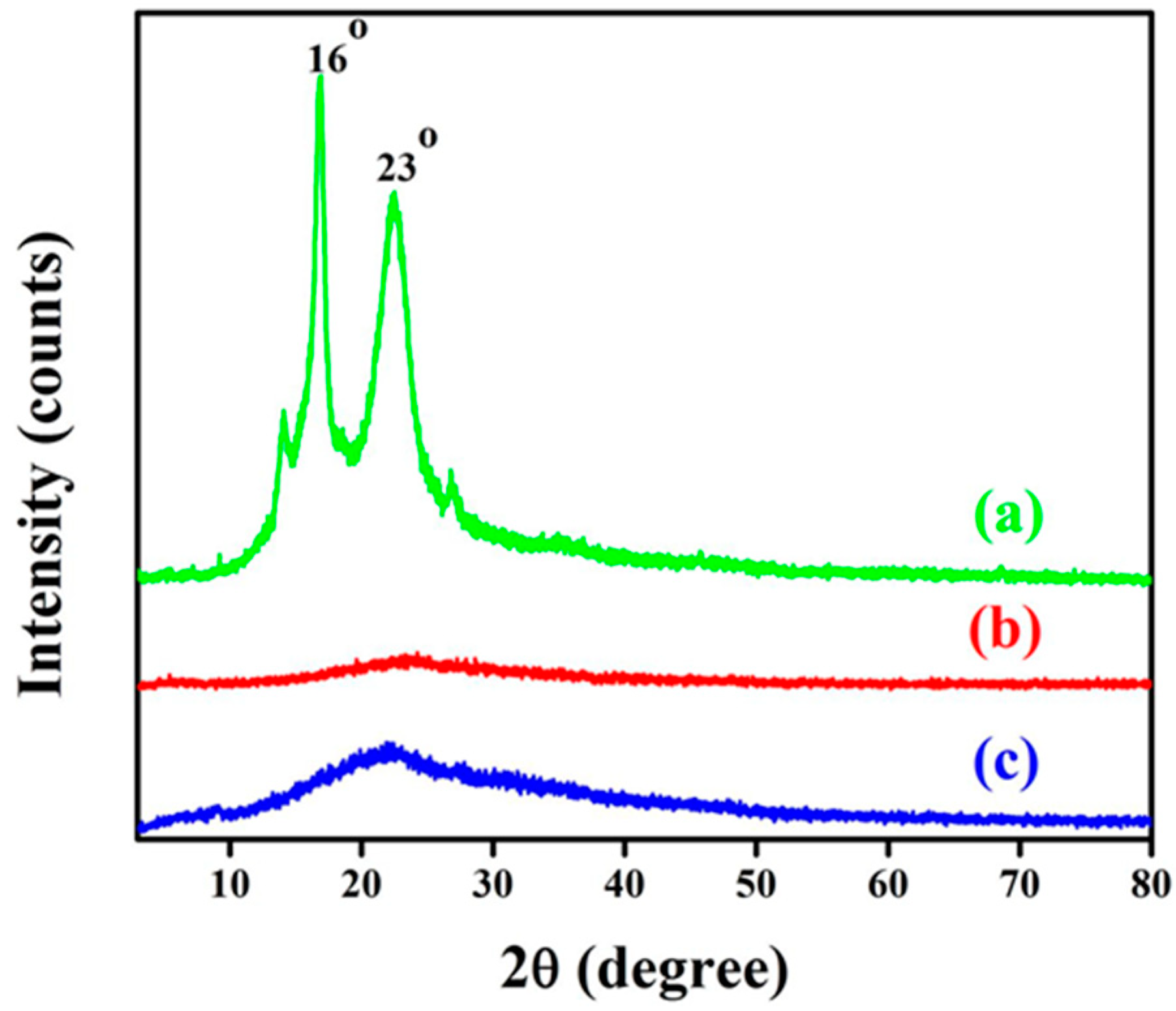
3.3. X-ray Diffraction (XRD)
3.4. Mechanical Properties
3.5. Thermal Properties by DSC
3.6. Antibacterial Activities of Bionanocomposites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Mishra, R.K.; Ha, S.K.; Verma, K.; Tiwari, S.K. Recent progress in selected bio-nanomaterials and their engineering applications: An overview. J. Sci. Adv. Mater. Dev. 2018, 3, 263–288. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.D.A.; Menegalli, F.C. Nanocomposites based on banana starch reinforced with cellulose nanofibers isolated from banana peels. J. Colloid Interface Sci. 2017, 505, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.M.A.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. Saudi Chem. Soc. 2018, in press. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Varma, K.; Jude, S.; Reddy, P.B.; Divya, C.; Haponiuk, J.T.; Thomas, S. Turmeric nanofiber-encapsulated natural product formulation act as a phytogenic feed additive—A study in broilers on growth performance, biochemical indices of blood, and E. coli in cecum. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 581–588. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Jude, S.; Varma, K.; Sreeraj, T.R.; Haponiuk, J.T.; Thomas, S. Preparation, characterization and anti-colitis activity of curcumin-asafoetida complex encapsulated in turmeric nanofiber. Mater. Sci. Eng. C 2017, 81, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Jude, S.; Varma, K.; Jacob, J.; Gopi, S.; Oluwafemi, O.S.; Thomas, S. Preparation of a novel bioavailable curcuminoid formulation (Cureit™) using Polar-Nonpolar-Sandwich (PNS) technology and its characterization and applications. Mater. Sci. Eng. C 2017, 75, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; George, R.; Thomas, M.; Jude, S. A pilot cross-over study to assess the human bio availability of “Cureit” A bio available curcumin in complete natural matrix. Asian J. Pharm. Technol. Innov. 2015, 3, 92–96. [Google Scholar]
- Gopi, S.; Amalraj, A.; Jacob, J.; Kalarikkal, N.; Thomas, S.; Guo, Q. Preparation, characterization and in vitro study of liposomal curcumin powder by cost effective nanofiber weaving technology. New J. Chem. 2018, 42, 5117–5127. [Google Scholar] [CrossRef]
- Prakobna, K.; Galland, S.; Berglund, L.A. High-performance and moisture-stable cellulose–starch nanocomposites based on bioinspired core–shell nanofibers. Biomacromolecules 2015, 16, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Dupeyre, D.; Vignon, M.R. Cellulose microfibrils from potato tuber cells: Processing and characterization of starch–cellulose microfibril composites. J. Appl. Polym. Sci. 2000, 76, 2080–2092. [Google Scholar] [CrossRef]
- Lobmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Worldatlas Canada. The Leading Ginger Producing Countries in the World. 1994. Available online: https://www.worldatlas.com/articles/the-leading-ginger-producing-countries-in-the-world.html (accessed on 25 April 2017).
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Khan, B.; Niazi, M.B.K.; Jahan, G.S.Z. Thermoplastic starch: A possible biodegradable food packaging material—A Review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Gutierrez, T.J.; Alvarez, V.A. Cellulosic materials as natural fillers in starch-containing matrix-based films: A review. Polym. Bull. 2017, 74, 2401–2430. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Prasad, V.S. Starch-based “Green” composites. In Biodegradable Green Composites, 1st ed.; Kalia, S., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; pp. 199–298. [Google Scholar]
- Alam, M.N.; Christopher, L.P. A novel, cost-effective and eco-friendly method for preparation of textile fibers from cellulosic pulps. Carbohydr. Polym. 2017, 173, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Roilo, D.; Maestri, C.A.; Scarpa, M.; Bettotti, P.; Checchetto, R. Gas barrier and optical properties of cellulose nanofiber coatings with dispersed TiO2 nanoparticles. Surf. Coat. Technol. 2018, 343, 131–137. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Negro, C.; Blanco, A. In situ production of bacterial cellulose to economically improve recycled paper properties. Int. J. Biol. Macromol. 2018, 118, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, F.M.; Sobral, P.J.D.A.; Menegalli, F.C. Isolation and characterization of cellulose nanofibers from banana peels. Cellulose 2014, 21, 417–432. [Google Scholar] [CrossRef]
- Kalita, D.; Kaushik, N.; Mahanta, C.L. Physicochemical, morphological, thermal and IR spectral changes in the properties of waxy rice starch modified with vinyl acetate. J. Food Sci. Technol. 2014, 51, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TiO2 and montmorillonite on potato starch nanocomposite film: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jung, J.; Zhao, Y. Development, characterization and validation of chitosan absorbed cellulose nanofiber (CNF) films as water resistant and antibacterial food contact packing. LWT–Food Sci. Technol. 2017, 83, 132–140. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid/chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Llanos, J.H.R.; Tandini, C.C. Preparation and characterization of bio-nanocomposite film based on cassava starch or chitosan, reinforced with montmorillonit or bamboo nanofibers. Int. J. Biol. Macromol. 2018, 107, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Silverajah, V.S.; Ibrahim, N.A.; Zainuddin, N.; Yunus, W.M.; Hassan, H.A. Mechanical, thermal and morphological properties of poly(lactic acid)/epoxidized palm olein blend. Molecules 2012, 17, 11729–11747. [Google Scholar] [CrossRef] [PubMed]
- Chundawat, S.P.; Bellesia, G.; Uppugundla, N.; da Costa, S.L.; Gao, D.; Cheh, A.M.; Agarwal, U.P.; Bianchetti, C.M.; Phillips, G.N., Jr.; Langan, P.; et al. Restructuring the crystalline cellulose hydrogen bond network enhances its depolymerization rate. J. Am. Chem. Soc. 2011, 133, 11163–11174. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, B. Thermal Analysis, 1st ed.; Academic Press Inc.: San Diego, CA, USA, 1990. [Google Scholar]


| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PS | 1.6 ± 0.6 | 19 ± 1 | 61 ± 2 |
| GNF–PS (1%) | 2.2 ± 0.5 | 22 ± 2 | 53 ± 1 |
| GNF–PS (3%) | 2.9 ± 1.2 | 24 ± 2 | 48 ± 2 |
| GNF–PS (5%) | 3.9 ± 0.9 | 32 ± 1 | 44 ± 1 |
| GNF–PS (7%) | 3.2 ± 0.9 | 29 ± 1 | 45 ± 2 |
| TS | 2.3 ± 0.8 | 22 ± 1 | 55 ± 2 |
| GNF–TS (1%) | 2.5 ± 0.3 | 25 ± 1 | 52 ± 2 |
| GNF–TS (3%) | 3.1 ± 0.8 | 29 ± 1 | 47 ± 2 |
| GNF–TS (5%) | 4.2 ± 0.4 | 33 ± 1 | 43 ± 2 |
| GNF–TS (7%) | 3.9 ± 0.7 | 29 ± 2 | 44 ± 2 |
| Sample | To (°C) | Tp (°C) | Te (°C) | ΔHm (J/g) |
|---|---|---|---|---|
| GNF | 33 | 69 | 107 | 109 |
| GNF–PS | 57 | 78 | 108 | 21 |
| GNF–TS | 44 | 81 | 147 | 25 |
| Samples | Minimum Inhibitory Concentration (µg/mL) | |||
|---|---|---|---|---|
| Bacillus cereus | Escherichia coli | Staphylococcus aureus | Salmonella typhimurium | |
| GNF | 14 ± 2 | 13 ± 1 | 18 ± 0 | 31 ± 0 |
| GNF–PS | 9.1 ± 1 | 8.1 ± 1 | 15 ± 1 | 24 ± 2 |
| GNF–TS | 8.3 ± 1 | 3.1 ± 0 | 12 ± 2 | 19 ± 1 |
| Positive Control | 1.6 ± 0 | 0.4 ± 0 | 6 ± 0 | 12 ± 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, J.; Haponiuk, J.T.; Thomas, S.; Peter, G.; Gopi, S. Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities. Fibers 2018, 6, 79. https://doi.org/10.3390/fib6040079
Jacob J, Haponiuk JT, Thomas S, Peter G, Gopi S. Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities. Fibers. 2018; 6(4):79. https://doi.org/10.3390/fib6040079
Chicago/Turabian StyleJacob, Joby, Józef T. Haponiuk, Sabu Thomas, Gregary Peter, and Sreeraj Gopi. 2018. "Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities" Fibers 6, no. 4: 79. https://doi.org/10.3390/fib6040079
APA StyleJacob, J., Haponiuk, J. T., Thomas, S., Peter, G., & Gopi, S. (2018). Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities. Fibers, 6(4), 79. https://doi.org/10.3390/fib6040079






