Concentration-Dependent Association of Flavylium Chloride with Differential Hydroxy Moieties in Ethanol
Abstract
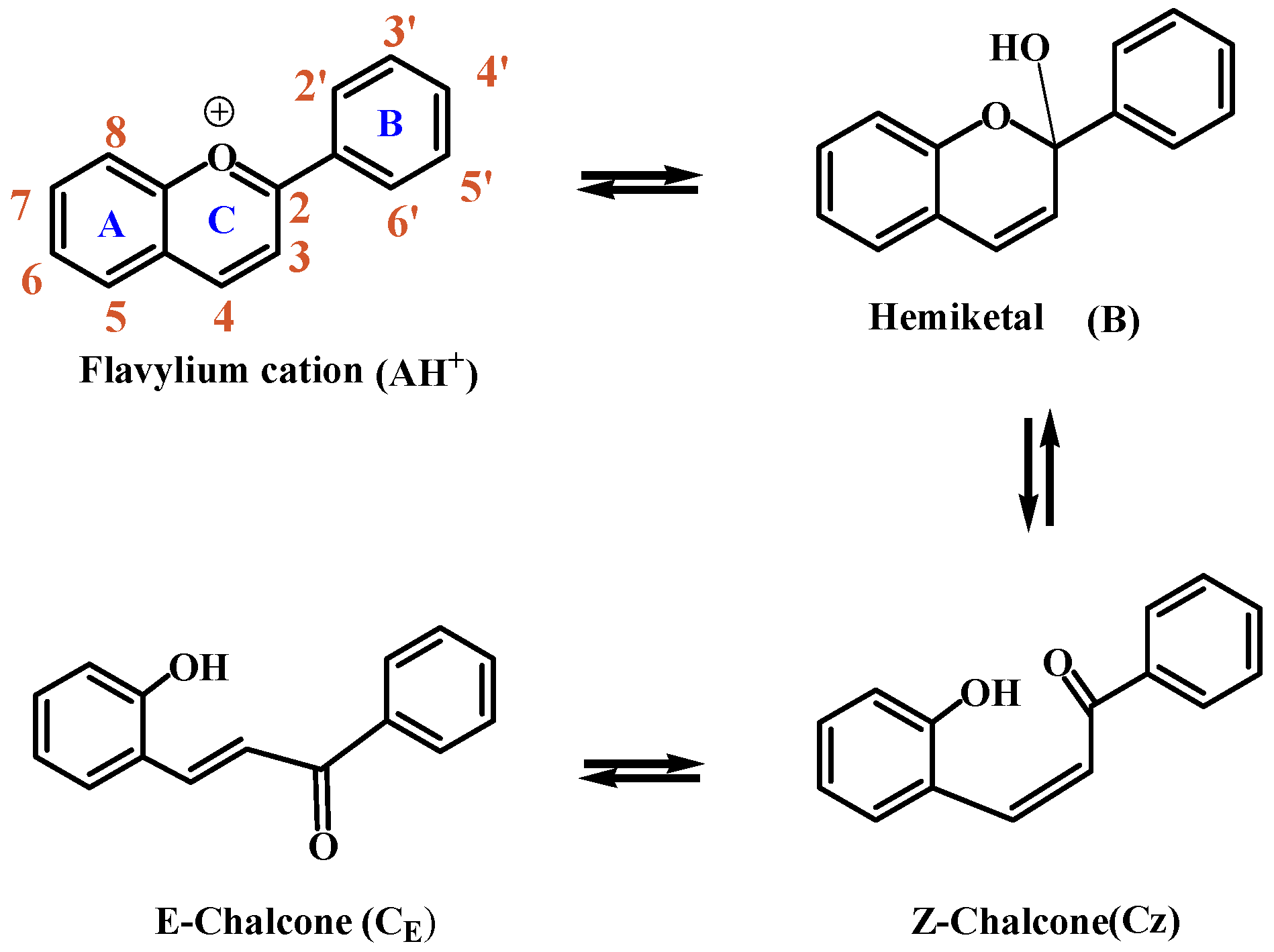
:1. Introduction
2. Materials and Methods
General Procedure
3. Results and Discussion
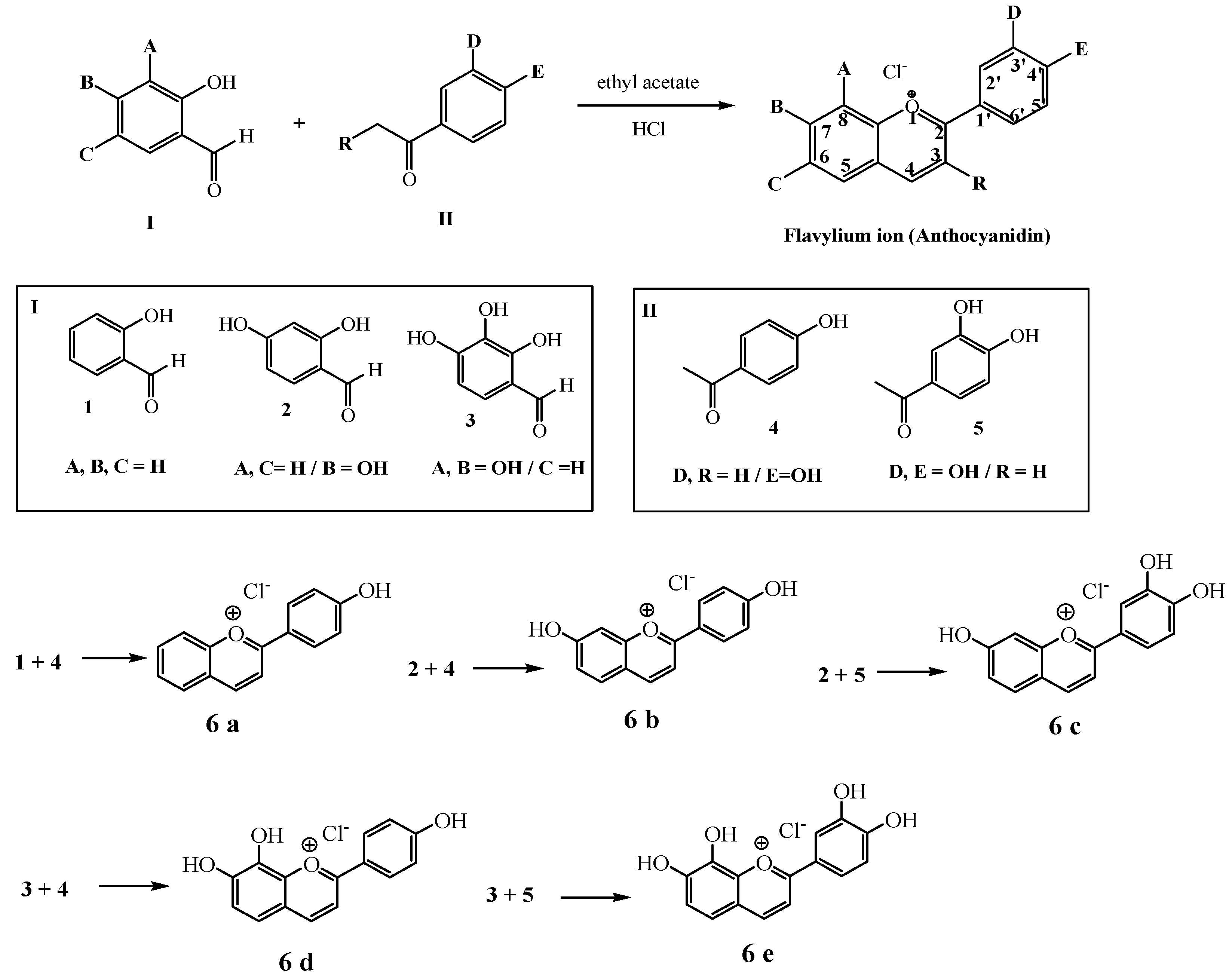
3.1. Synthesis
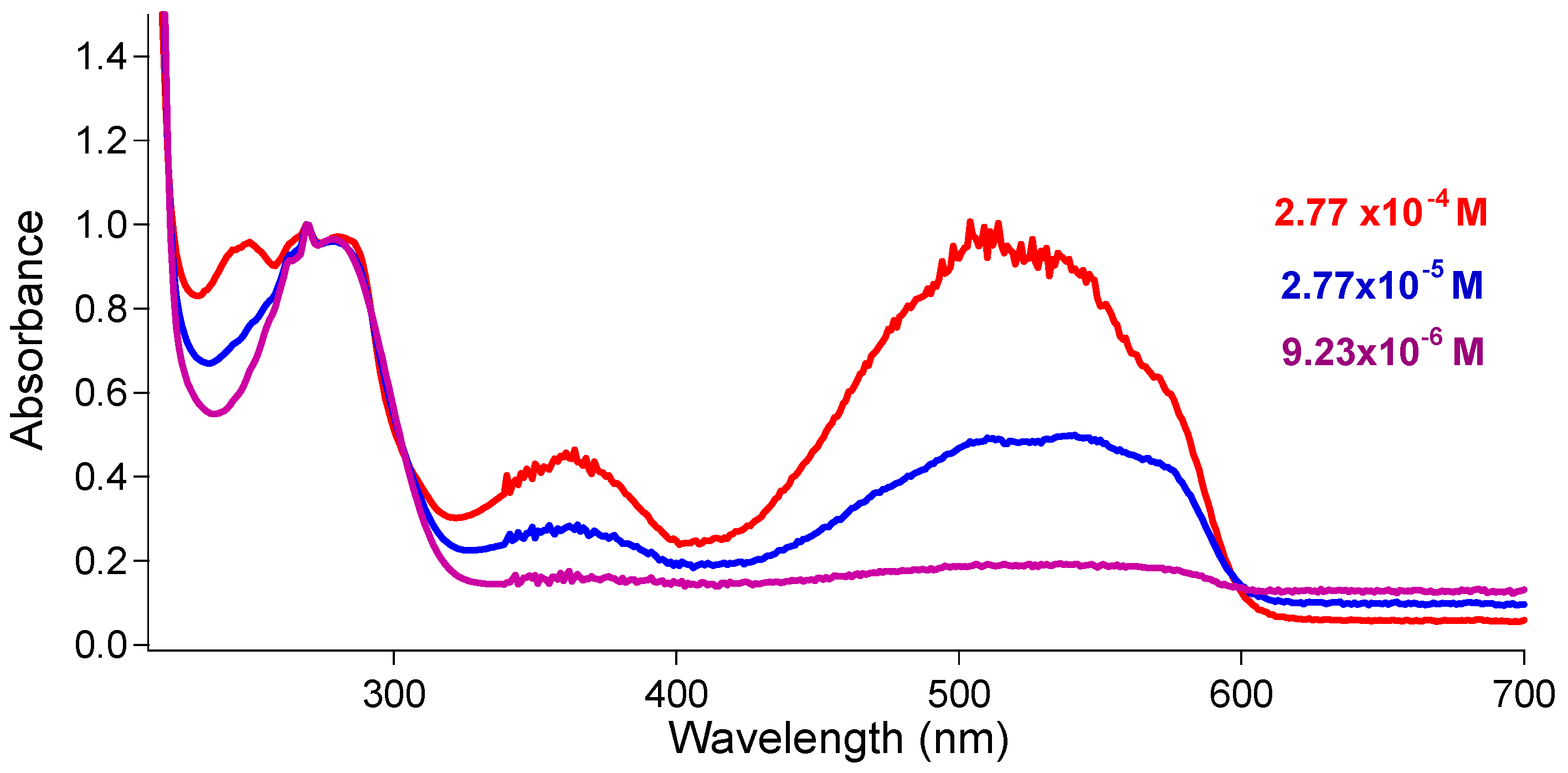
3.2. Effect of HCl on UV–Vis Spectra of 6a–6e in Ethanol
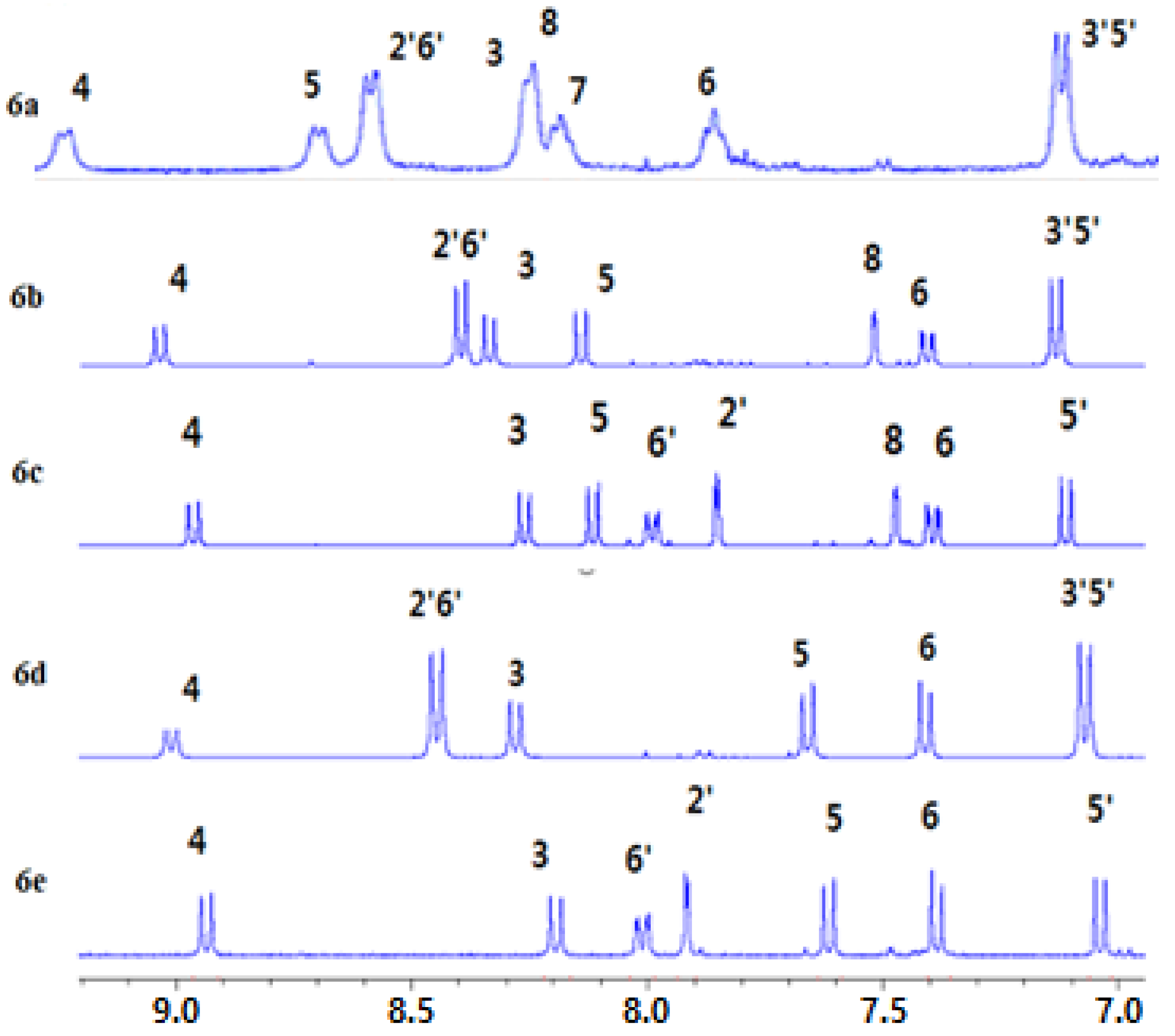
3.3. NMR Chemical Shift and Electron Density
3.4. 1H-NMR Chemical Shift Measuring Concentration Dependency of Ions
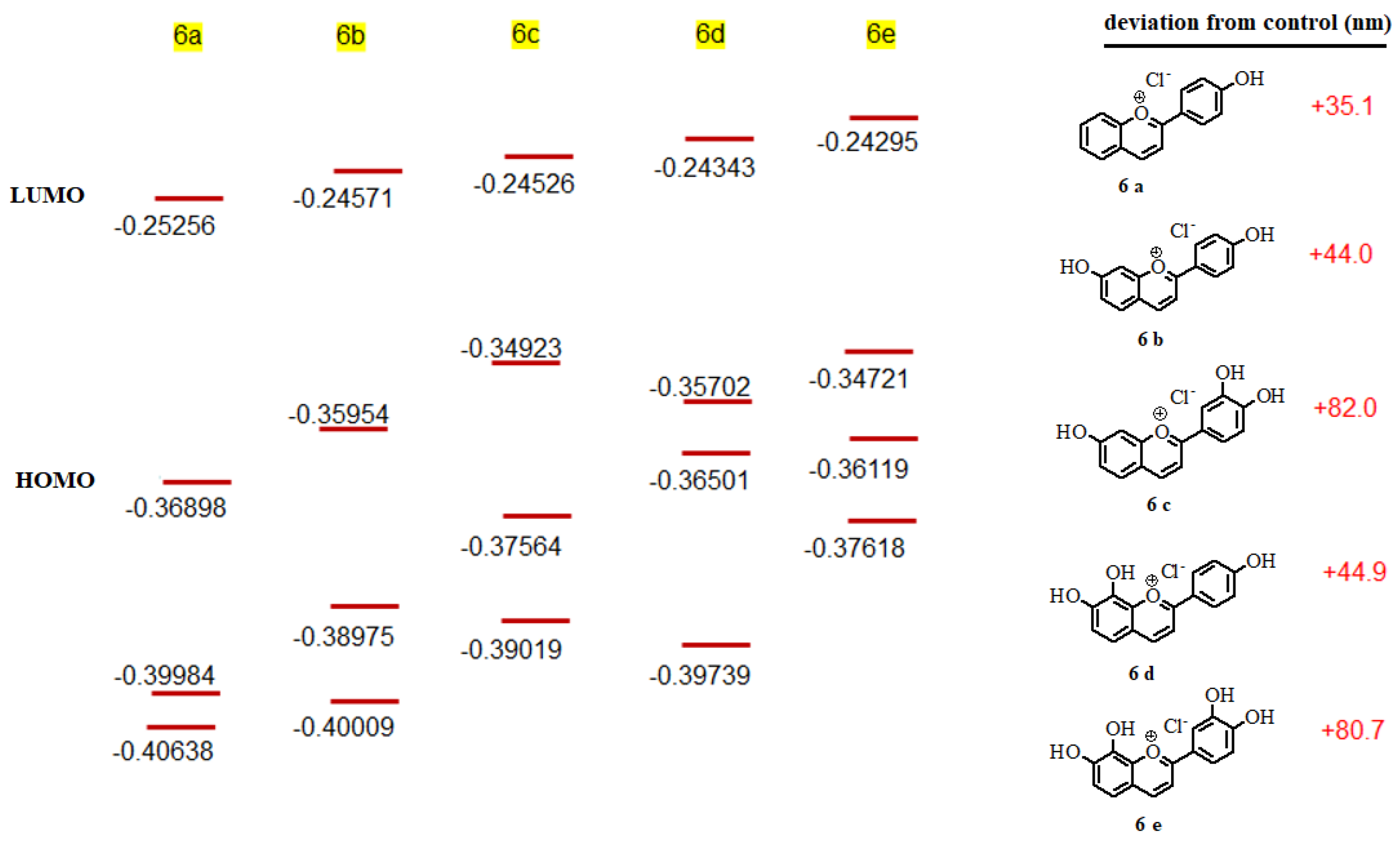
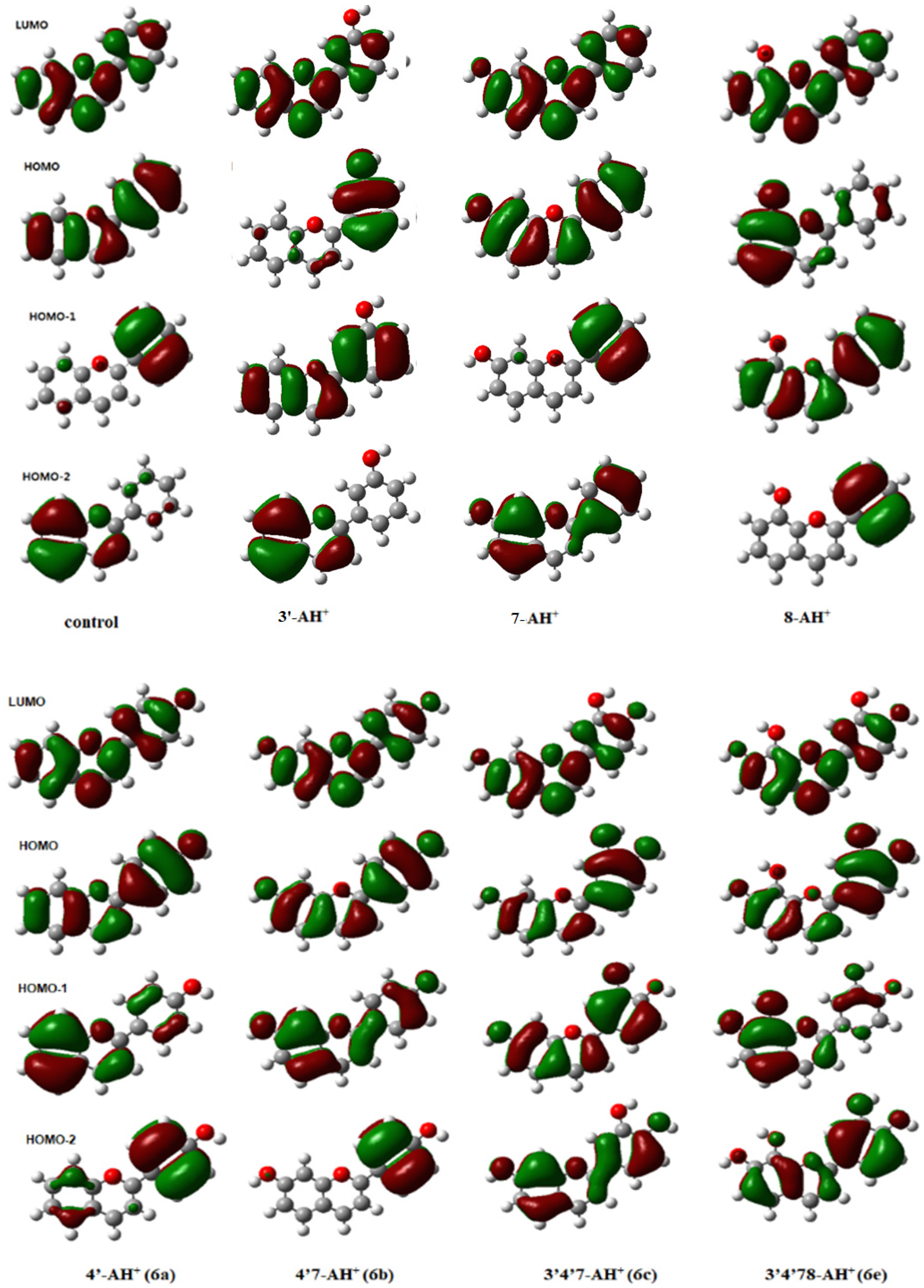
3.5. Correlation of UV Data and Calculation
3.6. Self-Aggregation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bridle, P.; Timberlake, C.F. Anthocyanins as natural food colours—Selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar] [CrossRef]
- Brouillard, R.; Figueiredo, P.; Elhabiri, M.; Dangles, O. Molecular Interactions of Phenolic Compounds in Relation to the Colour of Fruit and Vegetables. In Phytochemistry of Fruit and Vegetables; Tomas-Barberan, F.A., Robins, R.J., Eds.; Clarendon Press: Oxford, UK, 1997; Chapter 3; pp. 29–49. [Google Scholar]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2008, 25, 555–611. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Jordheim, M. Flavenoids, Chemistry, Biochemistry and Applications; Andersen, O.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 471–551. [Google Scholar]
- Hale, K.L.; Tufan, H.A.; Pickering, I.J.; George, G.N.; Terry, N.; Pilon, M.; Pilon-Smits, E.A.H. Anthocyanins facilitate tungsten accumulation in Brassica. Physiol. Plant 2002, 116, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Osakabe, A.; Saito, S.; Furuyama, D.; Tomita, A.; Kojima, Y.; Yamadera, M.; Sakuta, M. Components of protocyanin, a blue pigment from the blue flowers of Centaurea cyanus. Phytochemistry 2005, 66, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kitahara, S.D.; Ito, D.; Kondo, T. Ferric ions involved in the flower color development of the Himalayan blue poppy, Meconopsis grandis. Phytochemistry 2006, 67, 992. [Google Scholar] [CrossRef]
- Takeda, K. Blue metal complex pigments involved in blue flower color. Proc. Jpn. Acad. Ser. B Phys. Biol Sci 2006, 82, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Onslow, M.W. The Anthocyanin Pigments of Plants; Cambridge University Press: Cambridge, MA, USA, 1916. [Google Scholar]
- Gould, K.S.; Lee, D.W. Anthocyanins in leaves and other vegetative organs: An introduction. Adv. Bot. Res. 2002, 37, 1–16. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in Vegetative Tissues: A Proposed Unified Function in Photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J. Biomed. Biotechnol. 2004, 5, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, A.A.; Sussuchi, E.M.; Giovani, W.F. Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins. Croat. Chem. Acta 2007, 80, 29–34. [Google Scholar]
- Gould, K.S.; McKelvie, J.; Markham, K.R. Do anthocyanins function as antioxidants in leaves? Imaging of H2O2 in red and green leaves after mechanical injury. Plant Cell Environ. 2002, 25, 1261–1269. [Google Scholar] [CrossRef]
- Hoch, W.A.; Singsaas, E.L.; McCown, B.H. Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiol. 2003, 133, 1296–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskitalo, J.; Bergquist, G.; Gardeström, P.; Jansson, S. A Cellular Timetable of Autumn Senescence. Plant Physiol. 2005, 139, 1635–1648. [Google Scholar] [CrossRef] [Green Version]
- Feild, T.S.; Lee, D.W.; Holbrook, N.M. Why Leaves Turn Red in Autumn. The Role of Anthocyanins in Senescing Leaves of Red-Osier Dogwood. Plant Physiol. 2001, 127, 566–574. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L. The ecophysiology of foliar anthocyanin. Bot. Rev. 2003, 69, 149–161. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb Res. 2005, 116, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toufektsian, M.C.; De Lorgeril, M.; Nagy, N.; Salen, P.; Donati, M.B.; Giordano, L.; Mock, H.-P.; Peterek, S.; Matros, A.; Petroni, K.; et al. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J. Nutr. 2008, 138, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Bontempo, P.; de Masi, L.; Carafa, V.; Rigano, D.; Scisciola, L.; Iside, C.; Grassi, R.; Molinari, A.M.; Aversano, R.; Nebbioso, A.; et al. Anticancer activities of anthocyanin extract from genotyped Solanum tuberosum L. “Vitelotte”. J. Funct. Foods 2015, 19, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Faria, A.; Pestana, D.; Teixeira, D.; de Freitas, V.; Mateus, N.; Calhau, C. Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phytother Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef] [Green Version]
- Côté, J.; Caillet, S.; Doyon, G.; Dussault, D.; Sylvain, J.-F.; Lacroix, M. Antimicrobial effect of cranberry juice and extracts. Food Cont. 2011, 22, 1413–1418. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Hardin, B.E.; Hoke, E.T.; Amstrong, P.B.; Yum, J.H.; Comte, P.; Torres, T.; Frechet, J.M.J.; Nazeeruddin, M.K.; Gratzel, M.; McGehee, M.D. Increased light harvesting in dye-sensitized solar cells with energy relay dyes. Nat. Photonics 2009, 3, 406–411. [Google Scholar] [CrossRef]
- Calogero, G.; Yum, H.J.; Sinopoli, A.; Di Marco, G.; Gratzel, M.; Nazeeruddin, M.K. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol. Energy 2012, 86, 1563. [Google Scholar] [CrossRef]
- Dangles, O.; Fenger, J.A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Soumille, N.; Al Bittar, S.; Rosa, M.; Dangles, O. Analogs of anthocyanins with a 3′,4′-dihydroxy substitution: Synthesis and investigation of their acid–base, hydration, metal binding and hydrogen-donating properties in aqueous solution. Dyes Pigm. 2013, 96, 7–15. [Google Scholar] [CrossRef]
- Roque, A.; Lodeiro, C.; Pina, F.; Maestri, M.; Dumas, S.; Passaniti, P.; Balzani, V. Multistate/Multifunctional Systems. A Thermodaynamic, Kinetc, and Photochemical Inverstigation of the 4’-Dimethylaminoflavylium Compound. J. Am. Chem. Soc. 2003, 125, 987–994. [Google Scholar] [CrossRef]
- Moncada, M.C.; Fernandez, D.; Lima, J.C.; Parola, A.J.; Lodeiro, C.; Folgosa, F.; Melo, M.J.; Pina, F. Multistate properties of 7-(N,N-diethylamino)-4′-hydroxyflavylium. An example of an unidirectional reaction cycle driven by pH. Org. Biomol. Chem. 2004, 2, 2802–2808. [Google Scholar] [CrossRef]
- Giestas, L.; Folgosa, F.; Lima, J.C.; Parola, A.J.; Pina, F. Bio-Inspired Multistate Networks Responsive to Light, pH and Thermal Inputs–An Example of a Multistate System Operating Through Different Algorithms. Eur. J. Org. Chem. 2005, 19, 4187–4200. [Google Scholar] [CrossRef]
- Furtado, P.; Figueiredo, P.; Chaves das Neves, H.; Pina, F. Photochemical and thermal degradation of anthocyanidins. Photochemical and thermal degradation of anthocyanidins. J. Photochem. Photobiol. A 1993, 75, 113–118. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Parola, A.J.; Pina, F. pH-Dependent Multistate System Generated by a Synthetic Furanoflavylium Compound: An Ancestor of the Anthocyanin Multistate of Chemical Species. ACS Omega 2019, 4, 4091–4100. [Google Scholar] [CrossRef] [Green Version]
- Brouillard, R.; Iacobucci, G.A.; Sweeny, J.G. Chemistry of anthocyanin pigments. 9. UV-visible spectrophotometric determination of the acidity constants of apigeninidin and three related 3- deoxyflavylium salts. J. Am. Chem. Soc. 1982, 104, 7585–7590. [Google Scholar] [CrossRef]
- Melo, M.J.; Moura, S.; Roque, A.; Maestri, M.; Pina, F. Photochemistry of luteolinidin: “Write-lock-read-unlock-erase” with a natural compound. J. Photochem. Photobiol. A 2000, 135, 33–39. [Google Scholar] [CrossRef]
- Gomes, R.; Diniz, A.M.; Jesus, A.; Parola, A.J.; Pina, F. The synthesis and reaction network of 2-styryl-1-benzopyrylium salts: An unexploited class of potential colorants. Dyes Pigm. 2009, 81, 69–79. [Google Scholar] [CrossRef]
- Cabrita, L.; Petrov, V.; Pina, F. On the thermal degradation of anthocyanidins: Cyanidin. RSC Adv. 2014, 4, 18939–18944. [Google Scholar] [CrossRef]
- Sweeny, J.G.; Iacobucci, G.A. Effect of Substitution on the Stability of 3-Deoxyanthocyanidins in Aqueous Solutions. J. Agric. Food Chem. 1983, 31, 531–533. [Google Scholar] [CrossRef]
- Gavara, R.; Petrov, V.; Pina, F. Characterization of the 4’- Hydroxynaphthoflavylium Network of Chemical Reactions. Photochem. Photobiol. Sci. 2010, 9, 298–303. [Google Scholar] [CrossRef]
- Petrov, V.; Diniz, A.M.; Cunha-Silva, L.; Parola, A.J.; Pina, F. Kinetic and thermodynamic study of 2’-hydroxy-8-methoxyflavylium. Reaction network interconverting flavylium cation and flavanone. RSC Adv. 2013, 3, 10786–10794. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Laia, C.A.T.; Parola, A.J.; Lima, J.C. Chemistry and Applications of Flavylium Compounds: A Handful of Colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef] [PubMed]
- Forino, M.; Gambuti, A.; Luciano, P.; Moio, L. Malvidin-3-O-glucoside Chemical Behavior in the Wine pH Range. J. Agric. Food Chem. 2019, 67, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.A.; McGall, G.H. Hydration of the Flavylium Ion. 2. The 4’-Hydroxyflavylium Ion. J. Org. Chem. 1982, 47, 3730–3736. [Google Scholar] [CrossRef]
- Pina, F.; Roque, A.; Melo, M.J.; Maestri, M.; Belladelli, L.; Balzani, V. Multistate/Multifunctional Molecular-Level Systems: Light and pH Switching between the Various Forms of a Synthetic Flavylium Salt. Chem. Eur. J. 1998, 4, 1184–1191. [Google Scholar] [CrossRef]
- Quina, F.H.; Moreira, P.F., Jr.; Vautier-Giongo, C.; Rettori, D.; Rita, F.; Rodrigues, R.F.; Freitas, A.A.; Silva, P.F.; Maçanita, A.L. Photochemistry of anthocyanins and their biological role in plant tissues. Pure Appl. Chem. 2009, 81, 1687–1694. [Google Scholar] [CrossRef]
- Baranac, J.M.; Petranovic, N.A.; Dimitric-Markovic, J.M. Spectrophotometric study of anthocyan copigmentation reactions. 2. Malvin and the nonglycosidized flavone quercetin. J. Agric. Food Chem. 1997, 45, 1694–1697. [Google Scholar] [CrossRef]
- Lima, J.C.; Macanita, A.; Santos, H. Color stabilization of Malvidin 3-Glucoside: Self-aggregation of the Flavylium cation and copigmentation with the Z-Chalcone Form. J. Phys. Chem. B 1998, 102, 3578–3585. [Google Scholar]
- Ferreira da Silva, P.; Lima, J.C.; Quina, F.H.; Macüanita, A.L. Excited-state electron transfer in anthocyanins and related flavylium salts. J. Phys. Chem. A 2004, 108, 10133–10140. [Google Scholar] [CrossRef]
- Al Bittar, S.; Nathalie Mora, N.; Loonis, M.; Olivier Dangles, O. A simple synthesis of 3-deoxyanthocyanidins and their O-glucosides. Tetrahedron 2016, 72, 4294–4302. [Google Scholar] [CrossRef]
- Quina, F.H.; Pacheco da Silva, C.; Held, B.; Oliveira Silva, V. Challenges to the use of anthocyanins as natural coloring agents and antioxidants Abstracts of Papers. In Proceedings of the 250th ACS National Meeting & Exposition, Boston, MA, USA, 16–20 August 2015; p. 576. [Google Scholar]
- Asen, S.; Stewart, R.N.; Norris, K.H. Co-pigmentation of anthocyanins in plant tissues and its effect on color. Phytochemistry 1972, 11, 1139–1144. [Google Scholar] [CrossRef]
- Hoshino, T.; Matsumoto, U.; Goto, T.; Harada, N. Evidence for the self-association of anthocyanins IV. PMR spectroscopic evidence for the vertical stacking of anthocyanin molecules. Tetrahedron Lett. 1982, 23, 433–436. [Google Scholar] [CrossRef]
- Fernandes, A.; Natércia, F.; Brás, N.F.; Mateus, N.; de Freitas, V. A study of anthocyanin self-association by NMR spectroscopy. New J. Chem. 2015, 39, 2602–2611. [Google Scholar] [CrossRef]
- Dangles, O.; Elhajji, H. Synthesis of 3-Methoxy- and 3-(β-D-Glucopyranosyloxy) flavylium Ions. Influence of the flavylium substitution pattern on the reactivity of anthocyanins in aqueous solution. Helv. Chim. Acta 1994, 77, 1595–1610. [Google Scholar] [CrossRef]
- Freudenberg, V.K.; Weinges, K. Zur Kenntnis der Catechine und Catechingerbstoffe. Eur. J. Org. Chem. 1954, 590, 140–154. [Google Scholar] [CrossRef]
- George, F.; Fellague, T. Preparation of 3-Deoxyanthocyanidine Compounds and Their Use as Antimicrobial. Agents. Patent Number FR2855172, 26 November 2004. [Google Scholar]
- Saito, N.; Osawa, Y.; Hayashi, K. Platyconin, a new acylated anthocyanin in Chinese bell-flower, Platycodon grandiflorum. Phytochemistry 1971, 10, 445–447. [Google Scholar] [CrossRef]
- Hoshino, T. Self-association of flavylium cations of anthocyanidin 3,5-diglucosides studied by circular dichroism and 1H-NMR. Phytochemistry 1992, 31, 647–653. [Google Scholar] [CrossRef]
- Goulas, V.; Minas, I.S.; Kourdoulas, P.M.; Lazaridou, A.; Athanassios, N.; Molassiotis, A.N.; Gerothanassis, I.P.; Manganaris, G.A. 1H NMR Metabolic Fingerprinting to Probe Temporal Postharvest Changes on Qualitative Attributes and Phytochemical Profile of Sweet Cherry Fruit. Front. Plant Sci. 2015, 6, 959. [Google Scholar] [CrossRef] [Green Version]

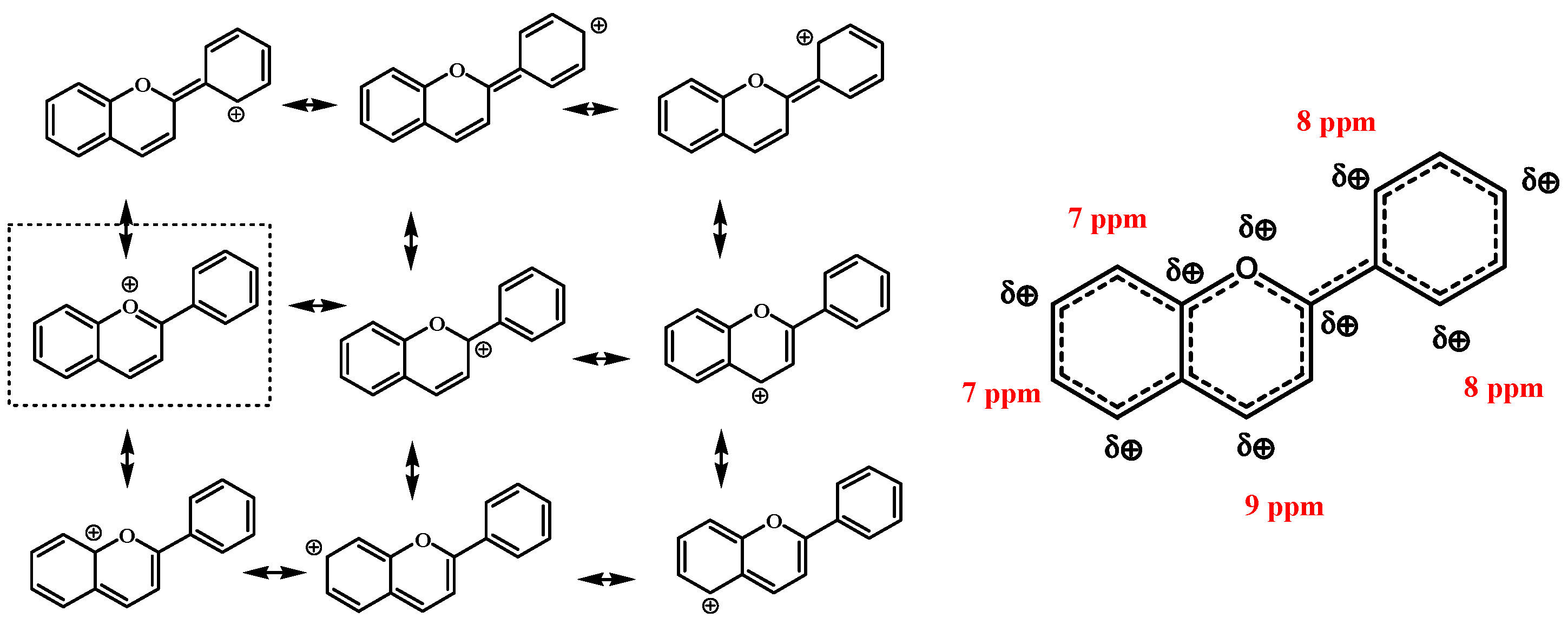





| Location on Ring | 1H-NMR (ppm) | 13C-NMR (ppm) | Charge Distribution on H (AO) | Charge Distribution on C (AO) |
|---|---|---|---|---|
| 3 | 8.2 | 146 | 0.251 | −0.1015 |
| 4 | 8.9 | 133 | 0.353 | −0.940 |
| 5 | 7.6 | 156 | 0.294 | −0.582 |
| 6 | 7.4 | 111 | 0.276 | 0.114 |
| 2′ | 7.9 | 116.7 | 0.350 | −0.167 |
| 5′ | 7.0 | 115.3 | 0.298 | −0.205 |
| 6′ | 8.0 | 125.2 | 0.198 | −0.734 |
| Compounds | Exp. Ab (nm) | Calc. (nm) Monomers | Calc. (nm) Dimers |
|---|---|---|---|
| 6a | 451.5 | 391.3 | 664.5 * |
| 6b | 466.5 | 400.2 | 456.7 |
| 6c | 497.5 | 438.2 | 493.3 * |
| 6d | 486.9 | 401.1 | 464.0 |
| 6e | 518.9 | 437.0 | 499.7 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagan, A.; Lee, J.I.; Kang, J. Concentration-Dependent Association of Flavylium Chloride with Differential Hydroxy Moieties in Ethanol. Colorants 2022, 1, 20-37. https://doi.org/10.3390/colorants1010004
Pagan A, Lee JI, Kang J. Concentration-Dependent Association of Flavylium Chloride with Differential Hydroxy Moieties in Ethanol. Colorants. 2022; 1(1):20-37. https://doi.org/10.3390/colorants1010004
Chicago/Turabian StylePagan, Anthony, Jong I. Lee, and Jeonghee Kang. 2022. "Concentration-Dependent Association of Flavylium Chloride with Differential Hydroxy Moieties in Ethanol" Colorants 1, no. 1: 20-37. https://doi.org/10.3390/colorants1010004





