Following the Light: Use of Multimodal Imaging and Fiber Optic Spectroscopy to Evaluate Aging in Daylight Fluorescent Artists’ Pigments
Abstract
:1. Introduction
1.1. Historic and Artistic Use
1.2. Composition
1.3. Fading and Color Shifts
1.4. Documentation and Compensation for Loss
1.5. Current Study
2. Materials and Methods
2.1. Pigments and Sample Preparation
2.2. Light Exposure
2.3. Fiber Optic Spectroscopy
2.4. Multimodal Imaging
3. Results and Discussion
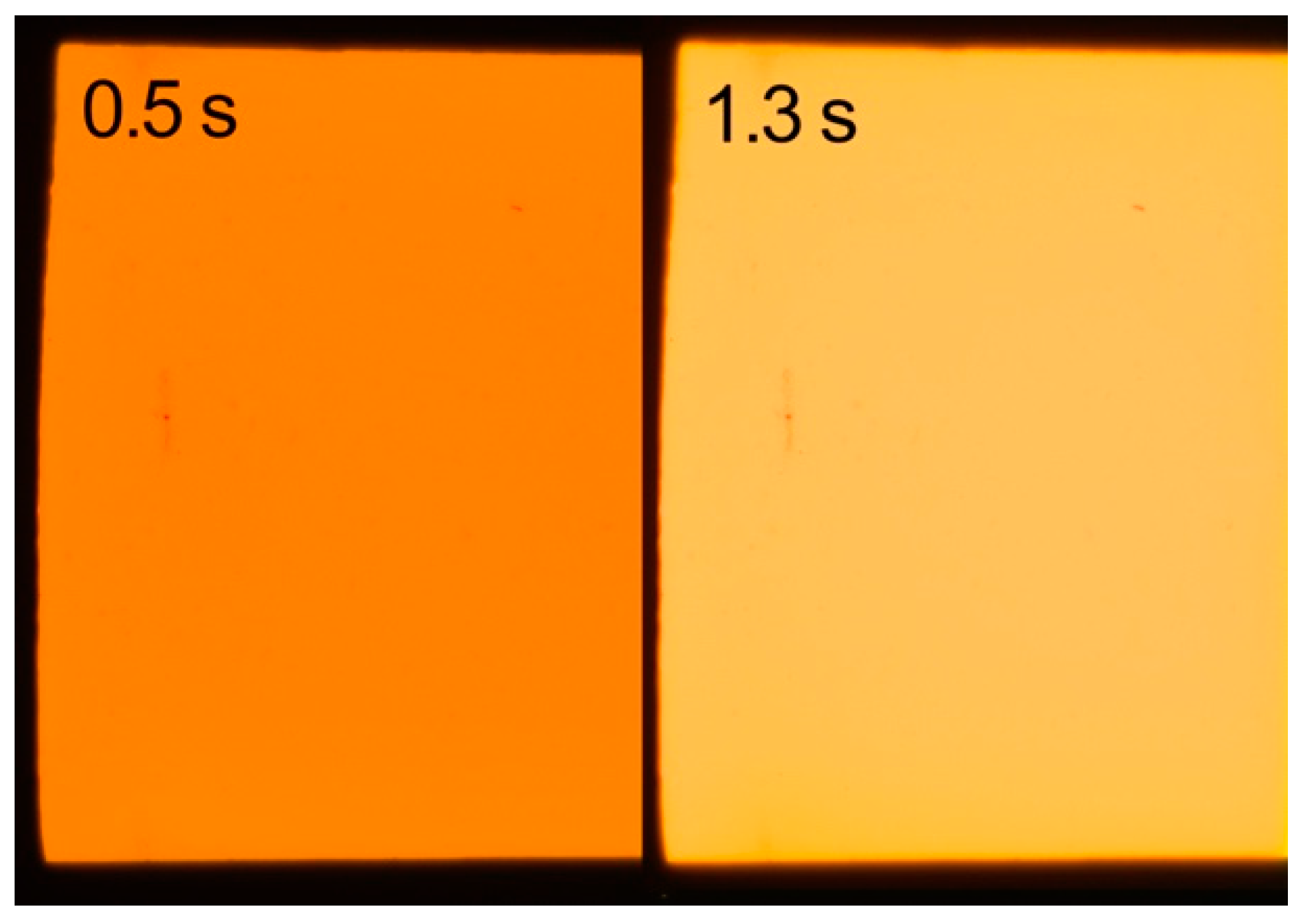
3.1. Light Exposure Results
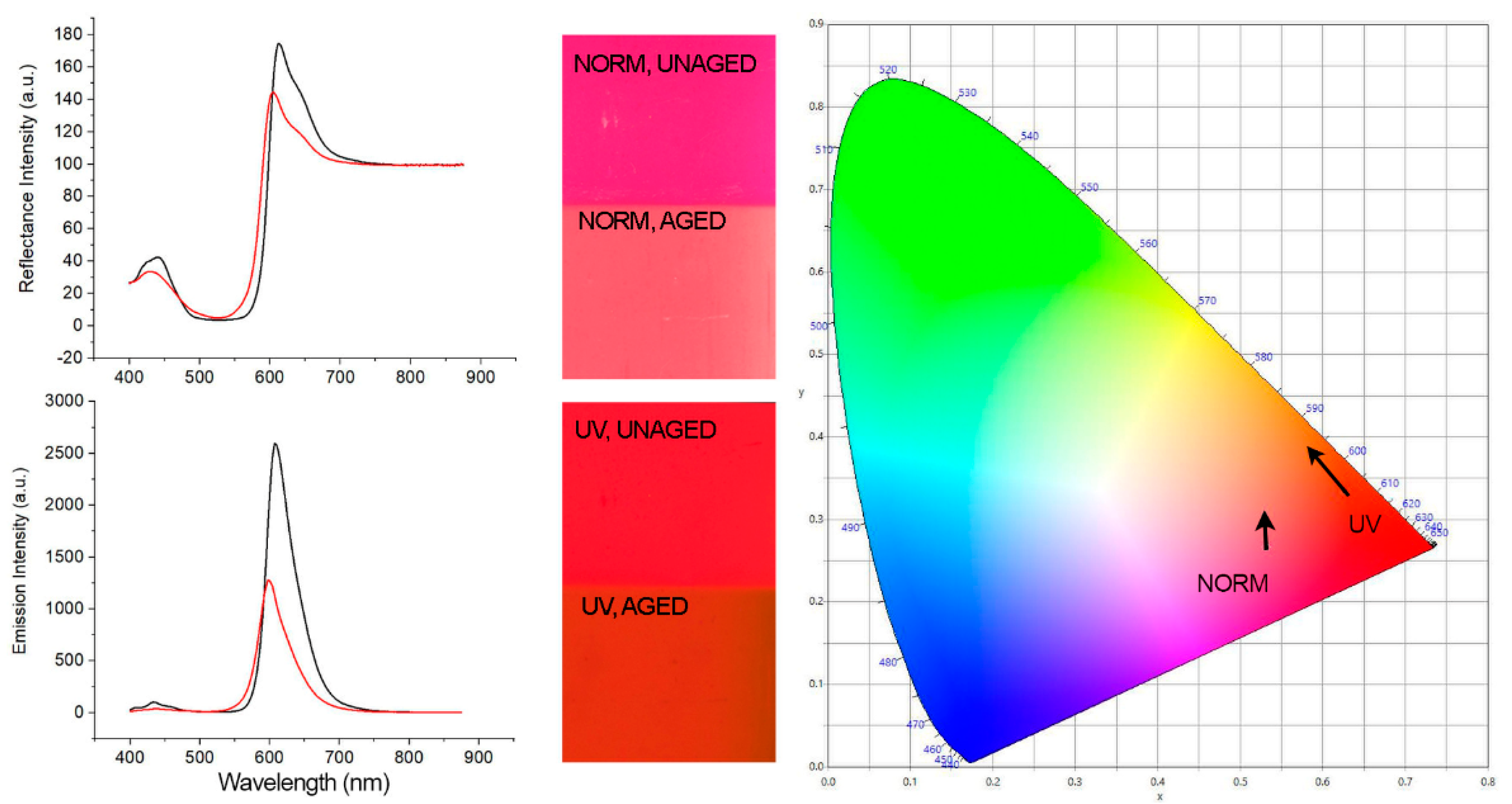
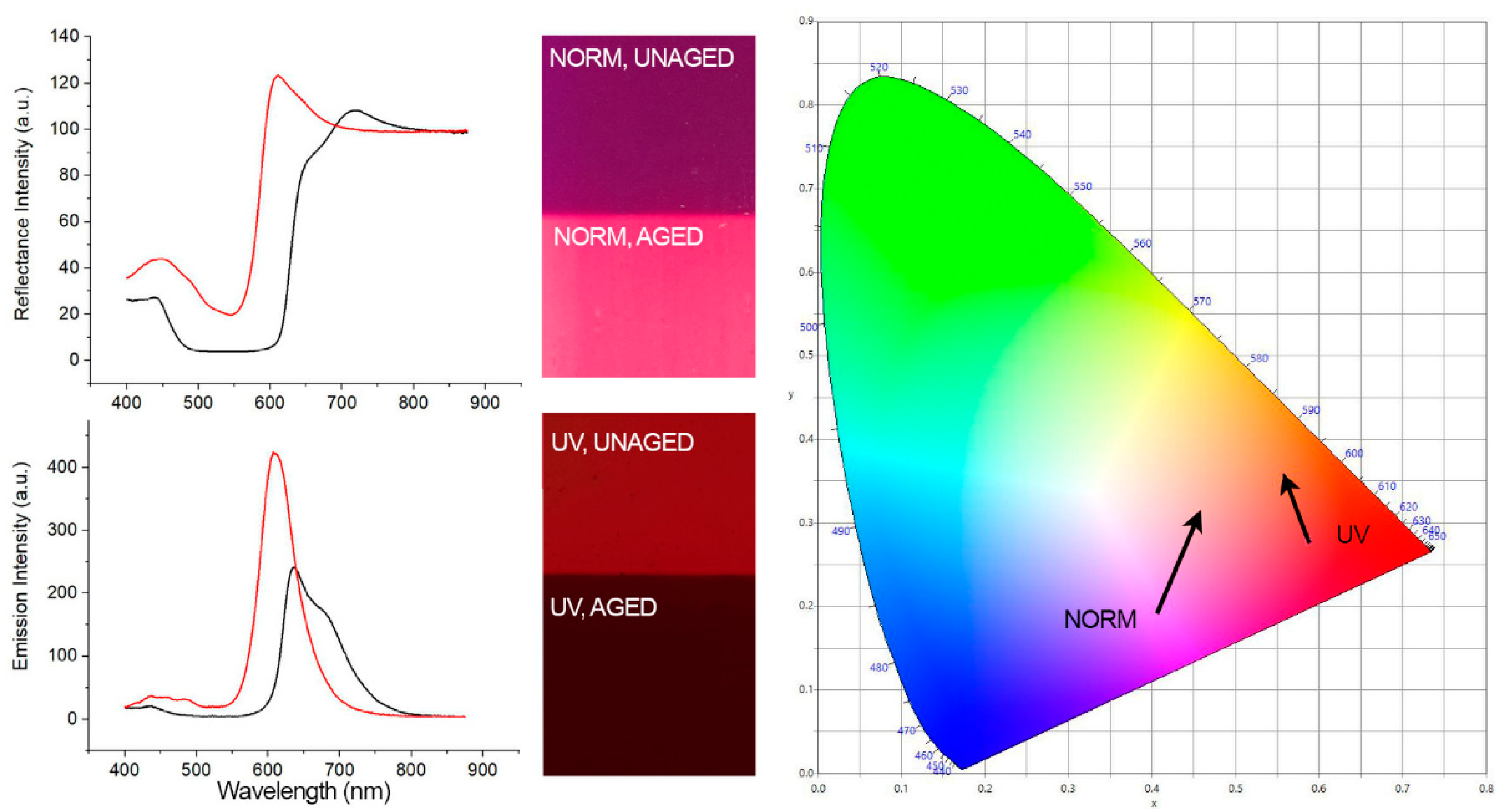
3.2. Spectroscopy Results
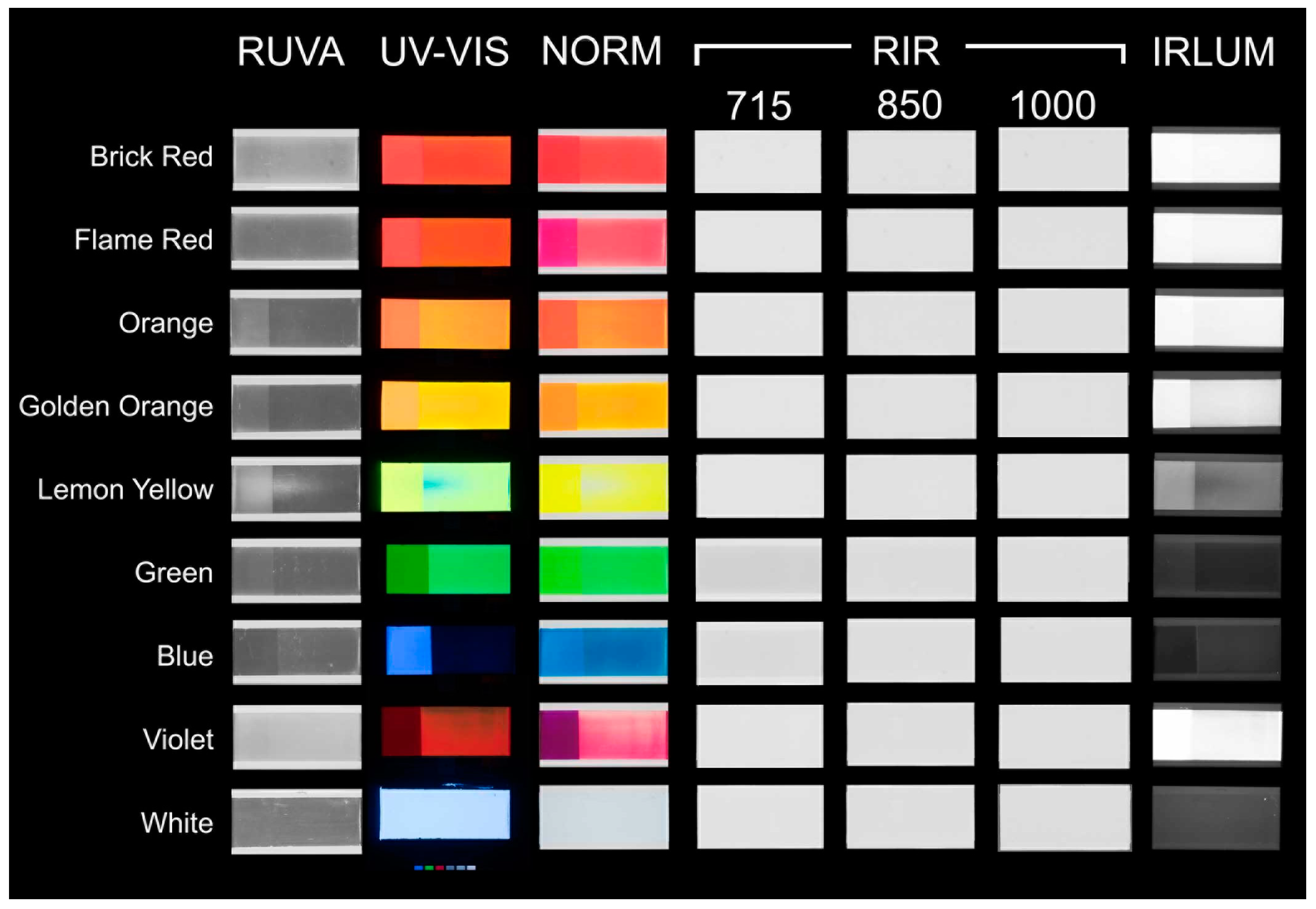
3.3. Imaging Results
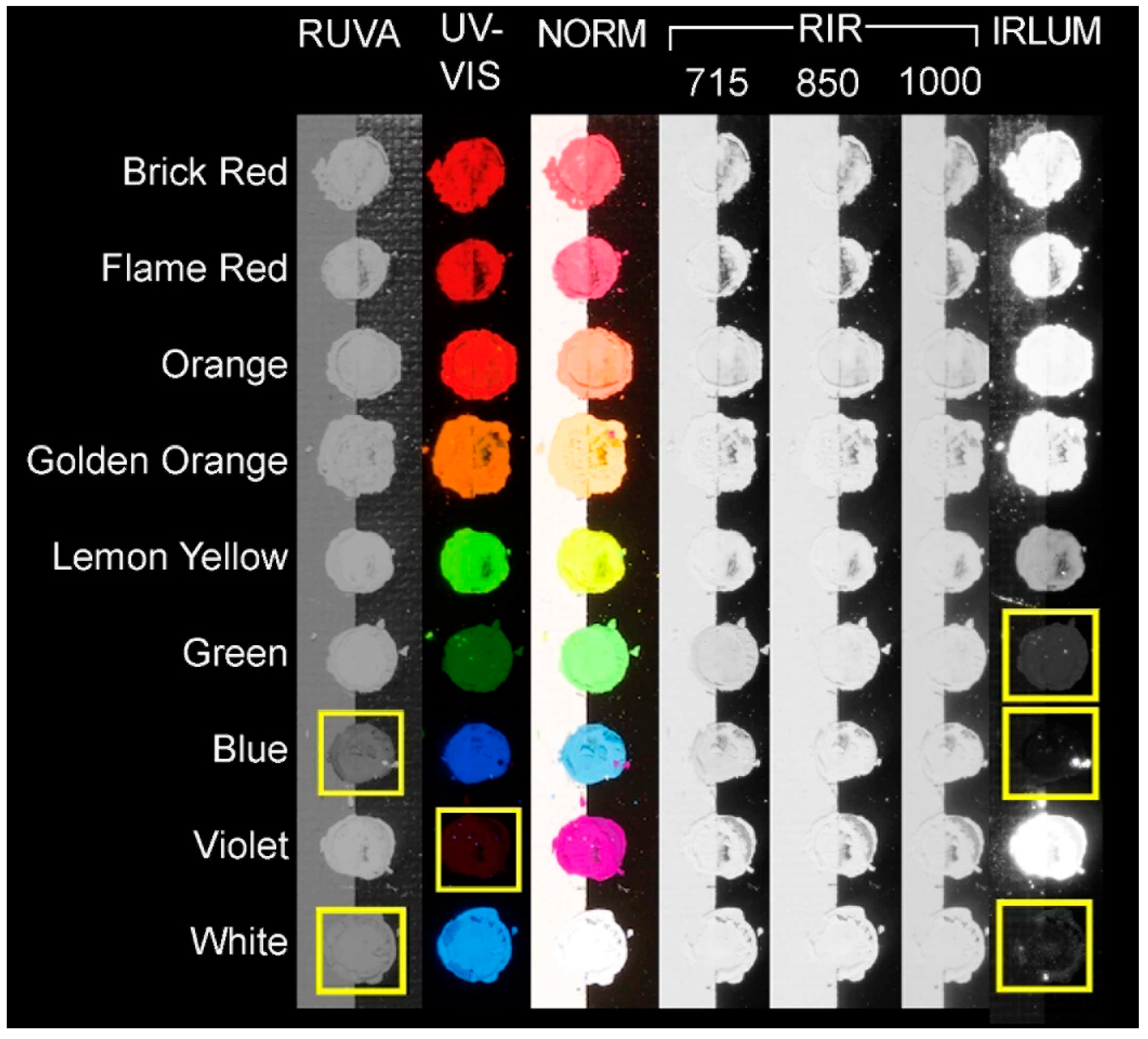
3.3.1. Normal Illumination
3.3.2. UV-Induced Visible Fluorescence
3.3.3. Reflected Ultraviolet Imaging
3.3.4. Reflected Infrared Imaging
3.3.5. Infrared Luminescence Imaging
3.4. Conservation Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DayGlo Color Corp. It’s Color. Only Better. Available online: https://www.dayglo.com/ (accessed on 27 January 2022).
- Lindblom, K. DayGlo Fluorescent Pigments: Brighter, Longer Lasting Color; American Chemical Society, Office of Communications, National Historic Chemical Landmarks: Washington, DC, USA, 2012.
- Baker, T.T. Fluorescent inks and paints. J. R. Soc. Arts 1951, 99, 763–776. [Google Scholar]
- Sexton, J.M.; Messier, P.; Chen, J.J.; Messier, J.M.S.P.; Chen, J.J. Development and Testing of a Fluorescence Standard for Documenting Ultraviolet Induced Visible Fluorescence. In Proceedings of the 42nd Annual Meeting of the American Institute for Conservation, San Francisco, CA, USA, 31 May 2014; pp. 28–31. [Google Scholar]
- Tsang, J.; Eleni Pinchin, S.; Almond, K.; Tumosa, C.S. Conservation of Murals in the Alameda Theatre: Reviving Former Cutting-Edge Fluorescent Paint and Black-Light Technology. Stud. Conserv. 2004, 49, 185–188. [Google Scholar] [CrossRef]
- Beckett, F.; Holden, A.; Smith, G.D. Seeing the Light: Conservation and Exhibition of a 1980s Day-Glo Painted Leather Jacket. In Proceedings of the AIC 43th Annual Meeting, Miami, FL, USA, 14 May 2015. [Google Scholar]
- De Winter, S. The Day Day-Glo Loses Its Glo (w): An Interdisciplinary Approach in Conserving Artworks Containing Daylight Fluorescent Paints. In Proceedings of the AIC 46th Annual Meeting, Houston, TX, USA, 1 June 2018. [Google Scholar]
- Kremer Pigmente GmbH & Co Daylight Fluorescent Pigments. Available online: https://shop.kremerpigments.com/us/shop/pigments/pearlescent-effect-pigments/daylight-fluorescent-pigments/ (accessed on 20 January 2022).
- Streitel, S.G. Fluorescent Pigments (Daylight). In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 1–25. ISBN 978-0-471-23896-6. [Google Scholar]
- Sobeck, S.J.S.; Chen, V.J.; Smith, G.D. Shedding Light on Daylight Fluorescent Artists’ Pigments, Part 1: Composition. J. Am. Inst. Conserv. 2021, 1–19. [Google Scholar] [CrossRef]
- Beckett, F.; Holden, A.; Smith, G.D. Seeing the Light: Research, Conservation and Exhibition of a 1980s Daylight Fluorescent Painted Leather Jacket Designed by Sprouse and Painted by Castronovo. J. Am. Inst. Conserv. 2019, 58, 233–247. [Google Scholar] [CrossRef]
- Hinde, E.; Nel, P.; Sloggett, R.; Roberts, A. Fluorimetric Analysis of the Constituent Dyes within Daylight Fluorescent Pigments: Implications for Display and Preservation of Daylight Fluorescent Artwork. J. Am. Inst. Conserv. 2013, 52, 97–106. [Google Scholar] [CrossRef]
- Fremout, W.; Saverwyns, S. Characterization of Daylight Fluorescent Pigments in Contemporary Artists’ Paints by Raman Spectroscopy. In Proceedings of the 11th Infrared and Raman Users Group Conference, Museum of Fine Arts, Boston, MA, USA, 5–7 November 2014; p. 39. [Google Scholar]
- Boscacci, M.; Francone, S.; Galli, K.; Bruni, S. The Brightest Colors: A Fourier-Transform Raman, Surface-Enhanced Raman, and Thin-Layer Chromatography-Surface-Enhanced Raman Spectroscopy Study of Fluorescent Artists’ Paints. J. Raman Spectrosc. 2020, 51, 1108–1117. [Google Scholar] [CrossRef]
- Campanella, B.; Botti, J.; Cavaleri, T.; Cicogna, F.; Legnaioli, S.; Pagnotta, S.; Poggialini, F.; Poli, T.; Scalarone, D.; Palleschi, V. The Shining Brightness of Daylight Fluorescent Pigments: Raman and SERS Study of a Modern Class of Painting Materials. Microchem. J. 2020, 152, 104292. [Google Scholar] [CrossRef]
- Francone, S.; Bruni, S.; Zaffino, C.; Galli, K.; Guglielmi, V.; Boscacci, M. The Issue of Metamerism in Mario Agrifoglio’s Paintings. Identification of Fluorescent Pigments through Raman Spectroscopy to Define a Methodology for Retouching. Postprints RECH4 Croatia 2017, 212–217. [Google Scholar]
- Longoni, M.; Cicala, N.; Guglielmi, V.; Poldi, G.; Bruni, S. The Art of Everyday Objects: A Non-Invasive In Situ Investigation of Materials and Techniques of Italian Pop Art Paintings on Aluminium. Heritage 2022, 5, 3. [Google Scholar] [CrossRef]
- Cosentino, A. Identification of Pigments by Multispectral Imaging; A Flowchart Method. Herit. Sci. 2014, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Castro, K.; Pérez-Alonso, M.; Rodríguez-Laso, M.D.; Fernández, L.A.; Madariaga, J.M. On-Line FT-Raman and Dispersive Raman Spectra Database of Artists’ Materials (e-VISART Database). Anal. Bioanal. Chem. 2005, 382, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Festa, G.; Scatigno, C.; Armetta, F.; Saladino, M.L.; Ciaramitaro, V.; Nardo, V.M.; Ponterio, R.C. Chemometric Tools to Point Out Benchmarks and Chromophores in Pigments through Spectroscopic Data Analyses. Molecules 2022, 27, 163. [Google Scholar] [CrossRef] [PubMed]
- Hochleitner, B.; Desnica, V.; Mantler, M.; Schreiner, M. Historical Pigments: A Collection Analyzed with X-Ray Diffraction Analysis and X-ray Fluorescence Analysis in Order to Create a Database. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 641–649. [Google Scholar] [CrossRef]
- Sobeck, S.J.S.; Smith, G.D. Shedding Light on Daylight Fluorescent Artists’ Pigments, Part 2: Spectral Properties and Light Stability. J. Am. Inst. Conserv. 2022, 1–17. [Google Scholar] [CrossRef]
- Golden Artist Colors, Inc. Available online: https://www.goldenpaints.com/products/colors/fluorescent---phosphorescent-colors (accessed on 24 January 2022).
- Connors-Rowe, S.A.; Morris, H.R.; Whitmore, P.M. Evaluation of Appearance and Fading of Daylight Fluorescent Watercolors. J. Am. Inst. Conserv. 2005, 44, 75–94. [Google Scholar] [CrossRef]
- Hinde, E.; Nel, P. A Novel Technique for the Photography of Daylight Fluorescent Artwork. In Proceedings of the ICOM Committee for Conservation, ICOM-CC, 15th Triennial Conference, New Delhi, India, 22–26 September 2008; Volume 1, pp. 475–483. [Google Scholar]
- Ellis, M.H.; Chao, E. Daylight Fluorescent Colors as Artistic Media. In The Broad Spectrum: Studies in the Materials, Techniques, and Conservation of Color on Paper; Archetype Publications: London, UK, 2002; pp. 160–166. [Google Scholar]
- UV InnovationsTM: Ultraviolet Photography Standards. Available online: https://www.uvinnovations.com (accessed on 20 January 2022).
- Whitmore, P.M.; Pan, X.; Bailie, C. Predicting The Fading of Objects: Identification of Fugitive Colorants Through Direct Nondestructive Lightfastness Measurements. J. Am. Inst. Conserv. 1999, 38, 395–409. [Google Scholar] [CrossRef]
- Ploeger, R.; Shugar, A.; Smith, G.D.; Chen, V.J. Late 19th Century Accounts of Indian Yellow: The Analysis of Samples from the Royal Botanic Gardens, Kew. Dyes Pigments 2019, 160, 418–431. [Google Scholar] [CrossRef]
- Chen, J.J.; Smith, T.J. Documentation of Salted Paper Prints with a Modified Digital Camera. J. Am. Inst. Conserv. 2020, 59, 271–285. [Google Scholar] [CrossRef]
- Bridgman, C.F.; Gibson, H.L. Infrared Luminescence in the Photographic Examination of Paintings and Other Art Objects. Stud. Conserv. 1963, 8, 77–83. [Google Scholar] [CrossRef]
- Kakoulli, I.; Radpour, R.; Lin, Y.; Svoboda, M.; Fischer, C. Application of Forensic Photography for the Detection and Mapping of Egyptian Blue and Madder Lake in Hellenistic Polychrome Terracottas Based on Their Photophysical Properties. Dyes Pigments 2017, 136, 104–115. [Google Scholar] [CrossRef]
- Dayglo Radiant Color, Manufacturer of Daylight Fluorescent Pigments. Available online: http://test.dayglo.com/radiant/company/about-radiant-color/ (accessed on 20 January 2022).
- Picollo, M.; Stols-Witlox, M.; Fuster-López, L. (Eds.) Introduction to the Volume. In UV-Vis Luminescence Imaging Techniques; CONSERVATION 360°; Editorial; Universitat Politècnia de València: Valencia, Spain, 2019; pp. 11–18. [Google Scholar]
- ASTM D4303-06; Standard Test Methods for Light Fastness of Colorants Used in Artists’ Materials. Micom Laboratories: Dorval, QC, Canada, 2006.
- Feller, R.L.; Johnston-Feller, R.M. Use of the International Standards Organization’s Blue-Wool Standards for Exposure to Light. I. Use as an Integrating Light Monitor for Illumination under Museum Conditions. In Proceedings of the Preprints of Papers Presented at the Sixth Annual Meeting of the American Institute for Conservation, Fort Worth, TX, USA, 1–4 June 1978; pp. 73–80. [Google Scholar]
- Picollo, M.; Bacci, M.; Casini, A.; Lotti, F.; Porcinai, S.; Radicati, B.; Stefani, L. Fiber Optics Reflectance Spectroscopy: A Non-Destructive Technique for the Analysis of Works of Art. In Optical Sensors and Microsystems; Springer: Berlin/Heidelberg, Germany, 2002; pp. 259–265. [Google Scholar]
- Warda, J.; Frey, F.; Heller, D.; Kushel, D.; Vitale, T.; Weaver, G. Digital Imaging Workflow for Treatment Documentation. AIC Guide to Digital Photography and Conservation Documentation, 3rd ed.; AIC: Washington, DC, USA, 2017. [Google Scholar]
- Kushel, D. Photographic Techniques for Conservation. In AIC Guide to Digital Photography and Conservation Documentation; Warda, J., Frey, F., Heller, D., Kushel, D., Vitale, T., Weaver, G., Eds.; AIC: Washington, DC, USA, 2011. [Google Scholar]
- Chen, J.J.; Ersenkal, A.; Walters, G. Characterizing Different UVA Lamps. In Proceedings of the AIC 47th Annual Meeting, Uncasville, CT, USA, 16 May 2019. [Google Scholar]
- McGlinchey Sexton, J.; Messier, P. Permanence of the Target-UVTM and UV-GrayTM 2015. Available online: https://www.uvinnovations.com/_files/ugd/750e25_77a29ae0fb3a459b9fe83189272b9bf1.pdf (accessed on 20 May 2022).




| Fluorescent Colorant | Known Dyes and Optical Brighteners |
|---|---|
| Brick Red 56300 | SRB, R6GD, R6G, R3B, SY172, FB 184, BP3 |
| Flame Red 56350 | SRB, R6GD, R6G, R3B, SY172, FB 184, BP3 |
| Orange 56250 | SRB, R6GD, R3B, SY172, FB 184, BP3 |
| Golden Orange 56200 | SRB, R6GD, R6G, R3B, SY172, FB 184, BP3 |
| Lemon Yellow 56150 | SY172, FB 184, BP3 |
| Green 56100 | SY 172, FB 61 |
| Blue 56050 | FB 61 |
| Violet 56450 | SRB, R6GD, R3B, FB 61, BP3, unknown (1) |
| White 56000 | FB 184 |
| Spectroscopy | Excitation Source, Geometry | Capture and Geometry | Integration Time, Scans | Data Collection, Interpretation |
|---|---|---|---|---|
| Method 1: FORS: Full-spectrum-induced reflectance | Ocean Insights HL-2000-HP-FSHA tungsten halogen light source Vis–NIR (400–2400 nm) fiber optic cable, 90-degree angle | Ocean Optics SD-2000 Fiber Optic Spectrometer using a Vis–NIR fiber optic cable, 45-degree angle | 1.5 ms/scan, 50 scans | Ocean Optics Oceanview software, Origin software, ColorCaculator |
| Method 2: Fiber optic UV-induced fluorescence emission | Ocean Insight LSM power source 365 nm LED UV–Vis–NIR (360–2400 nm) fiber optic with BG-38 filter, 90-degree angle, run between 2 mA and 5 mA | Ocean Optics SD-2000 Fiber Optic Spectrometer using a Vis–NIR fiber optic cable, 45-degree angle | 1.5 ms/scan, 50 scans | Ocean Optics Oceanview software, Origin software, ColorCalculator |
| Technique | Camera | Lens | Illumination/ Excitation Source | Filters on Lens |
|---|---|---|---|---|
| NORM | Nikon D810, modified UV–Vis–IR DSLR camera | Coastal Optics UV–Vis–IR 60 mm lens (apochromatic lens) | Profoto D1 500 W electronic flash, 25° angle to copy stand surface, one on each side for balanced illumination | X-Nite CC1 |
| UVA–Vis | Nikon D810, modified UV–Vis–IR DSLR camera | Coastal Optics UV–Vis–IR 60 mm apochromatic lens | UV Systems SuperBright UVA, peak 368 nm, 45° angle to copy stand surface, one on each side for balanced illumination | Kodak 2E + PECA 918 + X-Nite CC1 |
| RUVA | Nikon D810, modified UV–Vis–IR DSLR camera | Coastal Optics UV–Vis–IR 60 mm apochromatic lens | UV Systems SuperBright UVA, peak 368 nm, 45° angle to copy stand surface, one on each side for balanced illumination | B + W 403 and X-Nite CC1 |
| RIR | Nikon D810, modified UV–Vis–IR DSLR camera | Coastal Optics UV–Vis–IR 60 mm apochromatic lens | Profoto D1 500 W electronic flash, 25° angle to copy stand surface, one on each side for balanced illumination | X-Nite 715, X-Nite 850, and X-Nite 1000 |
| IRLUM | Nikon D810, modified UV–Vis–IR DSLR camera | Coastal Optics UV–Vis–IR 60 mm apochromatic lens | 1 Powersmith 50 W LED + Schott BG38 filter (6 mm-thick), axial position to subject | X-Nite 715, X-Nite 850, and X-Nite 1000 |
| Fluorescent Colorant | Maximum Intensity in a.u. (Unaged, Aged) | Percent Difference (%) | Highest Peak (nm) (Unaged, Aged) | Total Shift in nm |
|---|---|---|---|---|
| Brick Red 56300 | 170 | −13.53 | 619 | −9 |
| 147 | 608 | |||
| Flame Red 56350 | 174 | −17.24 | 613 | −9 |
| 144 | 604 | |||
| Orange 56250 | 188 | −19.68 | 608 | −10 |
| 151 | 598 | |||
| Golden Orange 56200 | 182 | −19.78 | 596 | −11 |
| 146 | 585 | |||
| Lemon Yellow 56150 | 159 | −13.84 | 524 | −11 |
| 137 | 513 | |||
| Green 56100 | 121 | −11.57 | 517 | −3 |
| 107 | 514 | |||
| Blue 56050 | 83 | −31.33 | 454 | 14 |
| 57 | 468 | |||
| Violet 56450 | 87 | 13.89 | 652 | −40 |
| 123 | 612 | |||
| White 56000 | 123 | 0 | 443 | 0 |
| 123 | 443 |
| Fluorescent Colorant (λexc. = 365 nm) | Maximum Intensity in a.u. (Unaged, Aged) | Percent Difference (%) | ~Highest Peak (nm) (Unaged, Aged) | Total Shift in nm |
|---|---|---|---|---|
| Brick Red 56300 | 2850 | −42.95 | 615 | −8 |
| 1626 | 607 | |||
| Flame Red 56350 | 2592 | −50.81 | 608 | −9 |
| 1275 | 599 | |||
| Orange 56250 | 3049 | −22.20 | 604 | −13 |
| 2372 | 591 | |||
| Golden Orange 56200 | 3788 | −11.77 | 591 | −18 |
| 3342 | 573 | |||
| Lemon Yellow 56150 Aged (3 mA *) | 3620 | −19.17 | 520 | −19 |
| 2926 | 501 | |||
| Green 56100 | 806 | 147.02 | 516 | −5 |
| 1991 | 511 | |||
| Blue 56050 aged (3 mA *) | 3819 | −97.28 | 453 | −10 |
| 104 | 443 | |||
| Violet 56450 | 242 | 74.79 | 636 | −28 |
| 423 | 608 | |||
| White 56000 aged (2 mA *) | 3832 | 0 | 441 | 0 |
| 3832 | 441 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beckett, F.; Shugar, A. Following the Light: Use of Multimodal Imaging and Fiber Optic Spectroscopy to Evaluate Aging in Daylight Fluorescent Artists’ Pigments. Colorants 2022, 1, 208-225. https://doi.org/10.3390/colorants1020013
Beckett F, Shugar A. Following the Light: Use of Multimodal Imaging and Fiber Optic Spectroscopy to Evaluate Aging in Daylight Fluorescent Artists’ Pigments. Colorants. 2022; 1(2):208-225. https://doi.org/10.3390/colorants1020013
Chicago/Turabian StyleBeckett, Fiona, and Aaron Shugar. 2022. "Following the Light: Use of Multimodal Imaging and Fiber Optic Spectroscopy to Evaluate Aging in Daylight Fluorescent Artists’ Pigments" Colorants 1, no. 2: 208-225. https://doi.org/10.3390/colorants1020013






