Abstract
Tb4+-doped sodium zirconate samples, Na2Zr1−xTbxO3, were synthesized as novel environmentally friendly inorganic yellow pigments by a conventional solid-state reaction method. Their crystal structures, optical properties, and colors were characterized. A single-phase form was obtained for the samples in the x range of x ≤ 0.18, while impurity phases were detected for the sample with x = 0.20. All samples showed strong optical absorption in the blue light region, due to the charge transfer transition between O2− and Tb4+. As a result, the sample color became yellow, which is the complementary color of blue, and the color became more vivid with increasing Tb4+ content in the single-phase region. Among the samples, Na2Zr0.82Tb0.18O3 was the optimal composition, with the highest yellowness (b* = +67.2) and pure yellow hue (h° = 90.1). Although the b* value was lower than commercial yellow pigments such as BiVO4 and ZrSiO4:Pr, this sample had a purer yellow hue. Since Na2Zr0.82Tb0.18O3 is composed of non-toxic elements, it could be a new environmentally friendly inorganic yellow pigment.
1. Introduction
Inorganic pigments have been widely applied to products such as inks, paints, ceramics, and so on. Particularly, yellow pigments are used in paints for warning signboards and traffic road markers owing to their high visibility []. Chrome yellow (PbCrO4), cadmium yellow (CdS), and nickel–titanium yellow (TiO2-NiO-Sb2O3) pigments have been used as industrial yellow pigments. However, these pigments contain elements (e.g., Pb, Cr, Cd, and Sb) that are toxic to humans and the environment. The use of these existing pigments has so far been prohibited or restricted, and there has been a need to develop novel yellow pigments. In light of this situation, a number of studies have been reported by several researchers [,,,,,,,,,,,]. However, there are few high-performance inorganic yellow pigments that are environmentally friendly, demonstrate sufficient chemical/thermal stability, and comparable to the conventional products. In addition, oxide materials that are highly stable and can be synthesized in processes that do not use toxic gases are desired to provide more sustainable and environmentally friendly materials.
In view of this situation, we focused on Tb4+ as a yellow coloring source and proposed a new concept in the development of yellow pigments. Some compounds such as A(Sn, Tb)O3 (A = Sr, Ba) [,,,], A2(B, Tb)O4 (A = Sr, Ba; B = Zr, Sn, Ce) [,,], and Bi3(Y, Tb)O6+δ [] have been proposed as environmentally friendly yellow pigments. These compounds absorb visible light in the blue light region due to the charge transfer (CT) transition between O2− and Tb4+ and exhibit a yellow color. In most cases of these materials, the Tb4+ ions are introduced into the octahedral [MO6] (M = metal) sites. Therefore, when Tb4+ ions are doped into [MO6] octahedra, a yellow color will be obtained.
In this study, we selected sodium zirconate, Na2ZrO3, as a host material. This compound is composed of only non-toxic and cost-effective elements. A Na2ZrO3 structure includes octahedral [ZrO6] sites [,,,] and the valence of Zr in this structure is tetravalent. The valence of Zr4+ is equivalent to Tb4+ and the ionic radius of Zr4+ (0.072 nm []) is close to that of Tb4+ (0.076 nm []) in size. For these reasons, we thought Tb4+ ions could be introduced into the octahedral Zr4+ site to exhibit a yellow color. Therefore, in this study, Tb4+-doped Na2ZrO3 samples were synthesized, and their optical and color properties were characterized as environmentally friendly inorganic yellow pigments. Considering the viewpoint of practical use, the sample color was compared with commercial inorganic yellow pigments, and the chemical stability was also evaluated.
2. Materials and Methods
2.1. Synthesis
The Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) samples were synthesized via a solid-state reaction method. Stoichiometric amounts of ZrO2 (FUJIFILM Wako Pure Chemical, 98.0%, Fujifilm, Tokyo, Japan) and Tb4O7 (FUJIFILM Wako Pure Chemical, 99.9%) and 1.6 times the stoichiometric amounts of Na2CO3 (Kanto Chemical, Tokyo, Japan, 99.5%) were mixed in an agate mortar for 30 min to obtain 2 g of the final product. The mixtures were calcined in an alumina crucible at 1200 °C for 5 h in air. Finally, the samples were ground in an agate mortar before characterization.
2.2. Characterization
The crystal phase and structure were identified by X-ray powder diffraction (XRD; Ultima IV, Rigaku, Tokyo, Japan). The XRD patterns were captured with Cu-Kα radiation operating at 40 kV and 40 mA. The data were collected by scanning over the 2θ range of 20–80°. The sampling width was 0.02° and the scan speed was 6° min−1. The lattice volumes were calculated from the XRD peak angles refined by α-Al2O3 as a standard and using the CellCalc Ver. 2.20 software. The morphologies were observed by using field-emission-type scanning electron microscopy (FE-SEM; JEOL, Tokyo, Japan, JSM-6701F).
To investigate optical properties of the powder samples, the optical reflectance spectra were recorded on an ultraviolet–visible–near-infrared (UV–Vis-NIR) spectrometer (JASCO, Tokyo, Japan, V-770 with an integrating sphere attachment) using a standard white plate as a reference. The step width was 1 nm and the scan rate was 1000 nm min−1. The color properties of the powder samples were evaluated in terms of the Commission Internationale de l’Éclairage (CIE) L*a*b*Ch° system, using a colorimeter (Konica-Minolta, Tokyo, Japan, CR-300). The L* parameter indicates the brightness or darkness in a neutral grayscale. The a* and b* values represent the red–green and yellow–blue axes, respectively. The chroma parameter (C) denotes the color saturation and is calculated with the formula, C = [(a*)2 + (b*)2]1/2. The hue angle (h°) ranges from 0 to 360° and is estimated according to the following formula: h° = arctan(b*/a*). The standard deviations of all values for the L*a*b*Ch° color coordinate data were less than 0.1.
3. Results and Discussion
3.1. X-ray Powder Diffraction (XRD) and Scanning Electron Microscopy (SEM)
Figure 1 shows the XRD patterns of the Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) samples. In the x range from 0 to 0.18, the Na2ZrO3 phase was obtained in a single-phase form. For x = 0.20, on the other hand, the target phase was observed as the primary phase, but additional impurity peaks indexed to ZrO2 and Tb11O20 were also detected.
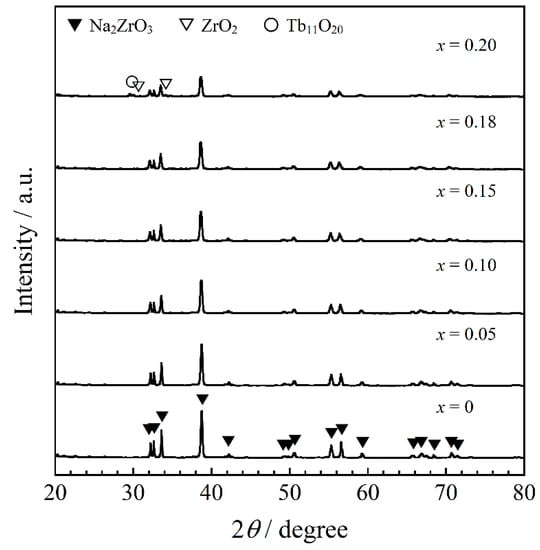
Figure 1.
XRD patterns of the Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) samples.
The crystal structure of Na2ZrO3 belongs to a monoclinic system with a symmetry of the C2/c (no. 15) space group. In the Na2ZrO3 structure, both Na+ and Zr4+ coordination numbers are six and there are [NaO6] and [ZrO6] octahedra in the lattice []. The composition dependence of the lattice volume of the samples calculated from each XRD pattern is shown in Figure 2. The lattice volume was found to increase linearly with increasing Tb4+ content in the x ≤ 0.18 range by introducing larger Tb4+ (ionic radius: 0.076 nm []) ions into the Zr4+ (ionic radius: 0.072 nm []) sites. The cell volume at x = 0.20 was almost equal to that at x = 0.18. Therefore, solid solutions of Na2Zr1−xTbxO3 were successfully synthesized in the region of x = 0 to 0.18.
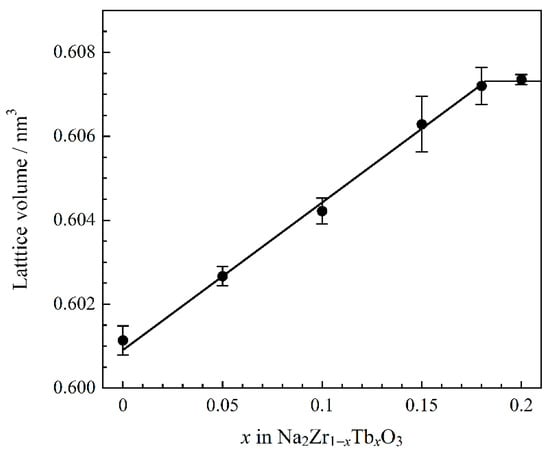
Figure 2.
Compositional dependence of the lattice volume for the Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) samples.
Figure 3 shows the SEM images of the Na2Zr1−xTbxO3 (x = 0 and 0.18) samples obtained in a single-phase form. The size of the primary particles in both samples was approximately 5 µm. The primary particles were thermochemically fused to form coarse secondary particles, due to the high calcination temperature of 1200 °C.
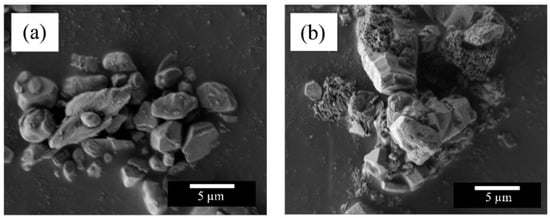
Figure 3.
SEM images of the Na2Zr1−xTbxO3 (x = 0 and 0.18) samples; (a) x = 0, (b) x = 0.18.
3.2. Ultraviolet-Visible (UV-Vis) Reflectance Spectra
The UV–Vis reflectance spectra of Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) are depicted in Figure 4. The host material exhibited high reflectance in the visible region and was white in color. Except for the Na2Zr0.80Tb0.20O3 (x = 0.20) sample, the Tb4+-doped samples strongly absorbed the blue light at wavelengths between 435 and 480 nm, and this optical absorption was attributed to the charge transfer (CT) transition between O2− and Tb4+ [,]. Since blue is the complementary color of yellow, these samples are yellow. On the other hand, the sample with x = 0.20 absorbed visible light in the longer wavelength region. The impurity phase, Tb11O20, detected in Figure 1 contains both Tb3+ and Tb4+. This compound absorbs visible light through charge transfer transitions between metal ions with different valence []. On the other hand, the other impurity, ZrO2, is white in color and generally shows no optical absorption in the visible light region. Therefore, the additional optical absorption observed in the sample with x = 0.20 was attributed to charge transfer transitions between Tb3+ and Tb4+.
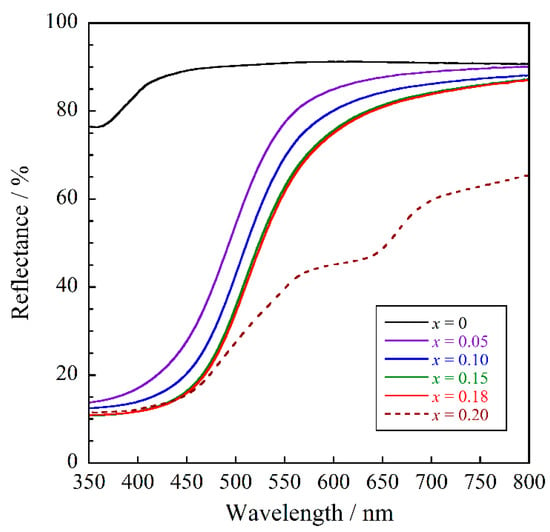
Figure 4.
UV–Vis reflectance spectra for the Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) samples.
As can be seen from Figure 4, the optical absorption intensity corresponding to the O2−–Tb4+ CT transition increased with increasing Tb4+ concentration. Among the samples with x ranging from 0 to 0.18 (solubility limit), the Na2Zr0.82Tb0.18O3 (x = 0.18) sample showed the highest absorption intensity.
3.3. Color Properties
The L*a*b*Ch° color parameters of the Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) samples and commercially available yellow BiVO4 (Dainichiseika Color & Chemicals Mfg., Tokyo, Japan), PbCrO4 (NIC, Osaka, Japan) and ZrSiO4:Pr (Kawamura Chemical, Yokkaichishi, Japan) pigments are summarized in Table 1. The photographs are also displayed in Figure 5. The host Na2ZrO3 sample had a high L* value (brightness), and both a* (redness) and b* (yellowness) values were almost equal to zero. This sample exhibited a white color.

Table 1.
Color coordinate data of the Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) samples and the commercially available yellow BiVO4, PbCrO4, and ZrSiO4:Pr pigments.
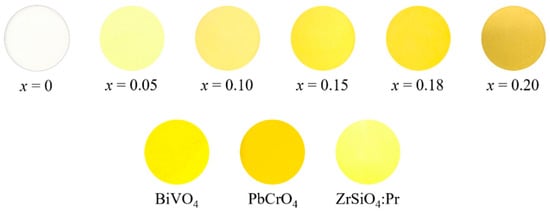
Figure 5.
Photographs of the Na2Zr1−xTbxO3 (0 ≤ x ≤ 0.20) samples and the commercial BiVO4, PbCrO4, and ZrSiO4 pigments.
When Zr4+ was partially replaced by Tb4+, the b* value increased dramatically due to the strong absorption of blue light, as shown in Figure 4. In addition, the h° parameter (hue angle) fell into the yellow region of 70 ≤ h° ≤ 105. As a result, the Tb4+-doped samples turned yellow. Furthermore, the color of the samples changed from pale to bright yellow as the absorption intensity in the blue light region increased with increasing Tb4+ content. Among the samples synthesized in this study, the highest b* and C (chroma) values were obtained for the Na2Zr0.82Tb0.18O3 sample, which exhibited the brightest yellow color.
The color coordinate data of the Na2Zr0.82Tb0.18O3 sample in this study were compared with those of the commercially available yellow pigments, as shown in Table 1 and Figure 5. The yellowness (b*) of this sample was slightly lower than that of the environmentally friendly ZrSiO4:Pr pigment. However, the h° value of this sample was closer to 90° (pure yellow) than the commercially available BiVO4 and ZrSiO4:Pr pigments. Because the standard deviations of all values for the L*a*b*Ch° color coordinate data were less than 0.1, there was a significance difference between the Na2Zr0.82Tb0.18O3 pigment and the commercial ones, and we concluded this pigment had a purer yellow color than them.
3.4. Chemical Stability Test
The chemical stability of the Na2Zr0.82Tb0.18O3 sample was evaluated using the powder sample. The powder samples were soaked in deionized water, 4% CH3COOH, and 4% NH4HCO3 aqueous solutions. The samples were left at room temperature for 5 h (for deionized water) and 2 h (for acid and base) and then washed with deionized water and ethanol. The samples were then dried at room temperature. The color coordinate data of the samples after the chemical stability tests are summarized in Table 2, and their photographs are also displayed in Figure 6. Unfortunately, the chemical stability of the Na2Zr0.82Tb0.18O3 sample was insufficient, and each treatment resulted in degradation of the color tone. Therefore, surface coating with a stable compound such as silica is considered necessary for the application of this sample to inorganic pigments. Silica is considered suitable as a coating material because it is a cost-effective, stable, and green compound. If the pigment in this study was coated with silica, we expect it would be a more environmentally friendly yellow pigment than uncoated pigments.

Table 2.
Color coordinate data of the Na2Zr0.82Tb0.18O3 sample before and after the chemical stability test.
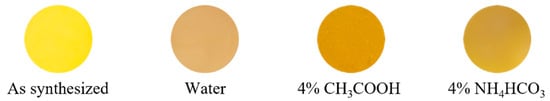
Figure 6.
Photographs of the Na2Zr0.82Tb0.18O3 sample before and after the chemical stability test.
4. Conclusions
Tb4+-doped sodium zirconate samples, Na2Zr1−xTbxO3, were synthesized as environmentally benign inorganic yellow pigments by a solid-state reaction method. In the x range from 0 to 0.18, the samples were obtained in a single-phase form and the lattice volume increased linearly. These results indicate that the solid solutions were successfully obtained in this x region. The Tb4+-doped samples exhibited optical absorption in the blue light region due to the charge transfer transition between O2− and Tb4+. The intensity of this optical absorption increased with increasing Tb4+ content. As a result, the color of the samples changed from pale to bright yellow. Among the samples synthesized, the Na2Zr0.82Tb0.18O3 sample showed the highest yellowness. The yellowness of this sample was inferior to those of commercially available yellow pigments. However, the yellow color purity of this sample was better than those of the existing environmentally friendly types. In addition, no toxic solvents or gases are required or generated in the synthesis of this pigment. Therefore, although the chemical stability of the present sample is not sufficient, it may become a new environmentally friendly inorganic yellow pigment by coating with a stable compound.
Author Contributions
Conceptualization, R.O. and T.M.; methodology, R.O., T.N. and T.M.; validation, R.O. and T.M.; investigation, R.O. and T.N.; data curation, R.O. and T.N.; writing—original draft preparation, R.O.; writing—review and editing, T.M.; supervision, T.M.; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant numbers JP22K04698 and JP20H02439.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Faulkner, E.B.; Schwartz, R.J. High Performance Pigments, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Thara, T.R.A.; Rao, P.P.; Raj, A.K.V.; Sreena, T.S. New series of brilliant yellow colorants in rare earth doped scheelite type oxides, (LiRE)1/2WO4- BiVO4 for cool roof applications. Sol. Energy Mater. Sol. Cells 2019, 200, 110015. [Google Scholar] [CrossRef]
- Kumari, L.S.; Rao, P.P.; Radhakrishnan, A.N.P.; James, V. Brilliant yellow color and enhanced NIR reflectance of monoclinic BiVO4 through distortion in VO43− tetrahedra. Sol. Energy Mater. Sol. Cells 2013, 112, 134–143. [Google Scholar] [CrossRef]
- Wendusu; Honda, T.; Masui, T.; Imanaka, N. Novel environmentally friendly (Bi, Ca, Zn, La)VO4 inorganic yellow pigments. RSC Adv. 2013, 3, 24941–24945. [Google Scholar] [CrossRef]
- Šulcová, P.; Trojan, M. Thermal synthesis and properties of the (Bi2O3)1–x(Ho2O3)x pigments. J. Therm. Anal. Calorim. 2006, 83, 557–559. [Google Scholar] [CrossRef]
- Laguna, M.; Núñez, N.O.; Fernández, M.; Ocaña, M. Synthesis and optical properties of environmentally benign and highly uniform NaCe(MoO4)2 based yellow nanopigments. J. Alloys Compd. 2018, 739, 542–548. [Google Scholar] [CrossRef]
- Nero, G.D.; Cappelletti, G.; Ardizzone, S.; Fermo, P.; Gilardoni, S. Yellow Pr-zircon pigments: The role of praseodymium and of the mineralizer. J. Eur. Ceram. Soc. 2004, 24, 3603–3611. [Google Scholar] [CrossRef]
- Guo, D.; Yang, Q.; Chen, P.; Chu, Y.; Zhang, Y.; Rao, P. The influence of micronization on the properties of Pr-ZrSiO4 pigment. Dyes Pigm. 2018, 153, 74–83. [Google Scholar] [CrossRef]
- Sumaletha, N.; Rajesh, K.; Mukundan, P.; Warrier, K.G.K. Environmentally benign sol–gel derived nanocrystalline rod shaped calcium doped cerium phosphate yellow-green pigment. J. Sol-Gel Sci. Technol. 2009, 52, 242–250. [Google Scholar] [CrossRef]
- Sameera, S.F.; Rao, P.P.; Kumari, L.S.; Koshy, P. New scheelite-based environmentally friendly yellow pigments: (BiV)x(CaW)1−xO4. Chem. Lett. 2009, 38, 1088–1089. [Google Scholar] [CrossRef]
- Calatayud, J.M.; Alarcón, J. V-containing ZrO2 inorganic yellow nano-pigments prepared by hydrothermal approach. Dyes Pigm. 2017, 146, 178–188. [Google Scholar] [CrossRef]
- Gorodylova, N.; Šulcová, P. Polymerizable precursor method vs solid state reaction for the synthesis of Ni3(PO4)2 yellow hue pigment with advanced characteristics. J. Alloys Compd. 2022, 903, 163854. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, J.; Liu, Z.; Wang, L.; Chen, L.; Zhuo, S.; Liu, Y.; Chen, W. High near-infrared reflective Zn1−xAxWO4 pigments with various hues facilely fabricated by tuning doped transition metal ions (A = Co, Mn, and Fe). Inorg. Chem. 2022, 61, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Pettini, F.; Seguelong, T.; Inventors; Chimie, R.; Assignee. Pigmented substrates comprising terbium compound colorants. U.S. Patent 5688316, 18th November 1997. [Google Scholar]
- Mesíková, Ž.; Šulcová, P.; Trojan, M. Synthesis and description of SrSn0.6Ln0.4O3 perovskite pigments. J. Therm. Anal. Calorim. 2008, 91, 163–166. [Google Scholar] [CrossRef]
- Lunáková, P.; Trojan, M.; Luxová, J.; Trojan, J. BaSn1−xTbxO3: A new yellow pigment based on a perovskite structure. Dyes Pigm. 2013, 96, 264–268. [Google Scholar] [CrossRef]
- Oka, R.; Tsukimori, T.; Inoue, H.; Mausi, T. Perovskite-type ALnO3 (A = Ca, Sr, Ba; Ln = Ce, Pr, Tb) oxides as environmentally friendly yellow pigments. J. Ceram. Soc. Jpn. 2017, 125, 652–656. [Google Scholar] [CrossRef] [Green Version]
- Raj, A.K.V.; Rao, P.P.; Sameera, S.; James, V.; Divya, S. Synthesis of novel nontoxic yellow pigments: Sr2Ce1−xTbxO4. Chem. Lett. 2014, 43, 985–987. [Google Scholar] [CrossRef]
- Raj, A.K.V.; Rao, P.P.; Divya, S.; Ajuthara, T.R. Terbium doped Sr2MO4 [M = Sn and Zr] yellow pigments with high infrared reflectance for energy saving applications. Powder Technol. 2017, 311, 52–58. [Google Scholar] [CrossRef]
- Raj, A.K.V.; Rao, P.P.; Sreena, T.S. Color tunable pigments with high NIR reflectance in terbium-doped cerate systems for sustainable energy saving applications. ACS Sustain. Chem. Eng. 2019, 7, 8804–8815. [Google Scholar] [CrossRef]
- Chen, J.; Xie, W.; Guo, X.; Huang, B.; Xiao, Y.; Sun, X. Near infrared reflective pigments based on Bi3YO6 for heat insulation. Ceram. Int. 2020, 46, 24575–24584. [Google Scholar] [CrossRef]
- Bastow, T.J.; Hobday, M.E.; Smith, M.E.; Whitfield, H.J. Structural characterisation of Na2ZrO3. Solid State Nucl. Magn. Reson. 1994, 3, 49–57. [Google Scholar] [CrossRef]
- Alcántar-Vázquez, B.; Diaz, C.; Romero-Ibarra, I.C.; Lima, E.; Pfeiffer, H. Structural and CO2 chemisorption analyses on Na2(Zr1−xAlx)O3 solid solutions. J. Phys. Chem. C 2013, 117, 16483–16491. [Google Scholar] [CrossRef]
- Song, S.; Kotobuki, M.; Zheng, F.; Xu, C.; Hu, N.; Lu, L.; Wang, Y.; Li, W.-D. Y-doped Na2ZrO3: A Na-rich layered oxide as a high-capacity cathode material for sodium-ion batteries. ACS Sustain. Chem. Eng. 2017, 5, 4785–4792. [Google Scholar] [CrossRef]
- Duan, Y.; Lekse, J.; Wang, X.; Li, B.; Alcántar-Vázquez, B.; Pfeiffer, H.; Halley, J.W. Electronic structure, phonon dynamical properties, and CO2 capture capability of Na2−xMxZrO3 (M = Li,K): Density-functional calculations and experimental validations. Phys. Rev. Appl. 2015, 3, 044013. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Found. Adv. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Verma, R.K.; Kumar, K.; Rai, S.B. Inter-conversion of Tb3+ and Tb4+ states and its fluorescence properties in MO–Al2O3: Tb (M = Mg, Ca, Sr, Ba) phosphor materials. Solid State Sci. 2010, 12, 1146–1151. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).