Tiles from Aosta: A Peculiar Glaze Roof Covering
Abstract
1. Introduction
2. Materials and Methods
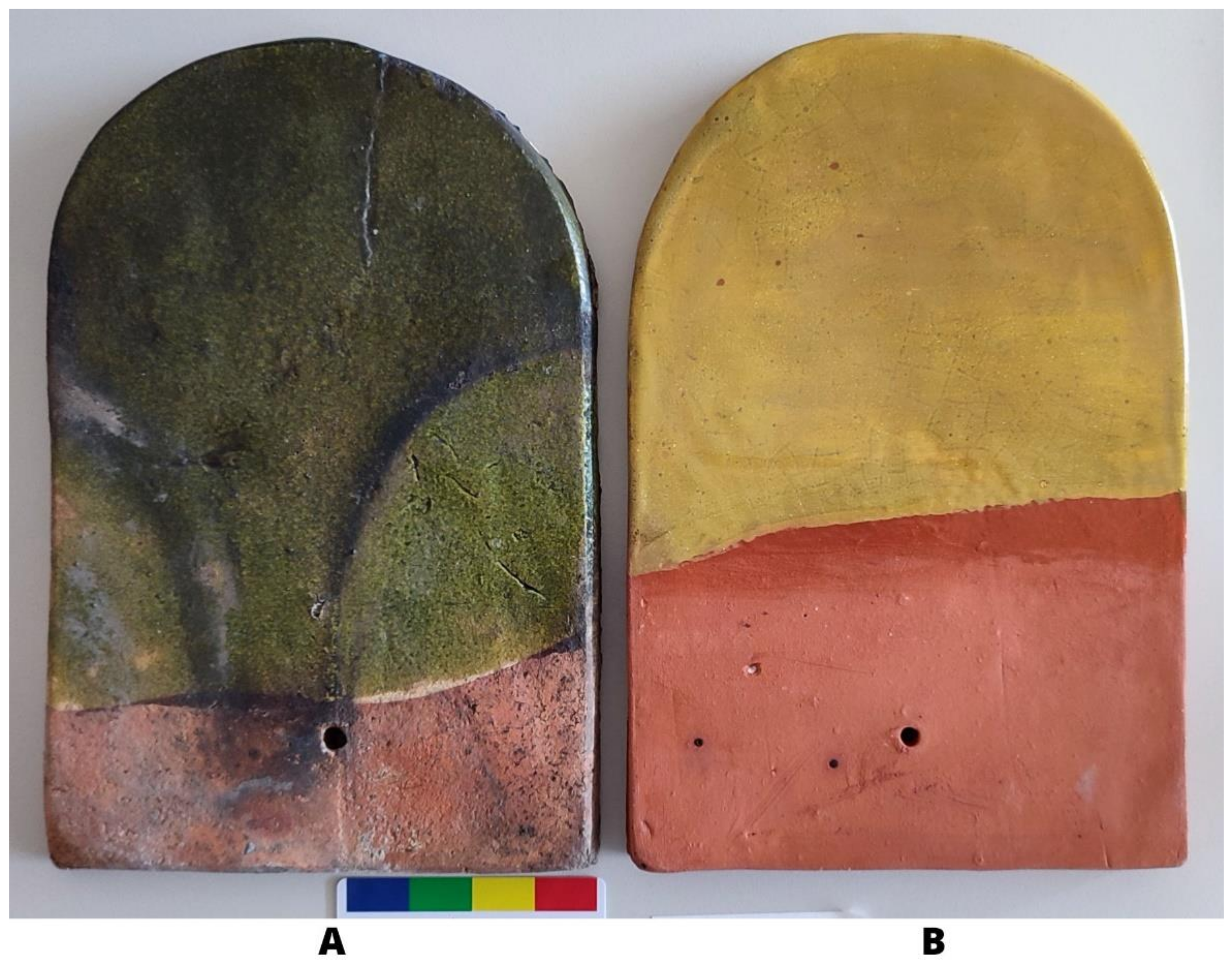
2.1. The Site and the Historical Tiles
2.2. Newly Made Tiles
2.3. Analytic Methodology
2.4. Freeze—Thaw Cycles
3. Results
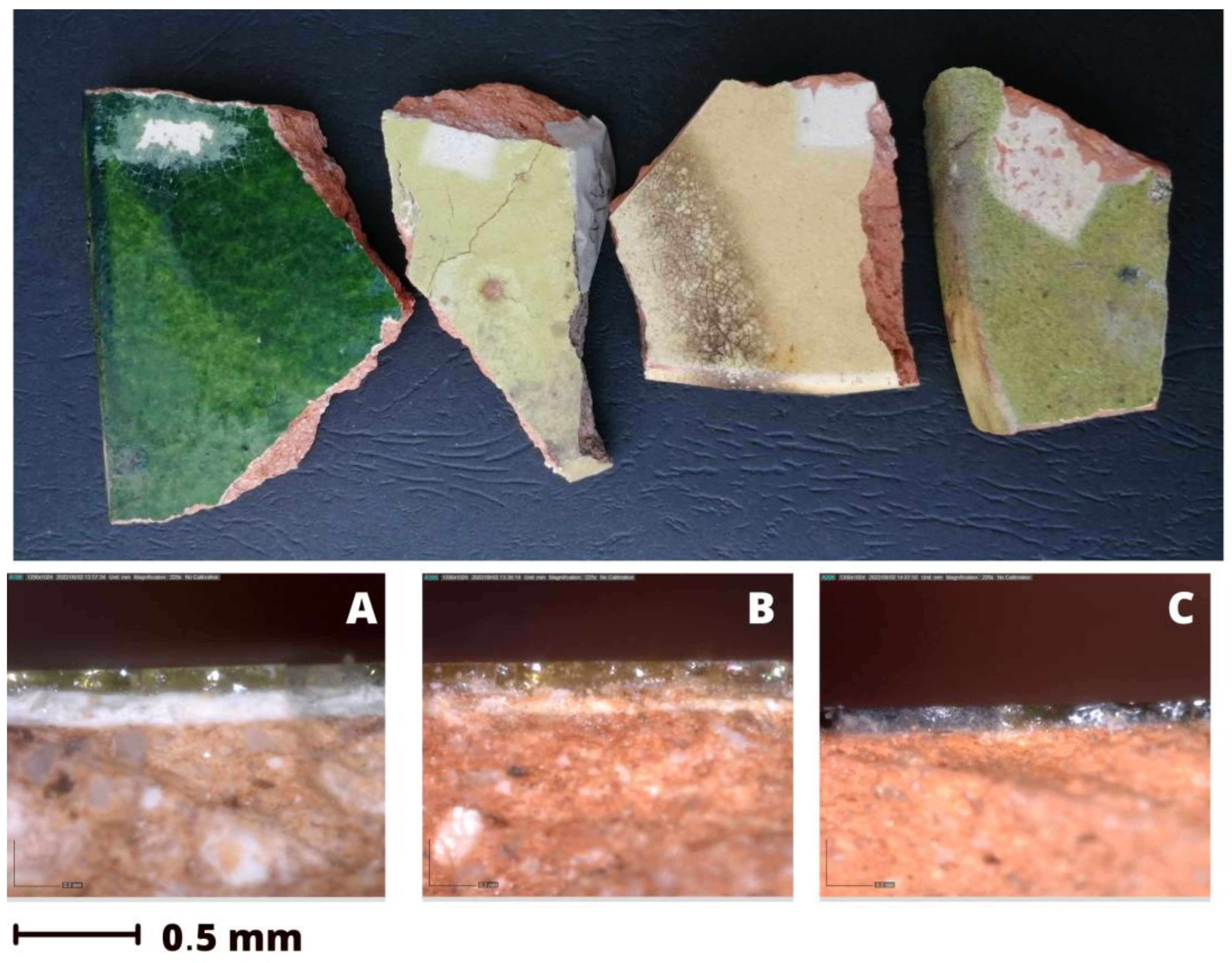
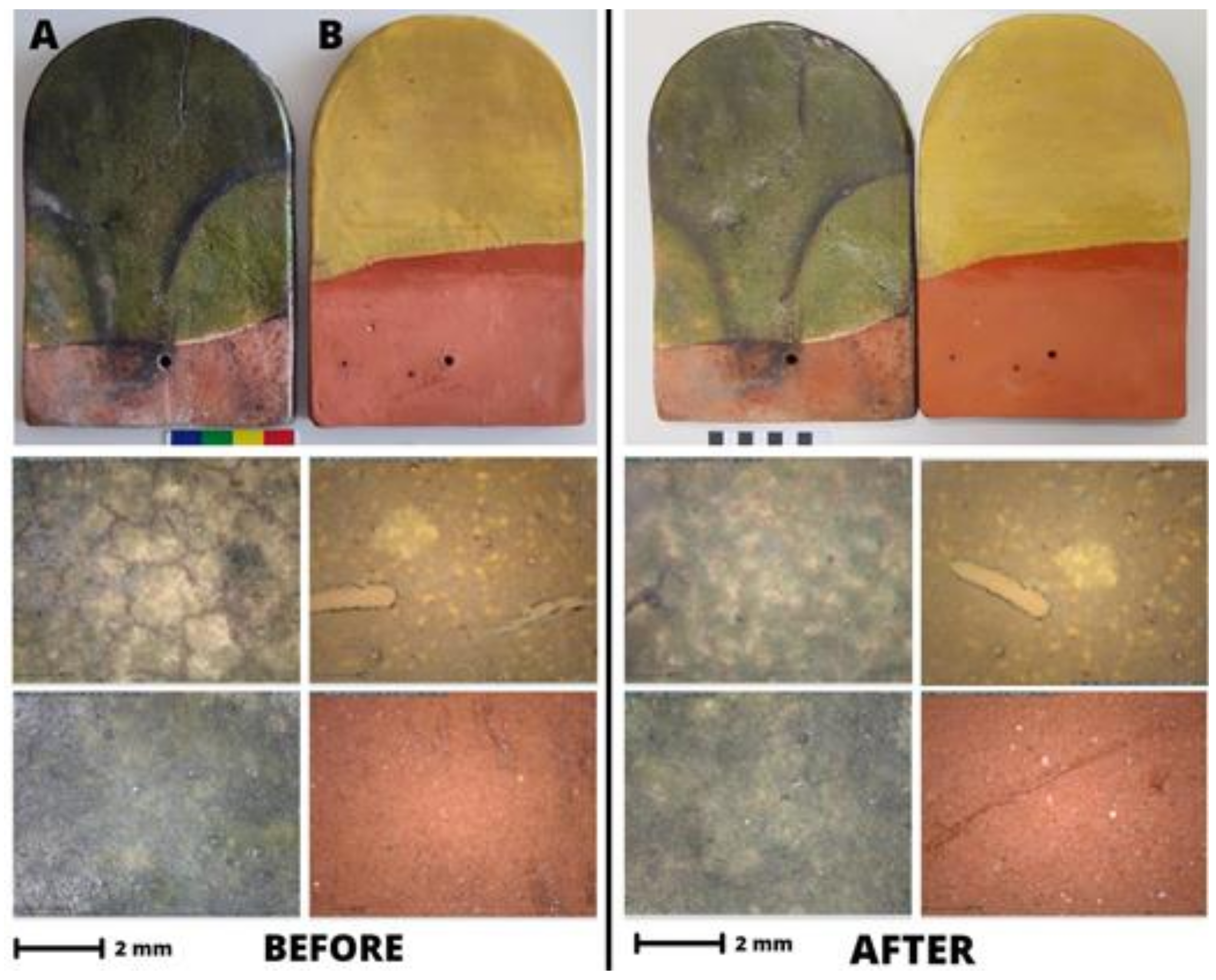
3.1. Optical Microscopy and Macro-Observations
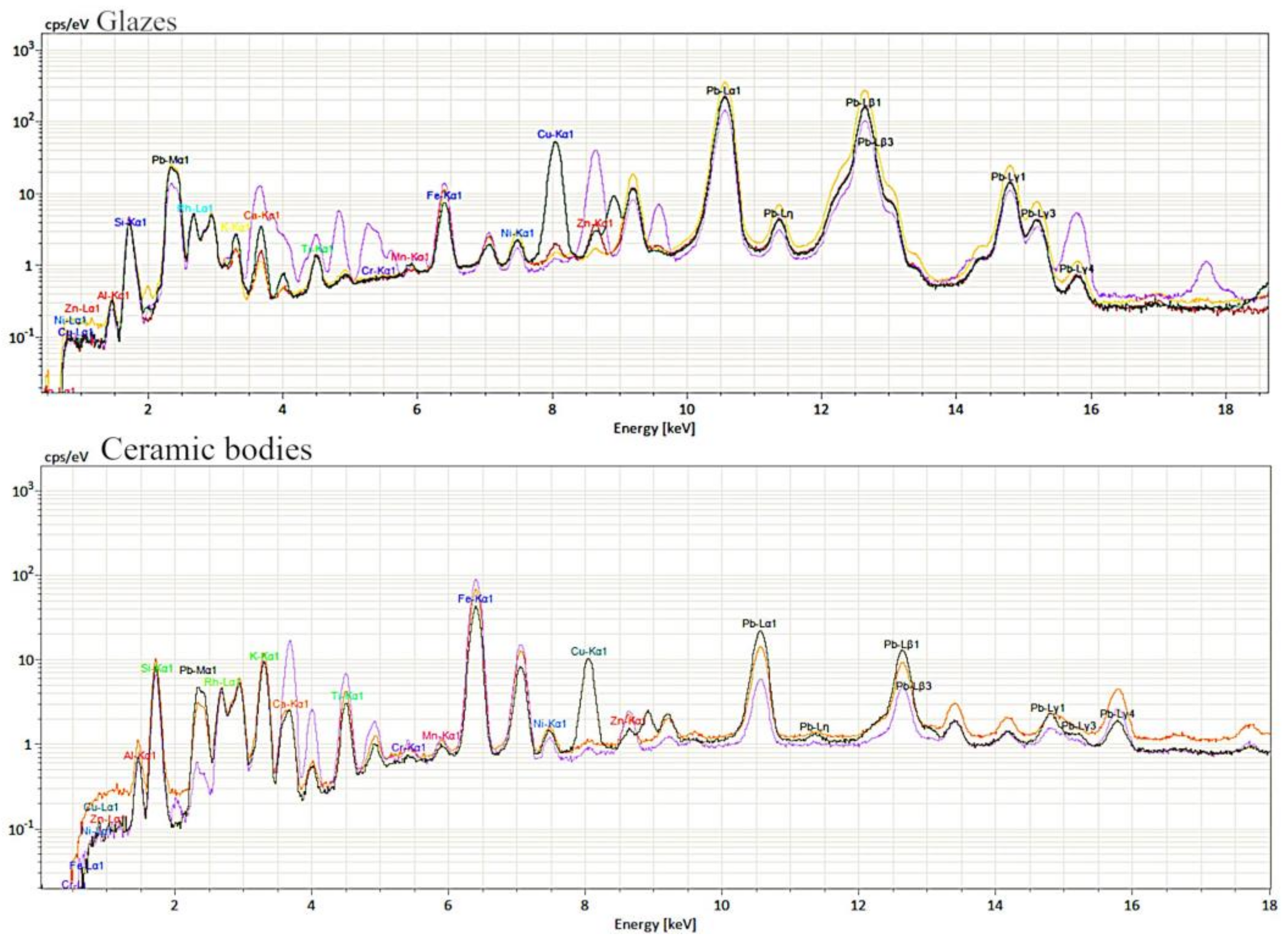
3.2. Elemental Analysis
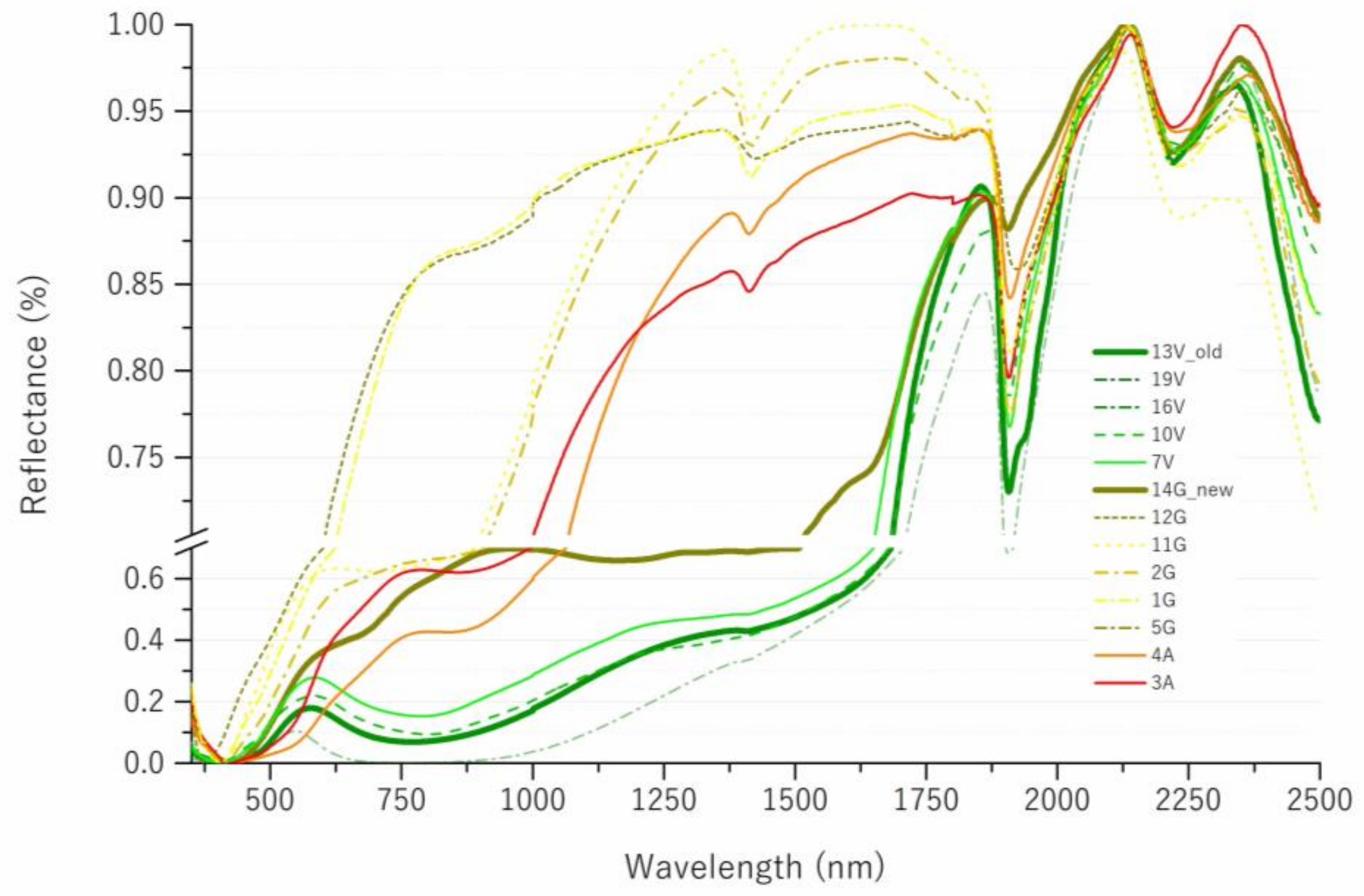
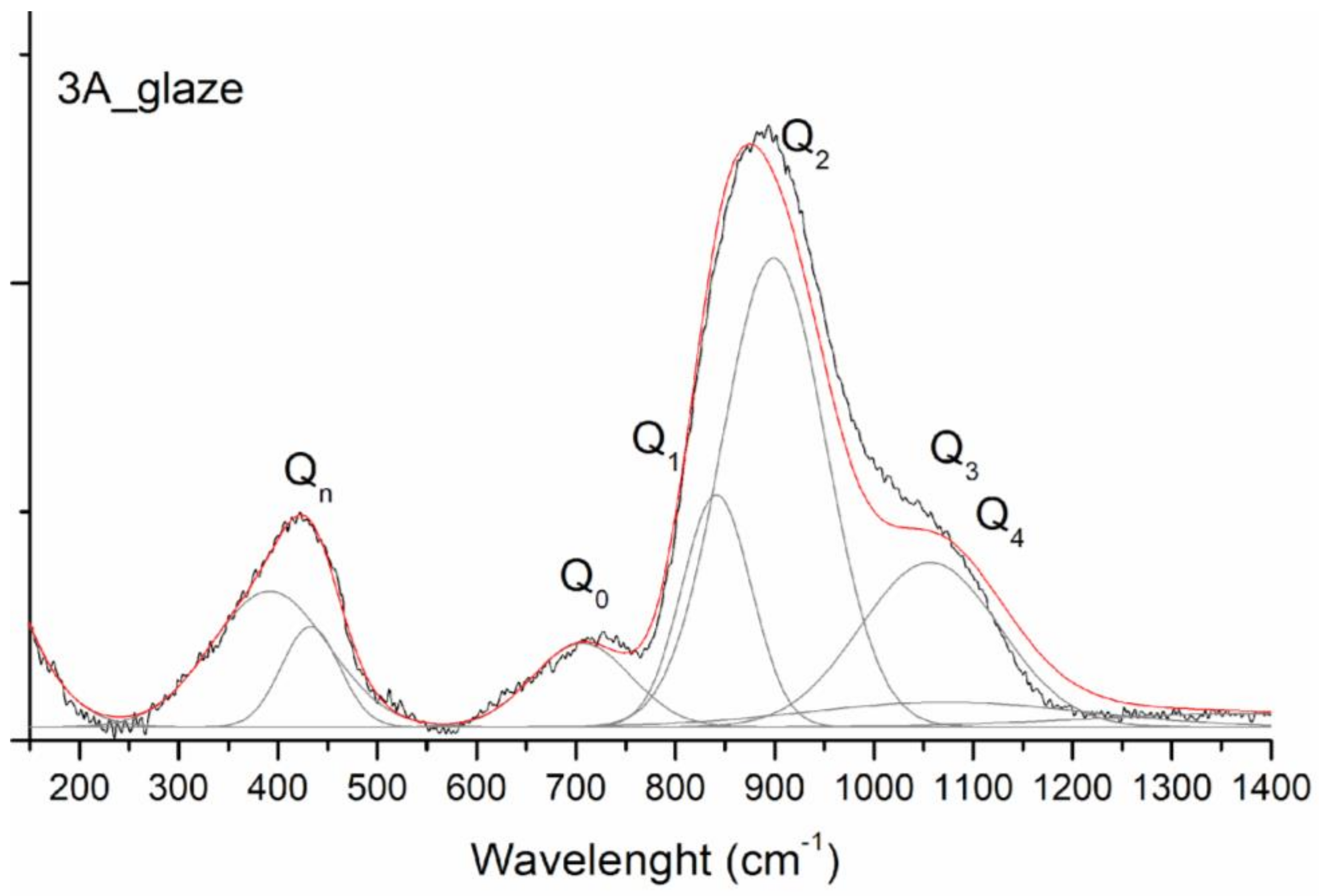
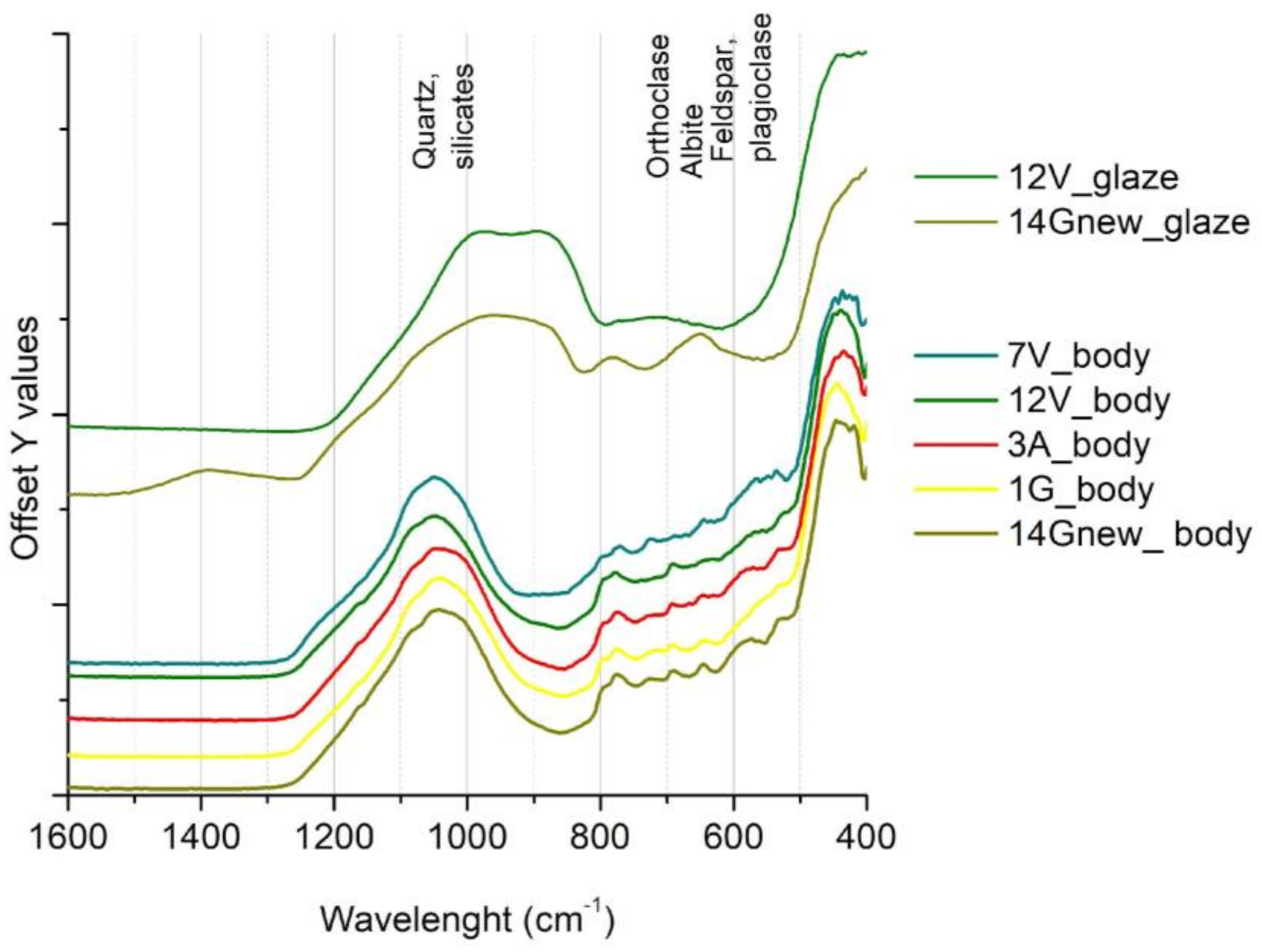
3.3. Spectroscopic Investigation of Old Tiles
3.4. TG-DSC
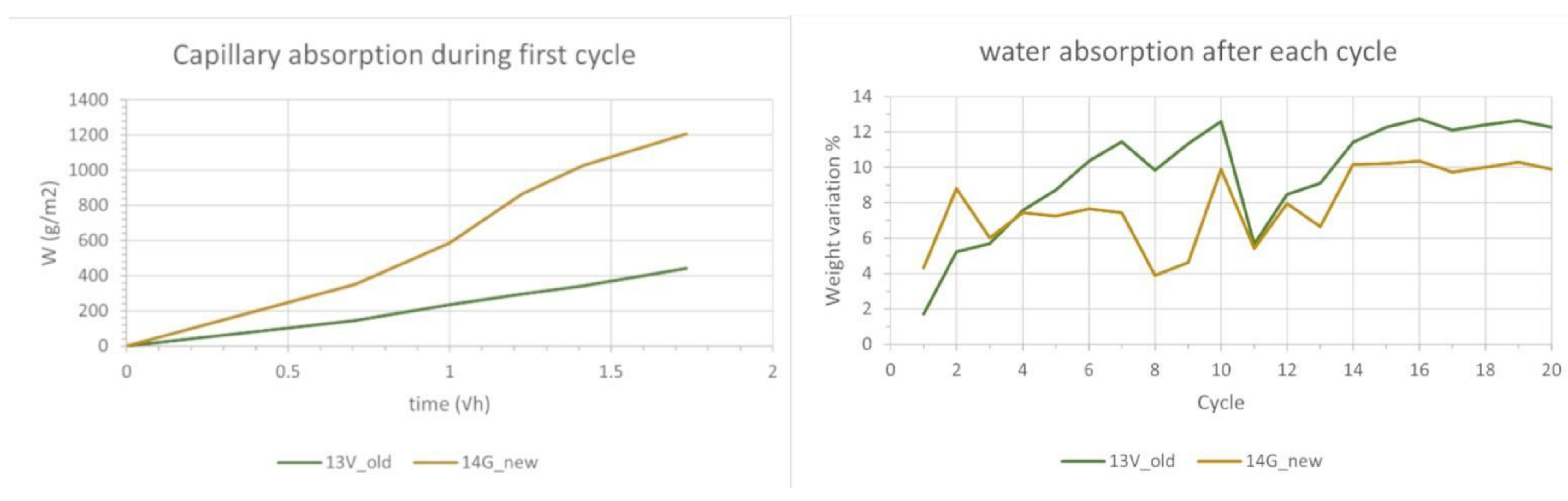
3.5. Freeze-Thaw Cycles
4. Discussion
5. Conclusions
- a common red ceramic body rich in hematite, probably cooked between 800 °C (absence of carbonates) and 950 °C (presence of feldspars);
- a white slip present in most of the tiles and collocated below a coloured final glaze. This white slip was probably added to enhance the final tile colour and was thinner for the lighter yellowish glazes and thicker for the greenish ones;
- a final coloured glaze particularly rich in lead, with optimal adhesion to the ceramic body. The colour of the glaze was modulated, adding different elements: iron in the yellow ones, iron and copper in the green ones.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casasola, R.; Rincón, J.M.; Romero, M. Glass–Ceramic Glazes for Ceramic Tiles: A Review. J. Mater. Sci. 2012, 47, 553–582. [Google Scholar] [CrossRef]
- Ansaldo, M. Storie Dimenticate: Testimonianze di Vita Sociale Nell’antica Valle d’Aosta; Tipografia Valdostana: Aosta, Italy, 2002. [Google Scholar]
- Brayda, R. Tegole Ed Embrici Antichi e Moderni—Memoria Del Socio Ing. R. Brayda. In Atti della Società Degli Ingegneri e Degli Industriali di Torino; Politecnico di Torino: Torino, Italy, 1886; pp. 56–65. Available online: https://digit.biblio.polito.it/173/ (accessed on 1 August 2023).
- Pantò, G. I Centri Produttori di Ceramica in Piemonte (Secoli XVII-XIX); All’Insegna del Giglio: Sesto Fiorentino, Italy, 2002. [Google Scholar]
- Cechi, D. Realization of New Scandole with Traditional Method. Available online: www.fornacepagliero.it (accessed on 25 June 2023).
- Gin, S.; Delaye, J.-M.; Angeli, F.; Schuller, S. Aqueous Alteration of Silicate Glass: State of Knowledge and Perspectives. Npj Mater. Degrad. 2021, 5, 42. [Google Scholar] [CrossRef]
- Ion, R.-M.; Fierascu, R.-C.; Teodorescu, S.; Fierascu, I.; Bunghez, I.-R.; Turcanu-Carutiu, D.; Ion, M.-L. Ceramic Materials Based on Clay Minerals in Cultural Heritage Study. In Clays, Clay Minerals and Ceramic Materials Based on Clay Minerals; InTech: Vienna, Austria, 2016. [Google Scholar]
- Porfido, C.; Rizzo, R.E.; Healy, D.; Spagnuolo, M.; Terzano, R.; Allegretta, I. Investigating the Evolution of Fractures in Clay–Based Ceramics during Repeated Freeze-Thawing Cycles Using X-ray Micro-Computed Tomography and Image Analysis. Mater. Charact. 2021, 177, 111185. [Google Scholar] [CrossRef]
- Colomban, P. Glass, Pottery and Enamelled Objects: Identification of Their Technology and Origin. In Conservation Science: Heritage Materials, 2nd ed.; Garside, P., Richardson, E., Eds.; RSC: Cambridge, UK, 2020; p. 978. [Google Scholar]
- Zhao, J.; Li, W.; Luo, H.; Miao, J. Research on Protection of the Architectural Glazed Ceramics in the Palace Museum, Beijing. J. Cult. Herit. 2010, 11, 279–287. [Google Scholar] [CrossRef]
- Holclajtner-Antunović, I.; Bajuk-Bogdanović, D.; Bikić, V.; Marić-Stojanović, M. Micro-Raman and Infrared Analysis of Medieval Pottery Findings from Braničevo, Serbia. J. Raman Spectrosc. 2012, 43, 1101–1110. [Google Scholar] [CrossRef]
- Raškovska, A.; Minčeva-Šukarova, B.; Grupče, O.; Colomban, P. Characterization of Pottery from Republic of Macedonia II. Raman and Infrared Analyses of Glazed Pottery Finds from Skopsko Kale. J. Raman Spectrosc. 2010, 41, 431–439. [Google Scholar] [CrossRef]
- Glass Trace Elements NIST-614. Available online: https://www.lgcstandards.com/US/en/Glass-Trace-elements/p/NIST-614 (accessed on 4 July 2023).
- Colomban, P.; Sagon, G.; Faurel, X. Differentiation of Antique Ceramics from the Raman Spectra of Their Coloured Glazes and Paintings. J. Raman Spectrosc. 2001, 32, 351–360. [Google Scholar] [CrossRef]
- Colomban, P.; Paulsen, O. Non-Destructive Determination of the Structure and Composition of Glazes by Raman Spectroscopy. J. Am. Ceram. Soc. 2005, 88, 390–395. [Google Scholar] [CrossRef]
- Colomban, P.; Arberet, L.; Kırmızı, B. On-Site Raman Analysis of 17th and 18th Century Limoges Enamels: Implications on the European Cobalt Sources and the Technological Relationship between Limoges and Chinese Enamels. Ceram. Int. 2017, 43, 10158–10165. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Ferreira, D.P.; Conceição, D.S.; Santos, L.F.; Pereira, M.F.C.; Casimiro, T.M.; Ferreira Machado, I. Portuguese Tin-Glazed Earthenware from the 17th Century. Part 2: A Spectroscopic Characterization of Pigments, Glazes and Pastes of the Three Main Production Centers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 149, 285–294. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Conceição, D.S.; Ferreira, D.P.; Santos, L.F.; Casimiro, T.M.; Ferreira Machado, I. Portuguese 16th Century Tiles from Santo António Da Charneca’s Kiln: A Spectroscopic Characterization of Pigments, Glazes and Pastes. J. Raman Spectrosc. 2014, 45, 838–847. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Thermal Analysis as a Method of Characterizing Ancient Ceramic Technologies. Thermochim. Acta 1995, 269–270, 743–753. [Google Scholar] [CrossRef]
- Cantelli, M.; Facchi, A.; Izzo, F.; Zendri, E. Characterization of Etruscan Non-Vascular Ceramic Fragments. In Proceedings of the 2020 IMEKO TC-4 International Conference on Metrology for Archaeology and Cultural Heritage, Trento, Italy, 22–24 October 2020; pp. 585–589. [Google Scholar]
- Alves de Oliveira, H.; Pereira dos Santos, C. Limestone Clays for Ceramic Industry. In Clay Science and Technology; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Cannillo, V.; Esposito, L.; Rambaldi, E.; Sola, A.; Tucci, A. Microstructural and Mechanical Changes by Chemical Ageing of Glazed Ceramic Surfaces. J. Eur. Ceram. Soc. 2009, 29, 1561–1569. [Google Scholar] [CrossRef]
- Padeletti, G.; Fermo, P.; Gilardoni, S.; Galli, A. Technological Study of Ancient Ceramics Produced in Casteldurante (Central Italy) during the Renaissance. Appl. Phys. A 2004, 79, 335–339. [Google Scholar] [CrossRef]
- Peng, I.; Hills-Kimball, K.; Lovelace, I.M.; Wang, J.; Rios, M.; Chen, O.; Wang, L.-Q. Exploring the Colors of Copper-Containing Pigments, Copper (II) Oxide and Malachite, and Their Origins in Ceramic Glazes. Colorants 2022, 1, 376–387. [Google Scholar] [CrossRef]
- Pee, J.H.; Kim, G.H.; Choi, Y.D.; Jung, D.S.; Kang, G.I. Effect of Flux Materials on the Melting Characteristics of Ash Glaze. Key Eng. Mater. 2014, 608, 21–25. [Google Scholar] [CrossRef]
- Aceto, M.; Fenoglio, G.; Labate, M.; Picollo, M.; Bacci, M.; Agostino, A. A Fast Non-Invasive Method for Preliminary Authentication of Mediaeval Glass Enamels Using UV–Visible–NIR Diffuse Reflectance Spectrophotometry. J. Cult. Herit. 2020, 45, 33–40. [Google Scholar] [CrossRef]
- Micheletti, F.; Orsilli, J.; Melada, J.; Gargano, M.; Ludwig, N.; Bonizzoni, L. The Role of IRT in the Archaeometric Study of Ancient Glass through XRF and FORS. Microchem. J. 2020, 153, 104388. [Google Scholar] [CrossRef]
- Reflectance Spectroscopy (350–950 Nm) (Gorgias) Pigments-Checker Database. Available online: https://Chsopensource.Org/Fors/ (accessed on 24 May 2023).
- Picollo, M.; Casini, A.; Cucci, C.; Cherubini, F.; Stefani, L. Application of hyper-spectral imaging technique for colorimetric analysis of paintings. SCIRES-IT 2022, 12, 69–76. [Google Scholar]
- Carvalho, F.; Coentro, S.; Costeira, I.; Trindade, R.A.A.; Alves, L.C.; da Silva, R.C.; Muralha, V.S.F. The Cistercian Glazed Tiles of the Monastery of Alcobaça: Characterization of the Colour Palette. J. Mediev. Iber. Stud. 2016, 8, 196–216. [Google Scholar] [CrossRef]
- Murad, E. Identification of Minor Amounts of Anatase in Kaolins by Raman Spectroscopy. Am. Mineral. 1997, 82, 203–206. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. 1. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; DE GRUYTER: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Colomban, P.; Tournie, A.; Bellot-Gurlet, L. Raman Identification of Glassy Silicates Used in Ceramics, Glass and Jewellery: A Tentative Differentiation Guide. J. Raman Spectrosc. 2006, 37, 841–852. [Google Scholar] [CrossRef]
- Dabanli, Ö.; Yildiz, D.; Bayazit, M. Composition and phase analysis on glazed tiles of southeast anatolia: Production process identification. Mediterr. Archaeol. Archaeom. 2021, 21, 1–22. [Google Scholar] [CrossRef]
- Leśniak, M.; Partyka, J.; Gajek, M.; Sitarz, M. FTIR and MAS NMR Study of the Zinc Aluminosilicate Ceramic Glazes. J. Mol. Struct. 2018, 1171, 17–24. [Google Scholar] [CrossRef]




| Samples | Ceramic Body | Bright Slip Presence | Glaze Colour * | Features and Degradation Patterns | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Paste Colour * | Crystals ≤ 100 μm | Crystals ≥ 100 μm | Cracks Network | Dark Deposition | Pitting | Opaque Areas | Stains | |||
| 1G | DR | ● | ● | Y | ● | ● | ● | ● | ||
| 2G | LR | ● | ● | Y | ● | |||||
| 3A | DR | ● | T | ● | ● | |||||
| 4A | DR | ● | T | ● | ● | ● | ● | |||
| 5G | DR | ● | ● | Y-T | ● | |||||
| 6V | DR | ● | ● | G | ● | ● | ||||
| 7V | LR | ● | ● | G | ● | ● | ||||
| 8V | DR | ● | ● | G | ● | ● | ● | |||
| 9G | LR | ● | ● | Y | ● | ● | ||||
| 10G | DR | ● | ● | T | ● | ● | ||||
| 11V | DR | ● | ● | G | ||||||
| 12V | DR | ● | Y | |||||||
| 13V | DR | ● | ● | ● | G | |||||
| 14G_new | R | ● | Y | ● | Bright stains | |||||
| Atomic Wt% | Al | Si | K | Ca | Ti | Fe | Pb | Cu | Zn | Others (Traces) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ceramic body | ||||||||||
| 3A_CC | 22 | 37 | 7 | 3 | 2 | 25 | 2 | tr | <1% | Cr, Mn, Ni |
| 10V_CC | 21 | 43 | 9 | 3 | 3 | 21 | tr | tr | <1% | |
| 13G_CC | 18 | 37 | 7 | 2 | 2 | 33 | tr | tr | <1% | |
| 14G_new | 15 | 39 | 8 | 13 | 3 | 21 | 1 | tr | <1% | |
| Slip | ||||||||||
| 1G_B1 | 20 | 56 | 10 | 1 | 1 | 10 | 1 | tr | tr | Cr, Mn |
| 1V_B1 | 25 | 48 | 10 | 2 | 2 | 10 | 1 | tr | tr | |
| 7V_B1 | 18 | 52 | 11 | 2 | 1 | 10 | 3 | 3 | tr | |
| 11G_B1 | 28 | 45 | 11 | 2 | 2 | 11 | 1 | tr | tr | |
| 16V_B | 25 | 43 | 8 | 2 | 2 | 20 | 1 | tr | tr | |
| Glaze | ||||||||||
| 1G_V | 7 | 35 | <1% | 1 | 1 | 3 | 52 | <1% | tr | Cr, Mn, Ni (0.2 < Ni < 1%) |
| 2G_V | 8 | 35 | 1 | 3 | 1 | 3 | 50 | <1% | <1% | |
| 3A_V | 8 | 34 | <1% | 2 | 1 | 3 | 51 | <1% | <1% | |
| 4A_V | 7 | 29 | 3 | 2 | 1 | 4 | 53 | <1% | tr | |
| 5G_V | 6 | 37 | 1 | 2 | 0 | 2 | 52 | <1% | tr | |
| 8V_V | 7 | 27 | 1 | 2 | 1 | 4 | 49 | 9 | <1% | |
| 10V_V | 6 | 34 | 1 | 2 | 1 | 3 | 46 | 7 | tr | |
| 11G_V | 6 | 29 | <1% | 1 | 1 | 1 | 61 | <1% | <1% | |
| 14G_new | 4 | 34 | 2 | 19 | 2 | 4 | 28 | tr | 6 | |
| 16V_V | 7 | 27 | 2 | 5 | 1 | 2 | 46 | 10 | <1% | |
| 19V_V | 7 | 25 | 1 | 2 | 1 | 6 | 50 | 8 | <1% | |
| 20V_V | 8 | 34 | 1 | 3 | 1 | 3 | 41 | 9 | <1% |
| Colour Variations ∆ | 10 Cycles | 20 Cycles | ||||||
|---|---|---|---|---|---|---|---|---|
| ∆L * 10 | ∆a * 10 | ∆b * 10 | ∆E 10 | ∆L * 20 | ∆a * 02 | ∆b * 20 | ∆E 20 | |
| 14G_new a | −0.2 | 1.2 | −0.5 | 1.3 | −2.0 | 1.5 | −0.6 | 2.6 |
| 14G_new b | −0.7 | −1.5 | −0.1 | 1.7 | 0.1 | −0.5 | 0.1 | 0.6 |
| 14G_new c | 3.6 | −1.8 | 6.1 | 7.3 | 3.5 | −2.0 | 6.0 | 7.2 |
| 13V_old a | −6.4 | −0.9 | −9.3 | 11.3 | −8.4 | −1.1 | −11.0 | 13.9 |
| 13V_old b | −3.8 | −0.2 | −4.2 | 5.7 | −5.2 | −1.2 | −5.1 | 7.3 |
| 13V_old c | 3.0 | −1.3 | 2.2 | 3.9 | −6.1 | 1.3 | −7.7 | 9.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balliana, E.; Caveri, E.M.C.; Falchi, L.; Zendri, E. Tiles from Aosta: A Peculiar Glaze Roof Covering. Colorants 2023, 2, 533-551. https://doi.org/10.3390/colorants2030026
Balliana E, Caveri EMC, Falchi L, Zendri E. Tiles from Aosta: A Peculiar Glaze Roof Covering. Colorants. 2023; 2(3):533-551. https://doi.org/10.3390/colorants2030026
Chicago/Turabian StyleBalliana, Eleonora, Eugénie Marie Claudine Caveri, Laura Falchi, and Elisabetta Zendri. 2023. "Tiles from Aosta: A Peculiar Glaze Roof Covering" Colorants 2, no. 3: 533-551. https://doi.org/10.3390/colorants2030026
APA StyleBalliana, E., Caveri, E. M. C., Falchi, L., & Zendri, E. (2023). Tiles from Aosta: A Peculiar Glaze Roof Covering. Colorants, 2(3), 533-551. https://doi.org/10.3390/colorants2030026








