Hydrogeochemical Characterization as a Tool to Recognize “Masked Geothermal Waters” in Bahía Concepción, Mexico
Abstract
1. Introduction
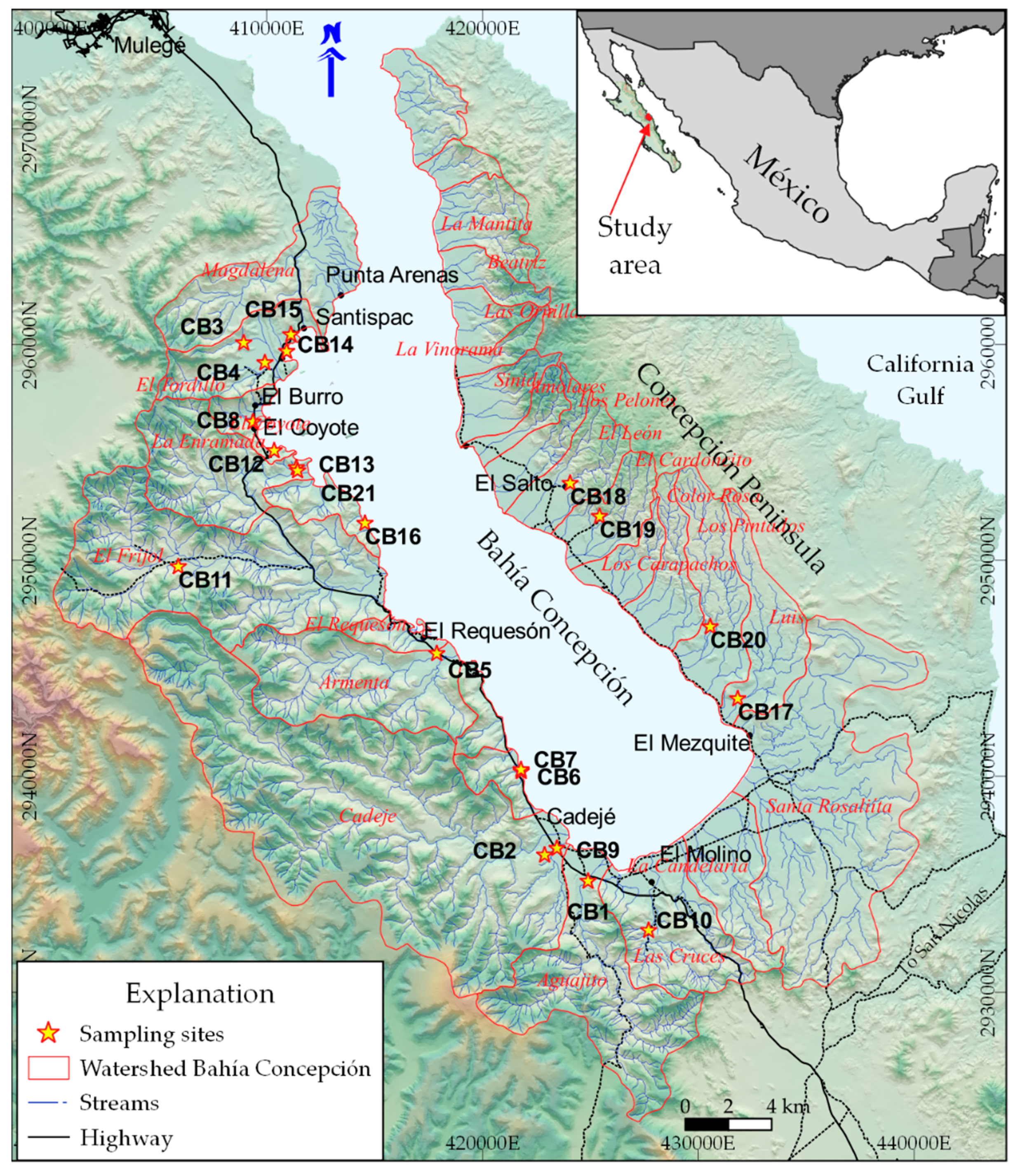
2. Study Area
2.1. Geological Units
2.2. Structural Setting
2.3. Climate and Hydrogeology
2.4. Geothermal Research in the Area
3. Methods
4. Results
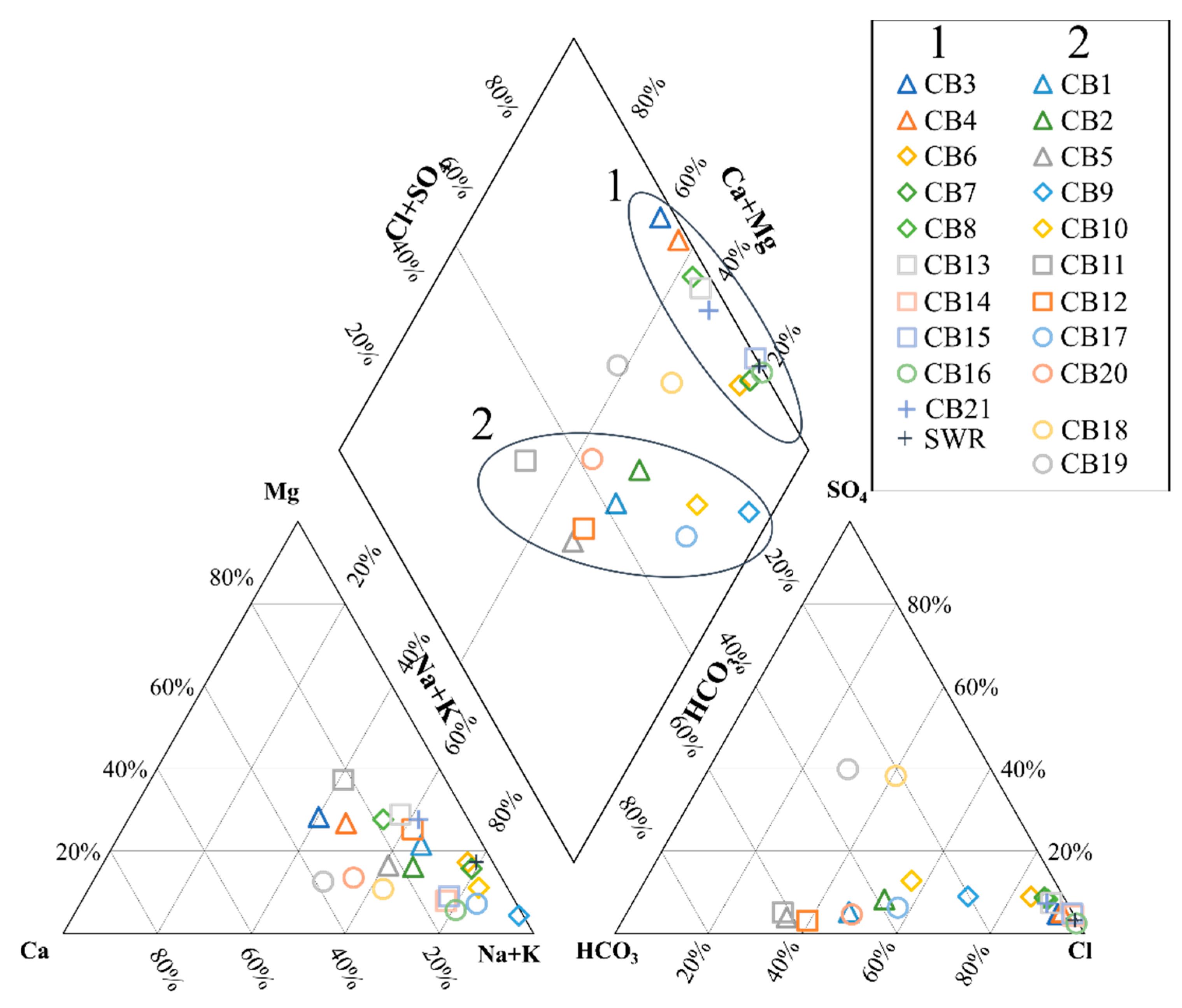
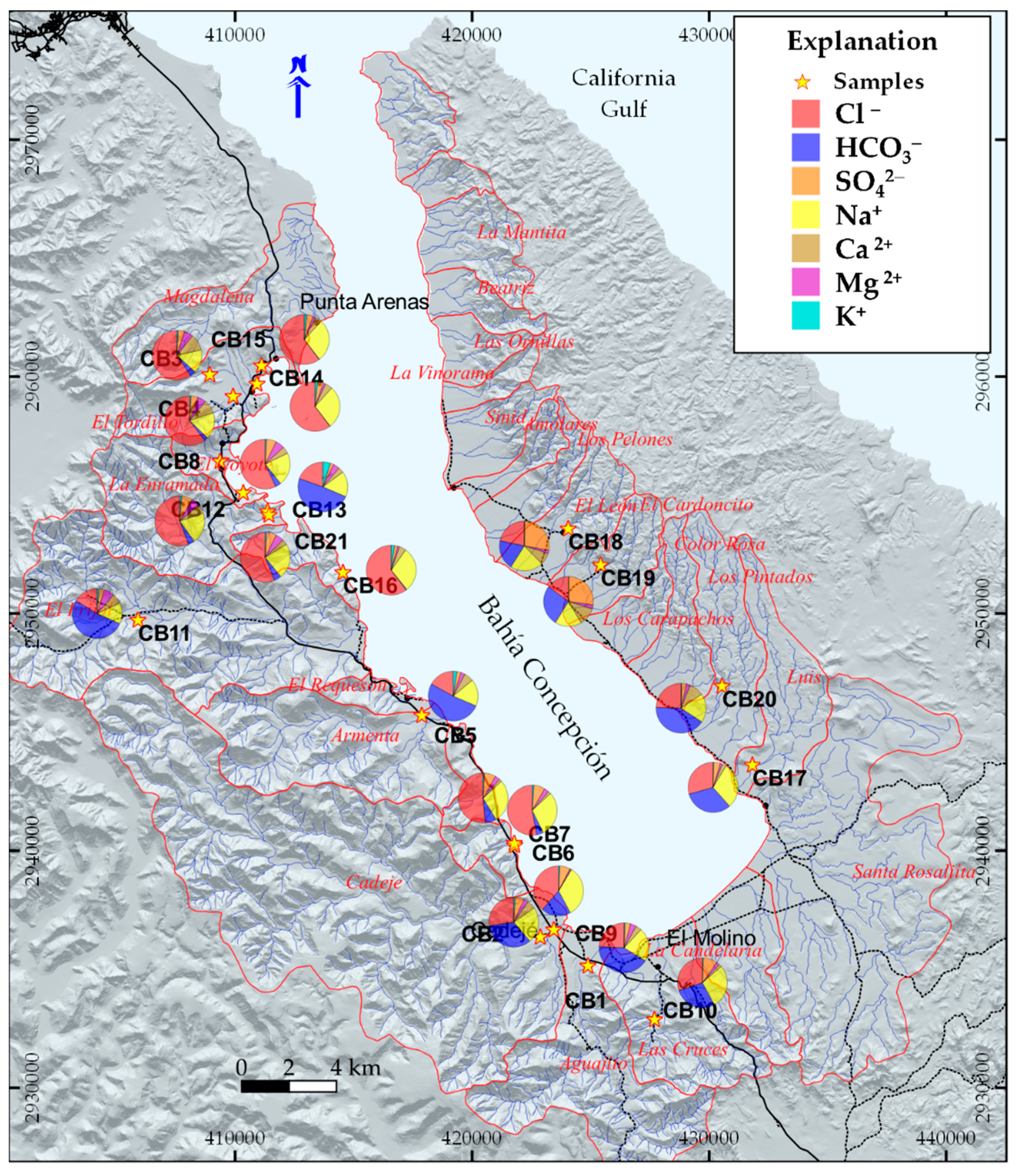
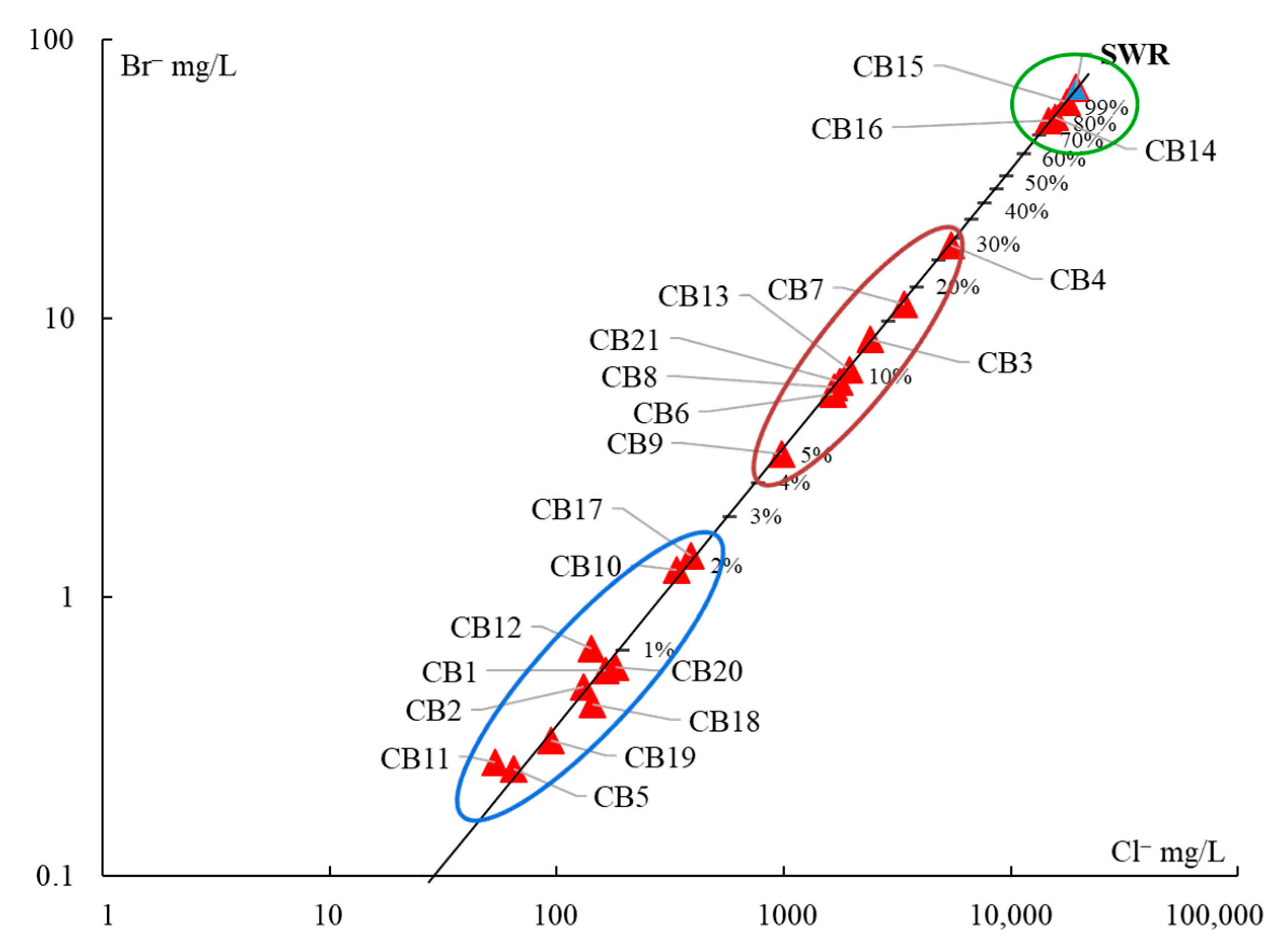
Hydrogeochemical Characterization
5. Discussion
- (1)
- The samples CB14, CB15, and CB16, with more than 75% of seawater, correspond to the intertidal hydrothermal springs in La Posada, Santispac, and Agua Caliente (Figure 1).
- (2)
- Three sites, located within the intertidal area (CB6, CB7, CB13), and five sites, located within a distance of less than 3 km to the coastline (CB3, CB4, CB8, CB9, and CB21), presented a fraction of seawater ranging between 5% and 30%. This proportion of seawater results from either tidal pump or groundwater extraction.
- (3)
- The remaining sites, with less than 2% seawater, are located at distances of more than 3 km to the coastline.
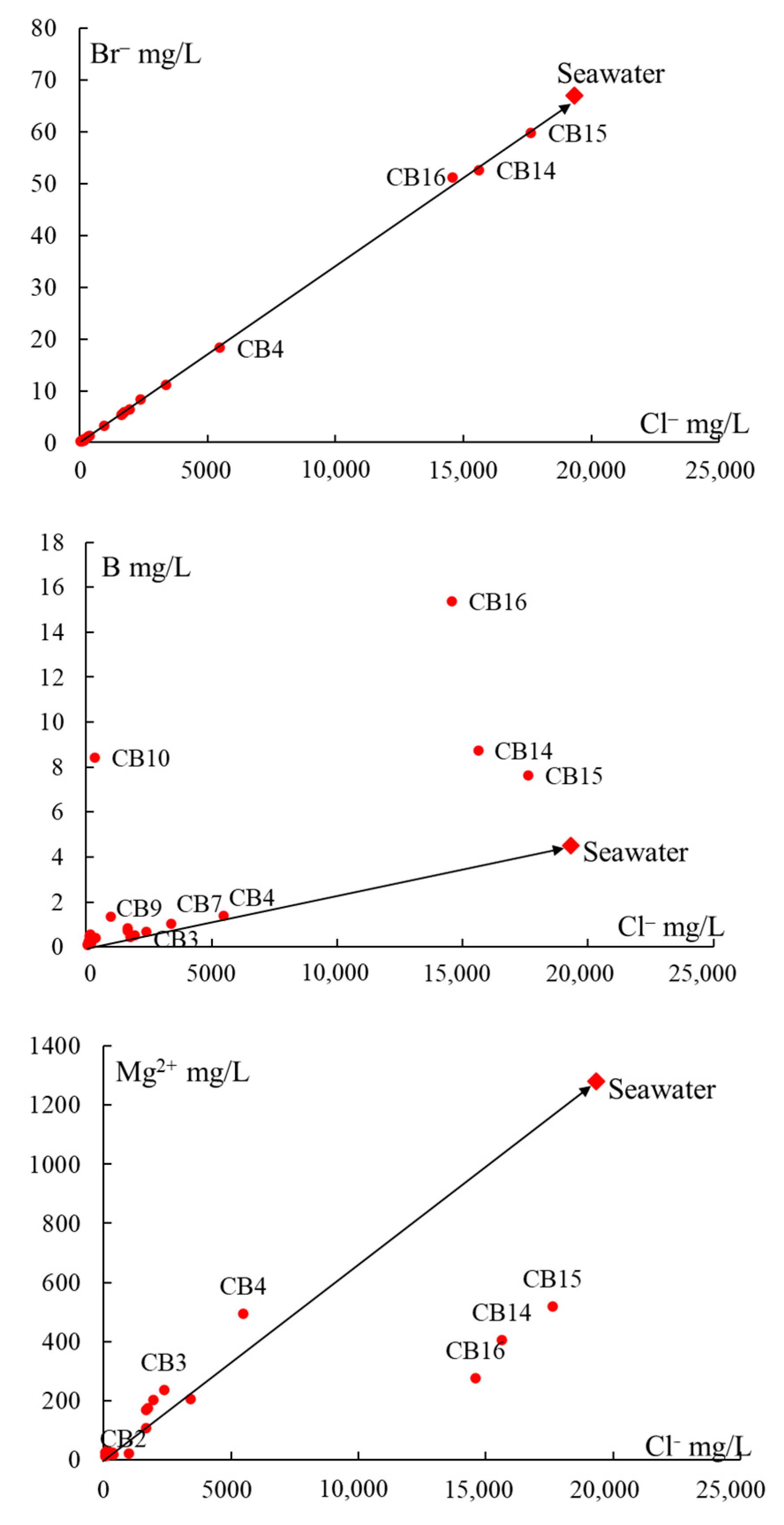
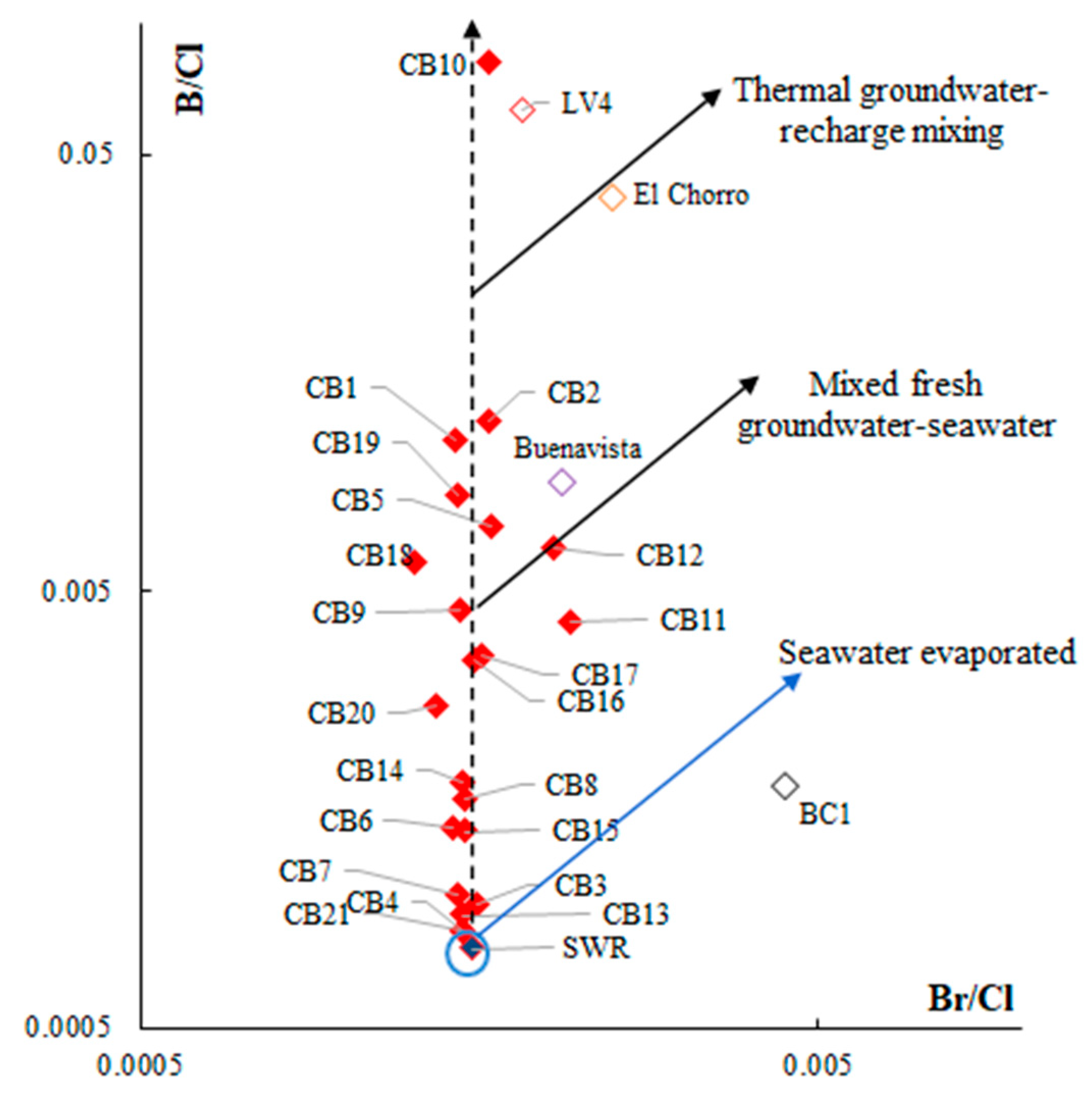
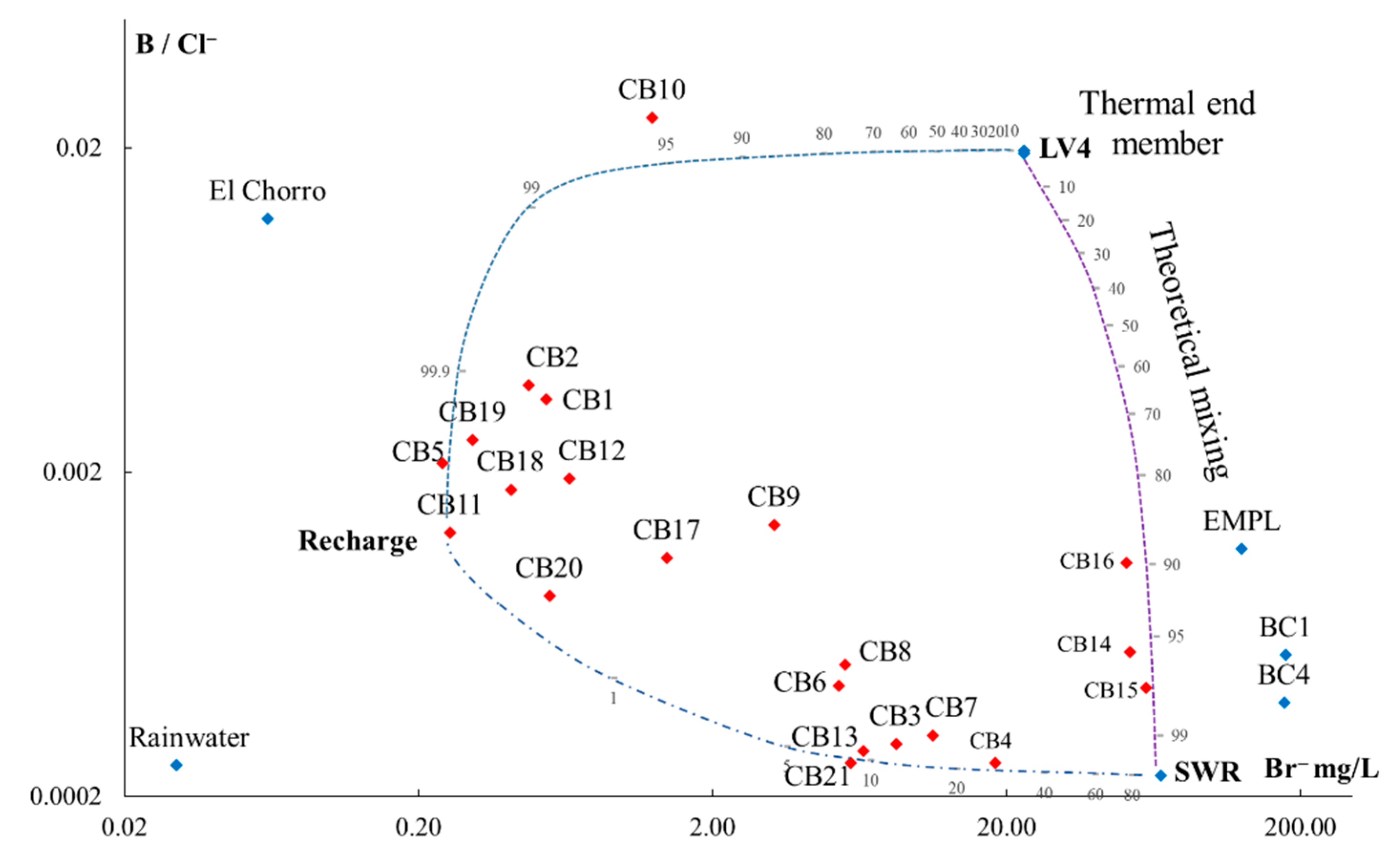
5.1. Origin of Water Salinity and Mixing of End Members
5.2. Masked Geothermal Water (MGW)
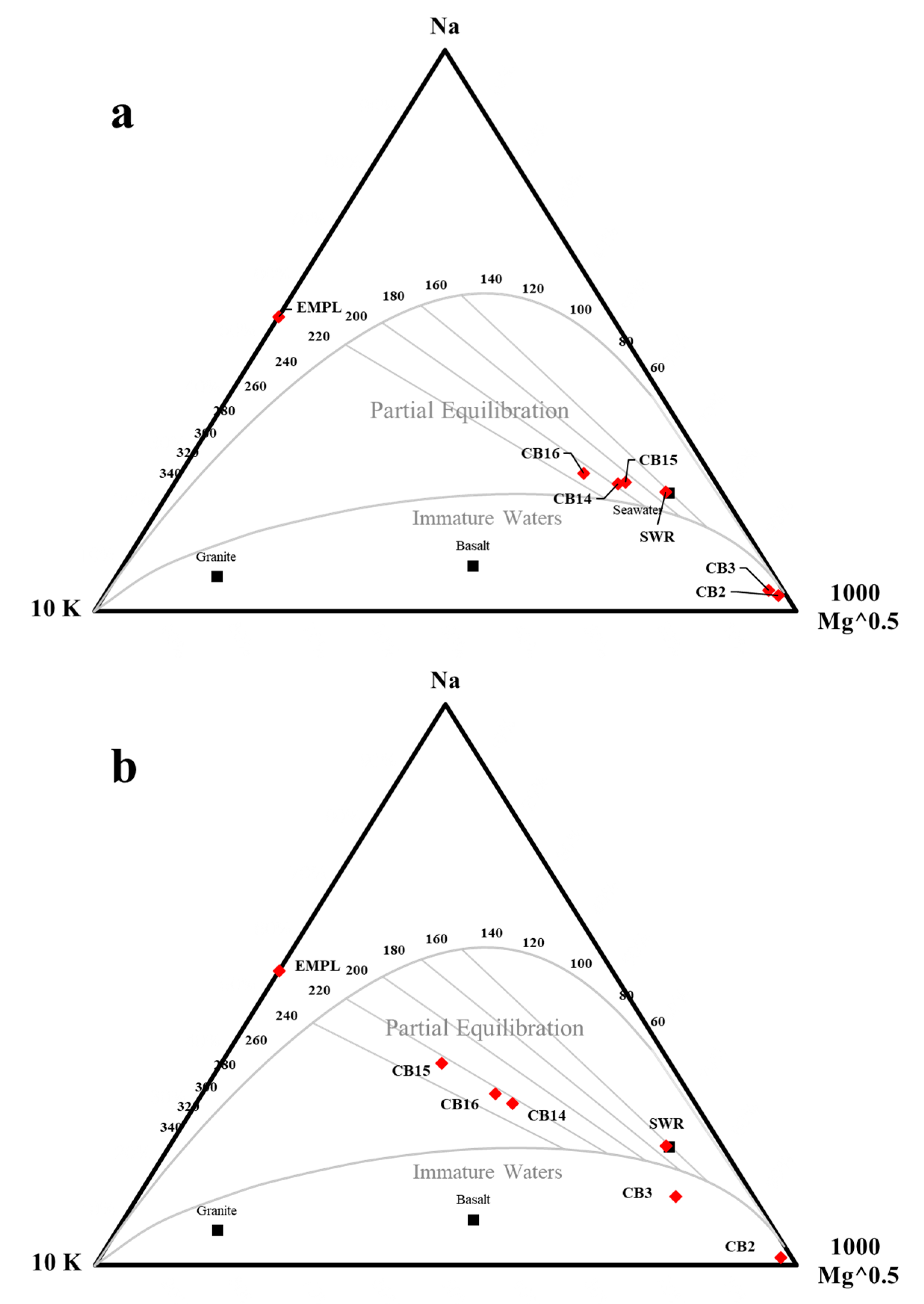
5.3. Geothermal Water and Geothermometry
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chepeliev, M.; Van der Mensbrugghe, D. Global fossil-fuel subsidy reform and Paris Agreement. Energy Econ. 2020, 85, 104598. [Google Scholar] [CrossRef]
- Sayed, E.T.; Wilberforce, T.; Elsaid, K.; Rabaia, M.K.H.; Abdelkareem, M.A.; Chae, K.J.; Olabi, A.G. A critical review on Environmental Impacts of Renewable Energy Systems and Mitigation Strategies: Wind, Hydro, Biomass and Geothermal. Sci. Total Environ. 2020, 766, 144505. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz-Zabłocka, J.; Łukasiewicz, E.; Guida, D. Analysis of the Sustainable Use of Geothermal Waters and Future Development Possibilities—A Case Study from the Opole Region, Poland. Sustainability 2019, 11, 6730. [Google Scholar] [CrossRef]
- Griebler, C.; Brielmann, H.; Haberer, C.M.; Kaschuba, S.; Kellermann, C.; Stumpp, C.; Hegler, F.; Kuntz, D.; Walker-Hertkorn, S.; Lueders, T. Potential impacts of geothermal energy use and storage of heat on groundwater quality, biodiversity, and ecosystem processes. Environ. Earth Sci. 2016, 75, 1391. [Google Scholar] [CrossRef]
- IRENA. Renewable Power Generation Costs in 2019; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates; Available online: https://www.irena.org/publications/2020/Jun/Renewable-Power-Costs-in-2019?fbclid=IwAR3UsYNJqcZta8YxP_VrXkYIIkHmK8f7Xt-_yMOExI29RH9coTRvpksMwP0 (accessed on 2 August 2020).
- Arango-Galván, C.; Prol-Ledesma, R.M.; Torres-Vera, M.A. Geothermal prospects in the Baja California peninsula. Geothermics 2015, 55, 39–57. [Google Scholar] [CrossRef]
- Carbajal-Martínez, D.; Peiffer, L.; Hinojosa-Corona, A.; Trasviña-Castro, A.; Arregui-Ojeda, S.M.; Carranza-Chávez, F.J.; Flores-Luna, C.; Méndez-Alonzo, R.; Inguaggiato, I.; Casallas-Moreno, K.L. UAV-based thermal imaging and heat output estimation of a coastal geothermal resource: La Jolla beach, Baja California, Mexico. Renew. Energy 2021, 168, 1364–1376. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Q.; Wu, G.; Guo, W.; She, W.; Yan, W. Boron geochemistry of the geothermal waters from two typical hydrothermal systems in Southern Tibet (China): Daggyai and Quzhuomu. Geothermics 2019, 82, 190–202. [Google Scholar] [CrossRef]
- Negri, A.; Daniele, L.; Aravena, D.; Muñoz, M.; Delgado, A.; Morata, D. Decoding fjord water contribution and geochemical processes in the Aysen thermal springs (Southern Patagonia, Chile). J. Geochem. Explor. 2018, 185, 1–13. [Google Scholar] [CrossRef]
- Lund, J.W.; Toth, A.N. Direct utilization of geothermal energy 2020 worldwide review. Geothermics 2021, 90, 101915. [Google Scholar] [CrossRef]
- Muffler, P.; Cataldi, R. Methods for regional assessment of geothermal resources. Geothermics 1978, 7, 53–89. [Google Scholar] [CrossRef]
- Towler, B.F. Geothermal energy. In The Future of Energy; Elsevier: Amsterdam, The Netherlands, 2014; p. 390. [Google Scholar] [CrossRef]
- Esteller, M.V.; Martínez-Florentino, A.K.; Morales-Reyes, G.P.; Cardona, A.; Expósito, J.L. Mixing processes between thermal waters and non-thermal waters: A case study in Mexico. Environ. Earth Sci. 2019, 78, 295. [Google Scholar] [CrossRef]
- Kaasalainen, H.; Stefánsson, A.; Giroud, N.; Arnórsson, S. The geochemistry of trace elements in geothermal fluids, Iceland. Appl. Geochem. 2015, 62, 207–223. [Google Scholar] [CrossRef]
- Navarro, A.; Font, X.; Viladevall, M. Geochemistry and groundwater contamination in the La Selva geothermal system (Girona, Northeast Spain). Geothermics 2011, 40, 275–285. [Google Scholar] [CrossRef]
- Tomaszewska, B.; Bundschuh, J.; Pająk, L.; Dendys, M.; Delgado-Quezada, V.; Bodzek, M.; Armienta, M.A.; Ormachea-Muñoz, M.; Kasztelewicz, A. Use of low enthalpy and waste geothermal energy sources to solve arsenic problems in freshwater production in selected regions of Latin America using a process membrane distillation—Research into model solutions. Sci. Total Environ. 2020, 714, 136853. [Google Scholar] [CrossRef]
- Wang, X.; Dan, Z.; Cui, X.; Zhang, R.; Zhou, S.; Wenga, T.; Yan, B.; Chen, G.; Zhang, Q.; Zhong, L. Contamination, ecological and health risks of trace elements in soil of landfill and geothermal sites in Tibet. Sci. Total Environ. 2020, 715, 136639. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.G.; Nordstrom, D.K. Geothermal Arsenic. In Arsenic in Ground Water; Welch, A.H., Stollenwerk, K.G., Eds.; Springer: Boston, MA, USA, 2003; pp. 101–125. [Google Scholar] [CrossRef]
- D’Amore, F.; Arnórsson, S. Geothermal manifestations and hydrothermal alteration. In Isotopic and Chemical Techniques in Geothermal Exploration, Development and Use: Sampling Methods, Data Handling, Interpretation; Arnórsson, S., Ed.; IAEA: Vienna, Austria, 2000; pp. 73–83. Available online: https://www.iaea.org/publications/5733/isotopic-and-chemical-techniques-in-geothermal-exploration-development-and-use-sampling-methods-data-handling-interpretation-edited-by-stefan-arnorsson (accessed on 17 August 2019).
- Prol-Ledesma, R.M. El Calor de la Tierra, 3rd ed.; FCE: Mexico City, Mexico; SEP: Mexico City, Mexico; CONACyT: Mexico City, Mexico, 2002; p. 99. [Google Scholar]
- Afsin, M.; Allen, D.M.; Kirste, D.; Durukan, U.G.; Gurel; A. ; Oruc, O. Mixing processes in hydrothermal spring systems and implications for interpreting geochemical data: A case study in the Cappadocia region of Turkey. Hydrogeol. J. 2014, 22, 7–23. [Google Scholar] [CrossRef]
- Amiri, V.; Nakhaei, M.; Lak, R.; Kholghi, M. Assessment of seasonal groundwater quality and potential saltwater intrusion: A study case in Urmia coastal aquifer (NW Iran) using the groundwater quality index (GQI) and hydrochemical facies evolution diagram (HFE-D). Stoch. Environ. Res. Risk Assess. 2016, 30, 1473–1484. [Google Scholar] [CrossRef]
- Arnórsson, S. The use of mixing models and chemical geothermometers for estimating underground temperatures in geothermal systems. J. Volc. Geotherm. Res. 1985, 23, 299–335. [Google Scholar] [CrossRef]
- Arnorsson, S.; Stefansson, A.; Bjarnason, J.O. Fluid-Fluid Interactions in Geothermal Systems. Rev. Mineral. Geochem. 2007, 65, 259–312. [Google Scholar] [CrossRef]
- Besser, H.; Mokadem, N.; Redhaounia, B.; Hadji, R.; Hamad, A.; Hamed, Y. Groundwater mixing and geochemical assessment of low-enthalpy resources in the geothermal field of southwestern Tunisia. Euro-Mediterr. J. Environ. Integr. 2018, 3, 16. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, J.J.; Liang, W.; Kuang, X. Hydrogeochemical characteristics in coastal groundwater mixing zone. Appl. Geochem. 2017, 85, 49–60. [Google Scholar] [CrossRef]
- Trezzi, G.; Garcia-Orellana, J.; Rodellas, V.; Santos-Echeandia, J.; Tovar-Sánchez, A.; Garcia-Solsona, E.; Masqué, P. Submarine groundwater discharge: A significant source of dissolved trace metals to the North Western Mediterranean Sea. Mar. Chem. 2016, 186, 90–100. [Google Scholar] [CrossRef]
- Bertani, R. Geothermal power generation in the world 2010–2014 update report. Geothermics 2016, 60, 31–43. [Google Scholar] [CrossRef]
- Gutiérrez-Negrín, L.C.A.; Canchola-Félix, I.; Romo-Jones, J.M.; Quijano-León, J.L. Geothermal Energy in Mexico: Update and Perspectives. In Proceedings of the World Geothermal Congress 2020, Reykjavik, Iceland, 26 April–2 May 2020. [Google Scholar]
- Prol-Ledesma, R.M.; Torres-Vera, M.A.; Rodolfo-Metalpa, R.; Angeles, C.; Lechuga-Deveze, C.H.; Villanueva-Estrada, R.E.; Shumilin, E.; Robinson, C. High heat flow and ocean acidification at a nascent rift in the northern Gulf of California. Nat. Commun. 2013, 4, 1388. [Google Scholar] [CrossRef]
- Prol-Ledesma, R.M.; Moran-Centeno, D.J. Heat flow and geothermal provinces in Mexico. Geothermics 2019, 78, 183–200. [Google Scholar] [CrossRef]
- Leal-Acosta, M.L.; Prol-Ledesma, R.M. Caracterización geoquímica de las manifestaciones termales intermareales de Bahía Concepción en la Península de Baja California. Bol. Soc. Geol. Mex. 2016, 68, 395–407. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Báncora-Alsina, C.; Prol-Ledesma, R.M.; Hiriart, G. A new geothermal resource in Los Cabos, Baja California Sur, México. In Proceedings of the 28th New Zealand Geothermal Workshop, Auckland, New Zealand, 16–17 November 2006; University of Auckland: Auckland, New Zealand, 2006; pp. S3–S6. Available online: https://www.geothermal-energy.org/pdf/IGAstandard/NZGW/2006/PDF/S3.pdf (accessed on 22 April 2019).
- Hernández-Morales, P.; Wurl, J. Hydrogeochemical characterization of the thermal springs in northeastern of Los Cabos Block, Baja California Sur, México. Environ. Sci. Pollut. Res. 2017, 24, 13184–13202. [Google Scholar] [CrossRef] [PubMed]
- Portugal, E.; Birkle, P.; Tello, E.; Tello, M. Hydrochemical–isotopic and hydrogeological conceptual model of the Las Tres Vírgenes geothermal field, Baja California Sur, México. J. Volcanol. Geotherm. Res. 2000, 101, 223–244. [Google Scholar] [CrossRef]
- Prol-Ledesma, R.M.; Canet, C.; Torres-Vera, M.A.; Forrest, M.J.; Armienta, M.A. Vent fluid chemistry in Bahía Concepción coastal submarine hydrothermal system, Baja California Sur, Mexico. J. Volcanol. Geotherm. Res. 2004, 137, 311–328. [Google Scholar] [CrossRef]
- Verma, S.P.; Pandarinath, K.; Santoyo, E.; González-Partida, E.; Torres-Alvarado, I.S.; Tello-Hinojosa, E. Fluid chemistry and temperaturas prior to exploitation at the Las Tres Vírgenes geothermal field, México. Geothermics 2006, 35, 156–180. [Google Scholar] [CrossRef]
- Wurl, J.; Rodríguez, L.M.; Cassassuce, F.; Gutiérrez, G.M.; Velázquez, E.R. Geothermal water in the San Juan Bautista Londó aquifer, BCS, Mexico. Procedia Earth Planet. Sci. 2013, 7, 900–903. [Google Scholar] [CrossRef]
- Canet, C.; Prol-Ledesma, R.M.; Proenza, J.A.; Rubio-Ramos, M.A.; Forrest, M.J.; Torres-Vera, M.A.; Rodríguez-Díaz, A.A. Mn-Ba-Hg mineralization at shallow submarine hydrothermal vents in Bahía Concepción, Baja California Sur, Mexico. Chem. Geol. 2005, 224, 96–112. [Google Scholar] [CrossRef]
- Leal-Acosta, M.L.; Shumilin, E.; Mirlean, N.; Lounejeva-Baturina, E.; Sánchez-Rodríguez, I.; Delgadillo-Hinojosa, F.; Borges-Souza, J. Intertidal geothermal hot springs as a source of trace elements to the coastal zone: A case study from Bahía Concepción, Gulf of California. Mar. Pollut. Bull. 2018, 128, 51–64. [Google Scholar] [CrossRef]
- Villanueva-Estrada, R.E.; Prol-Ledesma, R.M.; Rodríguez-Díaz, A.A.; Canet, C.; Torres-Alvarado, I.S.; González-Partida, E. Geochemical processes in an active shallow submarine hydrothermal system, Bahía Concepción, México: Mixing or boiling? Int. Geol. Rev. 2012, 54, 907–919. [Google Scholar] [CrossRef]
- Duque-Trujillo, J.; Ferrari, L.; Orozco-Esquivel, T.; López-Martínez, M.; Lonsdale, P.; Bryan, S.E.; Kluesner, J.; Piñero-Lajas, D.; Solari, L. Timing of rifting in the southern Gulf of California and its conjugate margins: Insights from the plutonic record. Geol. Soc. Am. Bull. 2015, 127, 702–736. [Google Scholar] [CrossRef]
- McFall, C.C. Reconnaissance Geology of the Concepcíon Bay Area, Baja California, Mexico; Stanford University Publications in Geological Sciences: Stanford, CA, USA, 1968; Volume 10, pp. 1–25. [Google Scholar]
- SGM. Carta Geológica-Minera y Geoquímica de Loreto G12-5, Escala 1:250000; Servicio Geológico Mexicano, Secretaria de Economia: Pachuca, Mexico, 2002. [Google Scholar]
- Ledesma-Vázquez, J.; Johnson, M.E. Miocene-Pleistocene Tectono-Sedimentary Evolution of Bahía Concepción Region, Baja California Sur (Mexico). Sediment. Geol. 2001, 144, 83–96. [Google Scholar] [CrossRef]
- Durán-Calderón, J.I. Estratigrafía regional y significado tectónico del Grupo Comondú en Baja California Sur, México. Tesis de Maestría, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2016; p. 193. [Google Scholar]
- Umhoefer, P.; Dorsey, R.; Willsey, S.; Mayer, L.; Renne, P. Stratigraphy and geochronology of the Comondu group near Loreto, Baja California Sur, Mexico. Sediment. Geol. 2001, 144, 125–147. [Google Scholar] [CrossRef]
- Ferrari, L.; Orozco-Esquivel, T.; Bryan, S.E.; López-Martínez, M.; Silva-Fragoso, A. Cenozoic magmatism and extension in western Mexico: Linking the Sierra Madre Occidental silicic large igneous province and the Comondú Group with the Gulf of California rift. Earth Sci. Rev. 2018, 183, 115–152. [Google Scholar] [CrossRef]
- Johnson, M.E.; Ledesma-Vázquez, J.; Mayall, M.A.; Minch, J. Upper Pliocene stratigraphy and depositional systems: The Peninsula Concepción Basin in Baja California Sur, Mexico. In Pliocene Carbonates and Related Facies Flanking the Gulf of California, Mexico; Johnson, M.E., Ledesma-Vázquez, J., Eds.; Geological Society of America: Boulder, CO, USA, 1997; Volume 318, pp. 57–72. [Google Scholar] [CrossRef]
- Ledesma-Vázquez, J.; Johnson, M.E.; Gutiérrez-Sanchez, S. El Mono chert: A shallow-water chert from the Pliocene Infierno Formation, Baja California Sur, Mexico. In Pliocene Carbonates and Related Facies Flanking the Gulf of California, Mexico; Johnson, M.E., Ledesma-Vázquez, J., Eds.; Geological Society of America: Boulder, CO, USA, 1997; Volume 318, pp. 73–81. Available online: https://pubs.geoscienceworld.org/books/chapter-pdf/966928/i0-8137-2318-3-318-0-73.pdf (accessed on 7 June 2019).
- Hausback, B.P. Cenozoic volcanic and tectonic evolution of Baja California Sur, Mexico. In Geology of the Baja California Peninsula: Pacific Section; Frizzel, V.A., Jr., Ed.; Society Economic Paleontologist and Mineralogist: Tulsa, OK, USA, 1984; Volume 39, pp. 219–236. [Google Scholar]
- Martín-Barajas, A. Vulcanism and extension of the extensional province of the gulf of California. Bol. Soc. Geol. Mex. 2000, 53, 72–83. [Google Scholar] [CrossRef]
- Sawlan, M.G.; Smith, J.G. Petrologic characteristics, age and tectonic setting of Neogene volcanic rocks in northern Baja California Sur, Mexico. In Geology of the Baja California Peninsula; Pacific Section; Frizzell, A.V., Ed.; Society of Economic Paleontologists and Mineralogists: Tulsa, OK, USA, 1984; Volume 39, pp. 237–251. Available online: http://archives.datapages.com/data/pac_sepm/055/055001/pdfs/237.htm (accessed on 3 October 2018).
- Umhoefer, P.J.; Mayer, L.; Dorsey, R.J. Evolution of the margin of the Gulf of California near Loreto, Baja California Peninsula, Mexico. Geol. Soc. Am. Bull. 2002, 114, 849–868. [Google Scholar] [CrossRef]
- Drake, W.R.; Umhoefer, P.J.; Griffiths, A.; Vlad, A.; Peters, L.; McIntosh, W. Tectono-stratigraphic evolution of the Comondú Group from Bahía de La Paz to Loreto, Baja California Sur, Mexico. Tectonophysics 2017, 719–720, 107–134. [Google Scholar] [CrossRef]
- Sutherland, F.H.; Kent, G.M.; Harding, A.J.; Umhoefer, P.J.; Driscoll, N.W.; Lizarralde, D.; Fletcher, J.M.; Axen, G.J.; Holbrook, W.S.; González-Fernández, A.; et al. Middle Miocene to early Pliocene oblique extension in the southern Gulf of California. Geosphere 2012, 8, 752–770. [Google Scholar] [CrossRef]
- Forrest, M.J.; Ledesma-Vázquez, J.; Ussler, W., III; Kulongoski, J.T.; Hilton, D.R.; Greene, H.G. Gas geochemistry of a shallow submarine hydrothermal vent associated with El Requesón fault zone in Bahía Concepción, Baja California Sur, México. Chem. Geol. 2005, 224, 82–95. [Google Scholar] [CrossRef]
- CONAGUA. Estadísticas del Agua en México; Comisión Nacional del Agua: Mexico City, Mexico, 2018; p. 303. Available online: http://sina.conagua.gob.mx/publicaciones/EAM_2018.pdf (accessed on 19 December 2020).
- CLICOM. Daily Weather Data from SMN Through its Web Platform CICESE. Available online: http://clicom-mex.cicese.mx/ (accessed on 8 December 2017).
- CONAGUA. Actualización de la Disponibilidad de Agua en el Acuífero Bahía Concepción (0331), Estado de Baja California Sur; Reporte Técnico; Comisión Nacional del Agua: Mexico City, Mexico, 2020; p. 24. Available online: https://sigagis.conagua.gob.mx/gas1/Edos_Acuiferos_18/BajaCaliforniaSur/DR_0331.pdf (accessed on 12 December 2020).
- Camprubí, A.; Canet, C.; Rodríguez-Diaz, A.A.; Prol-Ledesma, R.M.; Blanco-Florido, D.; Villanueva, R.E.; López-Sánchez, A. Geology, ore deposits and hydrothermal venting in Bahia Concepcion, Baja California Sur, Mexico. Island Arc 2008, 17, 6–25. [Google Scholar] [CrossRef]
- Leal-Acosta, M.L.; Shumilin, E.; Mirlean, N.; Delgadillo-Hinojosa, F.; Sánchez- Rodríguez, I. The impact of marine shallow-water hydrothermal venting on arsenic and mercury accumulation by seaweeds Sargassum sinicola in Concepcion Bay, Gulf of California. Environ. Sci. Process Impacts 2013, 15, 470–477. [Google Scholar] [CrossRef]
- Estradas-Romero, A.; Prol-Ledesma, R.M.; Zamudio-Reséndiz, M.E. Relación de las características geoquímicas de fluidos hidrotermales con la abundancia y riqueza de especies del fitoplancton de Bahía Concepción, Baja California Sur, México. Bol. Soc. Geol. Mex. 2009, 61, 87–96. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-33222009000100009&lng=es&nrm=iso (accessed on 22 September 2017). [CrossRef]
- Melwani, A.; Kim, S. Benthic infaunal distributions in shallow hydrothermal vent sediments. Acta Oecol. 2008, 33, 162–175. [Google Scholar] [CrossRef]
- Arnórsson, S.; D’Amore, F. Sampling of geothermal fluids: On-site measurements and sample treatment. In Isotopic and Chemical Techniques in Geothermal Exploration, Development and Use: Sampling Methods, Data Handling, Interpretation; Arnórsson, S., Ed.; IAEA: Vienna, Austria, 2000; pp. 84–142. Available online: https://www.iaea.org/publications/5733/isotopic-and-chemical-techniques-in-geothermal-exploration-development-and-use-sampling-methods-data-handling-interpretation-edited-by-stefan-arnorsson (accessed on 17 August 2019).
- Vengosh, A. Salinization and saline environments. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2003; Volume 9, pp. 333–365. [Google Scholar] [CrossRef]
- Arnórsson, S. Isotopic and Chemical Techniques in Geothermal Exploration, Development and Use: Sampling Methods, Data Handling, Interpretation; IAEA: Vienna, Austria, 2000; p. 351. Available online: https://www.iaea.org/publications/5733/isotopic-and-chemical-techniques-in-geothermal-exploration-development-and-use-sampling-methods-data-handling-interpretation-edited-by-stefan-arnorsson (accessed on 17 August 2019).
- Verma, S.P.; Pandarinath, K.; Santoyo, E. SolGeo: A new computer program for solute geothermometers and its application to Mexican geothermal fields. Geothermics 2008, 37, 597–621. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Powell, T.; Cumming, W. Spreadsheets for geothermal water and gas geochemistry. Proceedings of Thirty-Fifth Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 1–3 February 2010; Available online: https://earthsciences.stanford.edu/ERE/pdf/IGAstandard/SGW/2010/powell.pdf (accessed on 5 January 2019).
- Birkle, P.; Portugal Marín, E.; Pinti, D.L.; Clara Castro, M. Origin and evolution of geothermal fluids from Las Tres Vírgenes and Cerro Prieto fields, Mexico—Co-genetic volcanic activity and paleoclimatic constraints. J. Appl. Geochem. 2016, 65, 36–53. [Google Scholar] [CrossRef]
- Tomaszkiewicz, M.; Abou Najm, M.; El-Fadel, M. Development of a groundwater quality index for seawater intrusion in coastal aquifers. Environ. Model. Softw. 2014, 57, 13–26. [Google Scholar] [CrossRef]
- Nozaki, Y. Elemental Distribution: Overview. In Encyclopedia of Ocean Sciences, 2nd ed.; Steele, J.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 2, pp. 840–845. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Chapter A43. In Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations: US Geological Survey Techniques and Methods; US Department of the Interior: Washington, DC, USA, 2013; p. 497. Available online: http://pubs.usgs.gov/tm/06/a43 (accessed on 14 November 2020).
- Custodio, E.; Herrera, C. Utilización de la relación Cl/Br como trazador hidrogeoquímico en hidrología subterránea. Bol. Geol. Min. 2000, 111, 49–67. [Google Scholar]
- Vengoechea, A.M.; Rojano, R.E.; Arregoces, H.A. Dispersion and Concentration of PM 10 Particles in a Caribbean Coastal City. Inf. Tecnol. 2018, 29, 123–130. [Google Scholar] [CrossRef]
- Alcalá, F.J.; Custodio, E. Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. J. Hydrol. 2008, 359, 189–207. [Google Scholar] [CrossRef]
- Mendoza-Salgado, R.; Lechuga-Deveze, C.; Ortega-Rubio, A. First approach of a method to assess water quality for arid climate bay in the Gulf of California. Sci. Total Environ. 2005, 347, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Tello-Hinojosa, E.; Verma, M.P.; González-Partida, E. Geochemical characteristics of reservoir fluids in the Las Tres Virgenes, BCS, Mexico. In Proceedings of the World Geothermal Congress 2005, Antalya, Turkey, 24–29 April 2005. [Google Scholar]
- Nicholson, K. Geothermal Fluids Chemistry and Exploration Techniques; Springer: Berlin/Heidelberg, Germany, 1993; p. 255. [Google Scholar] [CrossRef]
- Farhadian Babadi, M.; Mehrabi, B.; Tassi, F.; Cabassi, J.; Pecchioni, E.; Shakeri, A.; Vaselli, O. Geochemistry of fluids discharged from mud volcanoes in SE Caspian Sea (Gorgan Plain, Iran). Int. Geol. Rev. 2020, 1–16. [Google Scholar] [CrossRef]
- Engle, M.A.; Doolan, C.A.; Pitman, J.A.; Varonka, M.S.; Chenault, J.; Orem, W.H.; McMahon, P.B.; Jubb, A.M. Origin and geochemistry of formation waters from the lower Eagle Ford Group, Gulf Coast Basin, south central Texas. Chem. Geol. 2020, 550, 119754. [Google Scholar] [CrossRef]
- Lipiec, I.; Wątor, K.; Kmiecik, E. The application of selected hydrochemical indicators in the interpretation of hydrogeochemical data—A case study from Busko-Zdrój and Solec-Zdrój (Poland). Ecol. Indic. 2020, 117, 106460. [Google Scholar] [CrossRef]
- Stefánsson, A.; Arnórsson, S.; Sveinbjörnsdóttir, Á.E.; Heinemaier, J.; Kristmannsdóttir, H. Isotope (δd, δ18o, 3h, δ13c, 14c) and chemical (B, Cl) Constrains on water origin, mixing, water-rock interaction and age of low-temperature geothermal water. Appl. Geochem. 2019, 108, 104380. [Google Scholar] [CrossRef]
- Arnórsson, S.; Andrésdóttir, A. Processes controlling the distribution of boron and chlorine in natural waters in Iceland. GCA 1995, 59, 4125–4146. [Google Scholar] [CrossRef]
- Lgourna, Z.; Warner, N.; Bouchaou, L.; Boutaleb, S.; Hssaisoune, M.; Tagma, T.; Ettayfi, N.; Vengosh, A. Elucidating the sources and mechanisms of groundwater salinization in the Ziz Basin of southeastern Morocco. Environ. Earth Sci. 2014, 73, 77–93. [Google Scholar] [CrossRef]
- Hao, Y.; Pang, Z.; Kong, Y.; Tian, J.; Wang, Y.; Liao, D.; Fan, Y. Chemical and isotopic constraints on the origin of saline waters from a hot spring in the eastern coastal area of China. Hydrogeol. J. 2020, 28, 2457–2475. [Google Scholar] [CrossRef]
- Sekuła, K.; Rusiniak, P.; Wątor, K.; Kmiecik, E. Hydrogeochemistry and Related Processes Controlling the Formation of the Chemical Composition of Thermal Water in Podhale Trough, Poland. Energies 2020, 13, 5584. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Chemical techniques in geothermal exploration. In Guidebook: Application of Geochemistry in Resources Development; UNITAR/UNDP: Geneva, Switzerland, 1991; pp. 119–144. [Google Scholar]
- Gómez Diaz, E.; Marín Cerón, M.I. Hydrogeochemical characteristics at Doña Juana Complex (SW Colombia): A new area for geothermal exploration in the Northern Andes region. Geothermics 2019, 101738. [Google Scholar] [CrossRef]
- Purnomo, B.J.; Pichler, T. Geothermal systems on the island of Java, Indonesia. J. Volcanol. Geotherm. 2014, 285, 47–59. [Google Scholar] [CrossRef]
- Reyes, A.G.; Christenson, B.W.; Faure, K. Sources of solutes and heat in low-enthalpy mineral waters and their relation to tectonic setting, New Zealand. J. Volcanol. Geotherm. 2010, 192, 117–141. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Liu, H.; Yu, M.; Hai, K.; Tan, M.; Huo, D. Hydrogeochemistry, Geothermometry, and Genesis of the Hot Springs in the Simao Basin in Southwestern China. Geofluids 2019, 1–23. [Google Scholar] [CrossRef]
- Santos, I.R.; Lechuga-Deveze, C.; Peterson, R.; Burnett, W. Tracing submarine hydrothermal inputs into a coastal bay in Baja California using radon. Chem. Geol. 2011, 282, 1–10. [Google Scholar] [CrossRef]
- Burnett, W.C.; Taniguchi, M.; Oberdorfer, J. Measurement and significance of the direct discharge of groundwater into the coastal zone. J. Sea Res. 2001, 46, 109–116. [Google Scholar] [CrossRef]
- Dimova, N.; Ganguli, P.M.; Swarzenski, P.W.; Izbicki, J.A.; O’Leary, D. Hydrogeologic controls on chemical transport at Malibu Lagoon, CA: Implications for land to sea exchange in coastal lagoon systems. J. Hydrol. Reg. Stud. 2017, 11, 219–233. [Google Scholar] [CrossRef]




| Parameter | Meteorological Station | Local Average in Bahia Concepción | ||
|---|---|---|---|---|
| San Nicolás | Mulegé | Ojo de Agua | ||
| Annual precipitation average (1980–2015) | 142.3 | 153.6 | 141.8 | 145.9 |
| Annual temperature average (1980–2015) | 23.73 | 22.31 | 22.37 | 22.80 |
| Sample | Locality | Electric Conductivity (μS/cm) | TDS (mg/L) | Salinity (UPS) | Temperature (°C) | pH |
|---|---|---|---|---|---|---|
| Western margin | ||||||
| CB1 | Casa de Piedra | 981 | 698 | -- | 27.4 | 7.7 |
| CB2 | Cadejé | 1160 | 506 | -- | 36.4 | 8.2 |
| CB3 | El Tordillo | 8300 | 4069 | -- | 32.7 | 7.7 |
| CB4 | El Llanito | 15,720 | 9133 | -- | 28.8 | 6.5 |
| CB5 | Armenta | 737 | 384 | -- | 25.8 | 7.2 |
| CB6 | Pocitos 2 | 4446 | 3231 | -- | 23.6 | 7.5 |
| CB7 | Pocitos 3 | 8160 | 6440 | -- | 30.3 | 7.3 |
| CB8 | Predio Adelaido | 6070 | 3014 | -- | 29.4 | 7.3 |
| CB9 | Arroyo Cadejé | 4020 | 2571 | -- | 27 | 7.3 |
| CB10 | Las Cruces | 1779 | 1143 | -- | 22.8 | 8.7 |
| CB11 | Las Cuevitas | 490 | 358 | -- | 30.7 | 7.9 |
| CB12 | La Enramada | 949 | 741 | -- | 22.2 | 8.1 |
| CB13 | El Coyote | 4660 | 3442 | -- | 31.6 | 7.2 |
| CB14 | La Posada | >20,000 | 25,942 | 22 | 53.4 | 6.9 |
| CB15 | Santispac | >20,000 | 29,320 | 25 | 42.1 | 7.8 |
| CB16 | Agua Caliente | >20,000 | 24,214 | 21 | 58.6 | 6.5 |
| CB21 | Santa Barbara | 4960 | 3167 | -- | 30.4 | 7.2 |
| Eastern margin | ||||||
| CB17 | El Mezquite | 2130 | 1338 | -- | 29.8 | 6.7 |
| CB18 | El Salto | 1135 | 675 | -- | 24.3 | 6.9 |
| CB19 | McFall | 1082 | 653 | -- | 24.7 | 6.9 |
| CB20 | La Pintada | 1161 | 755 | -- | 23.2 | 6.9 |
| Sample | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | Br− | B | SiO2 | Electrical Balance Error (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Western margin | |||||||||||
| CB1 | 148.0 | 3.6 | 26.2 | 26.1 | 164.7 | 24.8 | 285.0 | 0.5 | 0.6 | 42.3 | −1.2 |
| CB2 | 112.0 | 4.5 | 26.4 | 14.8 | 130.9 | 27.6 | 162.8 | 0.5 | 0.5 | 78.8 | 0.6 |
| CB3 | 615.0 | 32.4 | 434.0 | 235.5 | 2391.9 | 166.2 | 152.0 | 8.4 | 0.7 | 121.6 | −3.8 |
| CB4 | 1590.0 | 74.2 | 807.0 | 494.0 | 5472.6 | 414.5 | 231.0 | 18.4 | 1.4 | 129.5 | −4.9 |
| CB5 | 67.0 | 9.4 | 23.5 | 10.4 | 64.9 | 10.0 | 197.8 | 0.2 | 0.1 | 43.0 | −1.1 |
| CB6 | 900.0 | 26.0 | 54.8 | 108.0 | 1653.5 | 237.6 | 234.0 | 5.4 | 0.7 | 77.8 | −4.0 |
| CB7 | 1900.0 | 68.9 | 112.0 | 204.6 | 3397.0 | 460.4 | 278.7 | 11.2 | 1.0 | 103.0 | −1.6 |
| CB8 | 600.0 | 33.4 | 180.4 | 167.0 | 1659.6 | 212.3 | 122.9 | 5.6 | 0.8 | 53.0 | −4.0 |
| CB9 | 845.0 | 22.2 | 7.1 | 20.8 | 977.1 | 167.9 | 482.9 | 3.2 | 1.3 | 64.6 | −1.5 |
| CB10 | 320.0 | 6.3 | 20.6 | 23.0 | 338.9 | 103.3 | 312.7 | 1.3 | 8.4 | 83.1 | −0.9 |
| CB11 | 44.5 | 4.0 | 21.8 | 22.6 | 53.6 | 11.1 | 170.9 | 0.3 | 0.1 | 80.0 | −1.2 |
| CB12 | 120.2 | 36.1 | 26.1 | 30.7 | 142.3 | 15.1 | 356.1 | 0.7 | 0.3 | 63.2 | −3.2 |
| CB13 | 735.0 | 30.3 | 158.0 | 200.8 | 1943.1 | 216.6 | 127.3 | 6.5 | 0.5 | 72.7 | −4.0 |
| CB14 | 7225.0 | 446.3 | 1229.5 | 404.0 | 15,620.4 | 946.8 | 70.4 | 52.6 | 8.7 | 75.1 | −4.7 |
| CB15 | 8150.0 | 455.4 | 1271.0 | 519.0 | 17,629.5 | 1213.4 | 81.6 | 59.9 | 7.6 | 78.8 | −5.2 |
| CB16 | 7100.0 | 521.1 | 1092.5 | 276.0 | 14,585.9 | 495.0 | 143.3 | 51.1 | 15.4 | 211.7 | −3.0 |
| CB21 | 717.5 | 28.3 | 110.0 | 174.0 | 1757.3 | 197.0 | 150.9 | 5.9 | 0.4 | 72.0 | −4.6 |
| Eastern margin | |||||||||||
| CB17 | 395.0 | 4.8 | 34.5 | 18.0 | 388.3 | 57.6 | 428.3 | 1.4 | 0.4 | 66.4 | 2.9 |
| CB18 | 142.0 | 1.1 | 52.7 | 13.0 | 143.8 | 182.9 | 127.9 | 0.4 | 0.3 | 44.2 | −1.2 |
| CB19 | 109.0 | 0.6 | 74.6 | 14.9 | 93.8 | 170.7 | 165.4 | 0.3 | 0.2 | 44.4 | 2.1 |
| CB20 | 140.0 | 2.3 | 70.6 | 18.4 | 182.0 | 23.7 | 307.4 | 0.6 | 0.2 | 40.6 | 1.6 |
| Sample | EMPL 1 (%) | LV4 2 (%) | SWR 3 (%) | CB11 4 (%) | Original Data | Seawater Excluded | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | SiO2 | Na+ | K+ | Mg2+ | SiO2 | |||||
| CB2 | 99.1 | 0.6 | 0 | 0.4 | 112 | 4 | 15 | 79 | 65 | 8 | 22 | 83 |
| CB3 | 84.4 | 0 | 4 | 11.6 | 615 | 32 | 236 | 122 | 717 | 65 | 20 | 128 |
| CB14 | 16.5 | 0 | 72 | 11.5 | 7225 | 446 | 404 | 75 | 2357 | 213 | 14 | 243 |
| CB15 | 6.5 | 0 | 83.5 | 10 | 8150 | 455 | 519 | 79 | 3455 | 313 | 9 | 319 |
| CB16 | 20.5 | 0 | 62.5 | 17 | 7100 | 521 | 276 | 212 | 2595 | 235 | 13 | 259 |
| EMPL 1 | - | - | - | - | 5543.3 | 502.5 | 0.07 * | 463.3 | - | - | - | - |
| LV4 2 | - | - | - | - | 3484 | 603 | 0.07 | 484 | - | - | - | - |
| SWR 3 | - | - | - | - | 10780 | 399 | 1280 | 6 | - | - | - | - |
| CB11 4 | - | - | - | - | 44.5 | 4 | 22.6 | 80 | - | - | - | - |
| Site | Surface Temperature (°C) | Equilibrium Reservoir Temperature | |
|---|---|---|---|
| Original Data | Seawater Excluded | ||
| CB2 | 36.4 | 112 | 114.1 |
| CB3 | 32.7 | 137 | 140.3 |
| CB14 | 53.4 | 108 | 186.6 |
| CB15 | 42.1 | 110 | 209.9 |
| CB16 | 58.6 | 175 | 191.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Morales, P.; Wurl, J.; Green-Ruiz, C.; Morata, D. Hydrogeochemical Characterization as a Tool to Recognize “Masked Geothermal Waters” in Bahía Concepción, Mexico. Resources 2021, 10, 23. https://doi.org/10.3390/resources10030023
Hernández-Morales P, Wurl J, Green-Ruiz C, Morata D. Hydrogeochemical Characterization as a Tool to Recognize “Masked Geothermal Waters” in Bahía Concepción, Mexico. Resources. 2021; 10(3):23. https://doi.org/10.3390/resources10030023
Chicago/Turabian StyleHernández-Morales, Pablo, Jobst Wurl, Carlos Green-Ruiz, and Diego Morata. 2021. "Hydrogeochemical Characterization as a Tool to Recognize “Masked Geothermal Waters” in Bahía Concepción, Mexico" Resources 10, no. 3: 23. https://doi.org/10.3390/resources10030023
APA StyleHernández-Morales, P., Wurl, J., Green-Ruiz, C., & Morata, D. (2021). Hydrogeochemical Characterization as a Tool to Recognize “Masked Geothermal Waters” in Bahía Concepción, Mexico. Resources, 10(3), 23. https://doi.org/10.3390/resources10030023






