Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Pistachio Shells Extracts
2.3. Amberlite XAD-16 Fractionation
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of Total Flavonoid Content (TFC)
2.6. DPPH• Radical Scavenging Activity Assay
2.7. Determination of Oxygen Radical Absorbance Capacity (ORAC)
2.8. Determination of Trolox Equivalent Antioxidant Capacity (TEAC)
2.9. HPLC/ESI-MS/MS Analysis
2.10. H NMR Analysis
2.11. HPLC-UV Aunatification of Gallic Acid (1)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Different Extractions
3.2. Preparation of Polyphenol-Enriched Fractions by XAD-16 Fractionation
3.3. Correlation Analysis on Extracts and Fractions
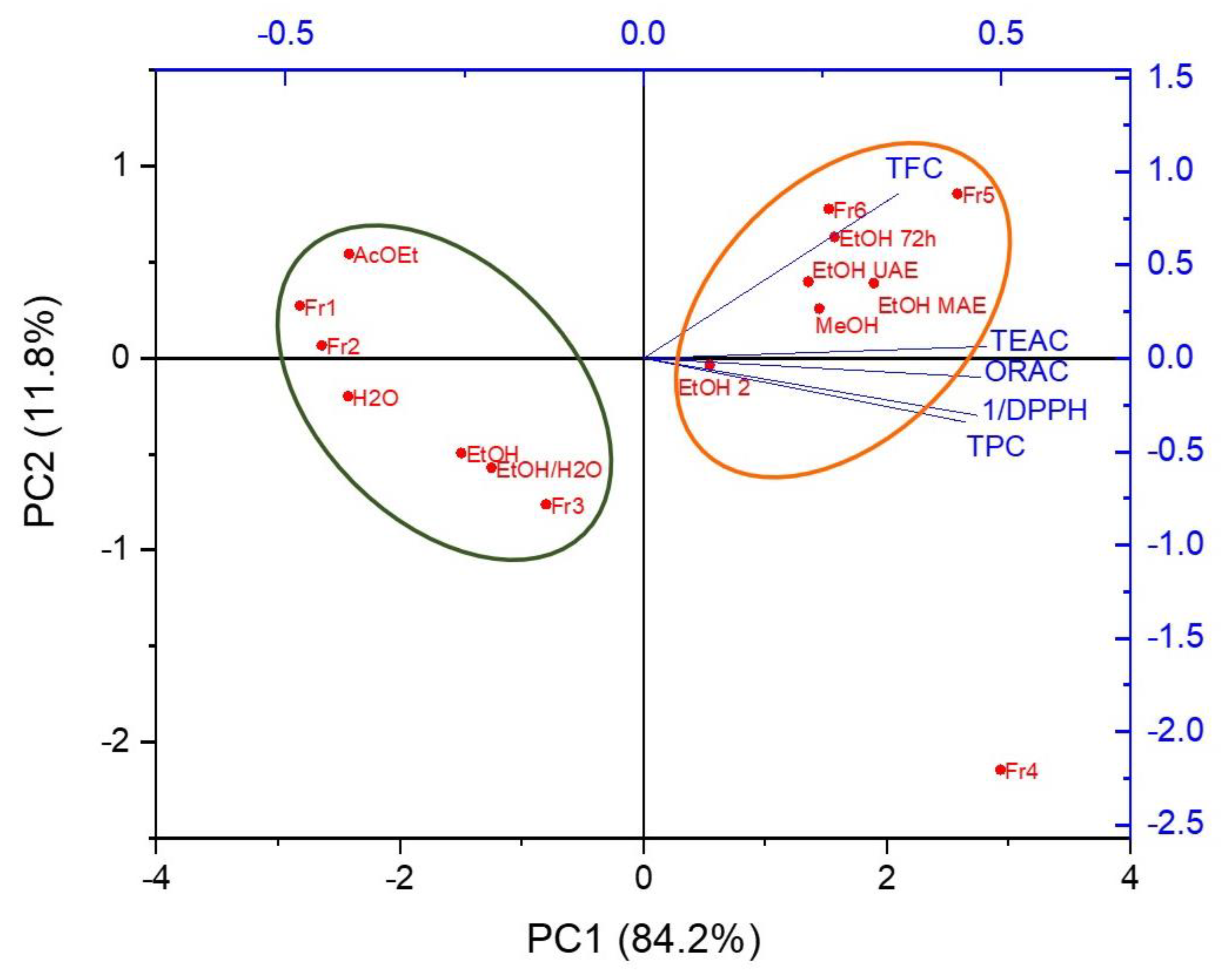
3.4. Principal Component Analysis (PCA)
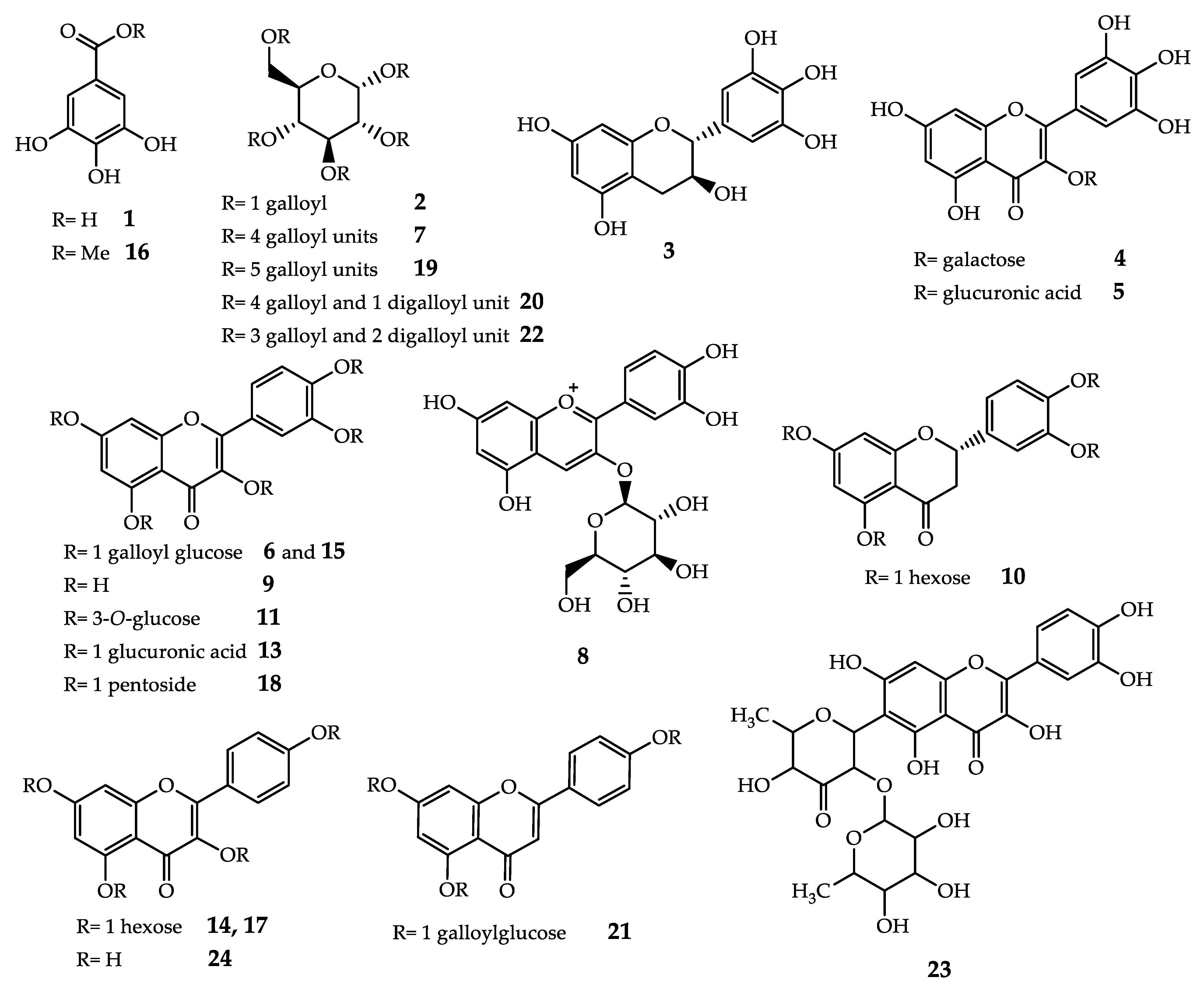
3.5. HPLC/ESI-MS/MS and 1H NMR Analysis of Fr1–Fr6 Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herrera, R.; Hemming, J.; Smeds, A.; Gordobil, O.; Willför, S.; Labidi, J. Recovery of Bioactive Compounds from Hazelnuts and Walnuts Shells: Quantitative–Qualitative Analysis and Chromatographic Purification. Biomolecules 2020, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Strong, P.J.; Xu, S.; Liu, S.; Lu, K.; Sheng, K.; Guo, J.; Che, L.; He, L.; et al. Thermal Properties of Biochars Derived from Waste Biomass Generated by Agricultural and Forestry Sectors. Energies 2017, 10, 469. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Chen, L.; Zhu, Q.; Wang, D.; Wang, W.; Sun, X.; Liu, X.; Du, F. Screening natural antioxidants in peanut shell using DPPH–HPLC–DAD–TOF/MS methods. Food Chem. 2012, 135, 2366–2371. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Campos, M.R.S. Bioactive Compounds. Health Benefits and Potential Applications; Woodhead Publishing Elsevier: Duxford, UK, 2019. [Google Scholar]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, J.F.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Vecchia, S.B.D.; Giovine, F.; Kbaier, H.B.H.; Bouzouita, N.; Pereira, L.B.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-Sustainable Recovery of Phenolic and Antioxidant Compounds from Industrial Chestnut Shells Using Ultrasound-Assisted Extraction: Optimization and Evaluation of Biological Activities In Vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kureck, I.; Policarpi, P.D.B.; Toaldo, I.M.; Maciel, M.V.D.O.B.; Bordignon-Luiz, M.T.; Barreto, P.L.M.; Block, J.M. Chemical Characterization and Release of Polyphenols from Pecan Nut Shell [Carya illinoinensis (Wangenh) C. Koch] in Zein Microparticles for Bioactive Applications. Plant Foods Hum. Nutr. 2018, 73, 137–145. [Google Scholar] [CrossRef]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Surmaghi, M.H.S.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A Review of Their Traditional Uses, Phytochemistry, and Pharmacology. Sci. World J. 2013, 2013, 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozden-Tokatli, Y.; Akdemir, H.; Tilkat, E.; Onay, A. Current status and conservation of Pistacia germplasm. Biotechnol. Adv. 2010, 28, 130–141. [Google Scholar] [CrossRef]
- Maalej, A.; Elloumi, W.; Angelov, I.; Kardaleva, P.; Dimitrov, V.; Chamkha, M.; Guncheva, M.; Sayadi, S. Pistacia lentiscus by-product as a promising source of phenolic compounds and carotenoids: Purification, biological potential and binding properties. Food Bioprod. Process. 2021, 126, 245–255. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef]
- Wilson, J.S.; Petino, G.; Knudsen, D.C. Geographic context of the Green Pistachio of Bronte, a protected designation of origin product. J. Maps 2018, 14, 144–150. [Google Scholar] [CrossRef]
- Arjeh, E.; Akhavan, H.-R.; Barzegar, M.; Carbonell-Barrachina, Á.A. Bio-active compounds and functional properties of pistachio hull: A review. Trends Food Sci. Technol. 2020, 97, 55–64. [Google Scholar] [CrossRef]
- Noorolahi, Z.; Sahari, M.A.; Barzegar, M.; Gavlighi, H.A. Tannin fraction of pistachio green hull extract with pancreatic lipase inhibitory and antioxidant activity. J. Food Biochem. 2020, 44, e13208. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Cunsolo, V.; Tringali, C. Mass Spectrometry and 1H-NMR Study of Schinopsis lorentzii (Quebracho) Tannins as a Source of Hypoglycemic and Antioxidant Principles. Molecules 2020, 25, 3257. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Stévigny, C.; Rolle, L.; Valentini, N.; Zeppa, G. Optimization of extraction of phenolic content from hazelnut shell using response surface methodology. J. Sci. Food Agric. 2007, 87, 2817–2822. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Erşan, S.; Üstündağ, Ö.G.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Erşan, S.; Üstündağ, Ö.G.; Carle, R.; Schweiggert, R.M. Identification of Phenolic Compounds in Red and Green Pistachio (Pistacia veraL.) Hulls (Exo- and Mesocarp) by HPLC-DAD-ESI-(HR)-MSn. J. Agric. Food Chem. 2016, 64, 5334–5344. [Google Scholar] [CrossRef]
- Erşan, S.; Üstündağ, Ö.G.; Carle, R.; Schweiggert, R.M. Determination of pistachio (Pistacia vera L.) hull (exo- and mesocarp) phenolics by HPLC-DAD-ESI/MSn and UHPLC-DAD-ELSD after ultrasound-assisted extraction. J. Food Compos. Anal. 2017, 62, 103–114. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Li, R.; Cui, Y.; Zhao, Y.; Yu, Z. Composition analysis and antioxidant activities of the Rhus typhina L. stem. J. Pharm. Anal. 2019, 9, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.J.; Swiecki, T.J.; Mitchell, A.E. Understanding the Native Californian Diet: Identification of Condensed and Hydrolyzable Tannins in Tanoak Acorns (Lithocarpus densiflorus). J. Agric. Food Chem. 2006, 54, 7686–7691. [Google Scholar] [CrossRef] [PubMed]
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oenological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Miketova, P.; Schram, K.H.; Whitney, J.; Li, M.; Huang, R.; Kerns, E.; Valcic, S.; Timmermann, B.N.; Rourick, R.; Klohr, S. Tandem mass spectrometry studies of green tea catechins. Identification of three minor components in the polyphenolic extract of green tea. J. Mass Spectrom. 2000, 35, 860–869. [Google Scholar] [CrossRef]
- Enomoto, H.; Nirasawa, T. Localization of Flavan-3-ol Species in Peanut Testa by Mass Spectrometry Imaging. Molecules 2020, 25, 2373. [Google Scholar] [CrossRef]
- Nonaka, G.; Kawahara, O.; Nishioka, I. Tannins and related compounds. XV. A new class of dimeric flavan-3-ol gallates, theasinensins A and B, and proanthocyanidin gallates from green tea leaf. (1). Chem. Pharm. Bull. 1983, 31, 3906–3914. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of Flavonoids and Phenolic Acids in Myrcia bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS Combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, W.L.R.; Peres, A.; Gallori, S.; Vincieri, F.F. Determination of myricetin derivatives in Chrysobalanus icaco L. (Chrysobalanaceae). Rev. Bras. de Farm. 2006, 16, 333–337. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Saletti, R.; Giovando, S.; Tringali, C. A mass spectrometry and 1H NMR study of hypoglycemic and antioxidant principles from a Castanea sativa tannin employed in oenology. Food Chem. 2018, 268, 585–593. [Google Scholar] [CrossRef]
- Li, Z.-H.; Guo, H.; Xu, W.-B.; Ge, J.; Li, X.; Alimu, M.; He, D.-J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeusL.) Leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Hou, J.; Fang, Q.; Sun, H.; Liu, X.; Zhang, L.; Wang, P.G. Synthesis of flavonol 3-O-glycoside by UGT78D1. Glycoconj. J. 2012, 29, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.A.; Rodrigues, E.; Mercadante, A.Z.; De Rosso, V.V. Phenolic Compounds and Carotenoids from Four Fruits Native from the Brazilian Atlantic Forest. J. Agric. Food Chem. 2014, 62, 5072–5084. [Google Scholar] [CrossRef] [PubMed]
- Yuzuak, S.; Ballington, J.; Xie, D.-Y. HPLC-qTOF-MS/MS-Based Profiling of Flavan-3-ols and Dimeric Proanthocyanidins in Berries of Two Muscadine Grape Hybrids FLH 13-11 and FLH 17-66. Metabolites 2018, 8, 57. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.-H.; He, X.-X.; Yan, M.-Z.; Chang, Q. Identification of in vivo components in rats after oral administration of lotus leaf flavonoids using ultra fast liquid chromatography with tandem mass spectrometry. Anal. Methods 2014, 6, 6088–6094. [Google Scholar] [CrossRef]
- Romeo, F.V.; Ballistreri, G.; Fabroni, S.; Pangallo, S.; Nicosia, M.G.L.D.; Schena, L.; Rapisarda, P. Chemical Characterization of Different Sumac and Pomegranate Extracts Effective against Botrytis cinerea Rots. Molecules 2015, 20, 11941–11958. [Google Scholar] [CrossRef] [Green Version]
- Velderrain-Rodríguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; González-Aguilar, G.A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’ Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Córdova, E.N.; Arvizu-Flores, A.A.; Valenzuela-Soto, E.M.; García-Orozco, K.D.; Wall-Medrano, A.; Alvarez-Parrilla, E.; Ayala-Zavala, J.F.; Domínguez-Avila, J.A.; González-Aguilar, G.A. Gallotannins are uncompetitive inhibitors of pancreatic lipase activity. Biophys. Chem. 2020, 264, 106409. [Google Scholar] [CrossRef]
- Kato-Schwartz, C.G.; Bracht, F.; Gonçalves, G.D.A.; Soares, A.A.; Vieira, T.F.; Brugnari, T.; Bracht, A.; Peralta, R.M. Inhibition of α-amylases by pentagalloyl glucose: Kinetics, molecular dynamics and consequences for starch absorption. J. Funct. Foods 2018, 44, 265–273. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Pulvirenti, L.; Cornu, A.; Pouységu, L.; Deffieux, D.; Quideau, S.; Tringali, C. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: A study of α-glucosidase and α-amylase inhibition. Food Chem. 2020, 313, 126099. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sarmiento, W.; Sáyago-Ayerdi, S.G.; Goñi, I.; Gutiérrez-Miceli, F.A.; Abud-Archila, M.; Rejón-Orantes, J.D.C.; Rincón-Rosales, R.; Peña-Ocaña, B.A.; Ruíz-Valdiviezo, V.M. Changes in Intestinal Microbiota and Predicted Metabolic Pathways During Colonic Fermentation of Mango (Mangifera indica L.)—Based Bar Indigestible Fraction. Nutrients 2020, 12, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [Green Version]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, S.; Conte, A.; Tagliazucchi, D. Comprehensive evaluation of phenolic profile in dark chocolate and dark chocolate enriched with Sakura green tea leaves or turmeric powder. Food Res. Int. 2018, 112, 1–16. [Google Scholar] [CrossRef]
- Ablajan, K.; Abliz, Z.; Shang, X.-Y.; He, J.-M.; Zhang, R.-P.; Shi, J.-G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef]
- Li, J.; Kuang, G.; Chen, X.; Zeng, R. Identification of Chemical Composition of Leaves and Flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS. Molecules 2016, 21, 947. [Google Scholar] [CrossRef] [Green Version]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Ferreres, F.; Gil-Izquierdo, A.; Andrade, P.; Valentão, P.; Tomás-Barberán, F. Characterization of C-glycosyl flavones O-glycosylated by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2007, 1161, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Rho, H.S.; Kim, D.H.; Chang, I.S. Enzymatic Preparation of Kaempferol from Green Tea Seed and Its Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 2951–2956. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef] [PubMed]
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Ojha, S.; Arya, D.S. Molecular Pathways Involved in the Amelioration of Myocardial Injury in Diabetic Rats by Kaempferol. Int. J. Mol. Sci. 2017, 18, 1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Sun, J.; George, J.; Ye, H.; Cui, Z.; Li, Z.; Liu, Q.; Zhang, Y.; Ge, D.; Liu, Y. Study of neuroprotective function of Ginkgo bilobaextract (EGb761) derived-flavonoid monomers using a three-dimensional stem cell-derived neural model. Biotechnol. Prog. 2016, 32, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, L.; Igarashi, K.; Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]


| Code | %w/w 1 | TPC 2 (mg GAE/g) | TFC 3 (mg/CatEg) | DPPH 4 EC50 (µg/mL) | TEAC 5 (µmol TE/g) | ORAC 5 (µmol TE/g) |
|---|---|---|---|---|---|---|
| Evaluation of Different Extraction Procedures | ||||||
| EtOH 1 | 0.94 ± 0.08 a | 189 ± 10 a | 157 ± 10 a | 15.1 ± 1.2 a | 1348 ± 9.8 a | 399 ± 11 a |
| MeOH | 0.81 ± 0.03 a | 381 ± 17 b | 359 ± 12 b | 7.8 ± 1.0 b,f | 3188 ± 18 b | 798 ± 23 b |
| H2O pH 4 | 2.21 ± 0.09 b | 146 ± 12 c | 162 ± 11 a | 239 ± 3.7 c | 166 ± 3.7 c | 203 ± 15 c |
| EtOH/H2O | 1.78 ± 0.10 c | 272 ± 5 d | 191 ± 17 c | 11.0 ± 2.7 b | 697 ± 4.1 d | 240 ± 8 d |
| EtOAc | 0.37 ± 0.02 d | 100 ± 5 e | 234 ± 5.4 d | 22.5 ± 5.9 a | 106 ± 5.4 e | 161 ± 13 e |
| EtOH 2 6 | 0.54 ± 0.01 7,e | 293 ± 10 f | 283 ± 18 e | 8.9 ± 1.2 b | 2721 ± 9.9 f | 779 ± 8 b |
| Optimization of Ethanol Extraction | ||||||
| Hex | 1.43 ± 0.10 f | - | ||||
| EtOH 72h 8 | 2.43 ± 0.09 9,b | 309 ± 9 f,g | 383 ± 15 b,f | 7.3 ± 0.3 b | 3677 ± 9.7 g | 916 ± 27 f |
| EtOH UAE 8 | 1.48 ± 0.11 9,f | 311 ± 7 g | 351 ± 17 b | 7.6 ± 1.3 b | 3682 ± 6.7 g | 849 ± 22 b |
| EtOH MAE8 | 3.00 ± 0.12 9,g | 332 ± 11 h | 376 ± 22 b,f | 6.1 ± 0.9 b | 4001 ± 7.5 h | 879 ± 17 f |
| Fractions from XAD-16 Chromatographic Separation | ||||||
| Fr1 | 40.26 | 101 ± 1.3 e | 182 ± 9 c,d | 192 ± 7.1 d | 104 ± 29 e | 202 ± 20 c,e |
| Fr2 | 9.11 | 110 ± 2.3 f | 170 ± 11a,b,c | 52.7 ± 3.3 e | 172 ± 32 c,e | 256 ± 18 d |
| Fr3 | 4.48 | 197 ± 5.6 a | 153 ± 4.6 a,c | 10.0 ± 0.2 f | 1823 ± 93 i | 647 ± 15.4 g |
| Fr4 | 12.57 | 458 ± 9.1 i | 179 ± 13 a,b,c | 4.1 ± 0.5 g | 4155 ± 9.6 l | 1344 ± 21 h |
| Fr5 | 22.24 | 310 ± 6.2 f,g | 445 ± 15 g | 5.5 ± 1.5 b,g | 3921 ± 5.2 h | 1243 ± 33 i |
| Fr6 | 11.33 | 274 ± 5.3 d | 395 ± 11 f | 6.9 ± 0.3 b,g | 3475 ± 61 m | 965 ± 18 g |
| Que | - | 3.6 ± 0.1 g | 1.8 ± 0.6 10 | 7.9 ± 0.2 10 | ||
| Coefficients of PC1 | Coefficients of PC2 | |
|---|---|---|
| 1/DPPH | 0.46674 | −0.30523 |
| TEAC | 0.4802 | 0.06349 |
| ORAC | 0.47144 | −0.10192 |
| TPC | 0.45003 | −0.34135 |
| TFC | 0.35607 | 0.88085 |
| TR (min) | Identification | MW | [M-H]− | MS/MS Fragments, m/z (Relative Intensity) | Fraction |
|---|---|---|---|---|---|
| 2.30 | Gallic acid (1) | 170 | 169 | 125 (100) | Fr3; Fr4; Fr5; Fr6 |
| 2.30 | Monogalloylglucose isomer (2) | 332 | 331 | 271 (100); 169 (90); 241 (40); 211 (40); 125 (10) | Fr4; Fr5 |
| 3.56 | (−)-Gallocatechin (3) | 306 | 305 | 179 (100); 261 (45); 125 (10); 287 (10); | Fr5; Fr6 |
| 26.70 | Myricetin hexoside (4) | 480 | 479 | 316 (100); 179 (10); 271 (5); | Fr5; Fr6 |
| 27.50 | Myricetin hexuronide (5) | 494 | 493 | 317 (100); | Fr5 |
| 30.00 | Quercetin galloyl hexoside isomer (6) | 616 | 615 | 463 (100) | Fr5; Fr6 |
| 30.00 | Tetragalloyl glucose isomer (7) | 788 | 787 | 617 (100); 623 (20); 465 (10); | Fr5; Fr6 |
| 31.90 | Cyanidin-hexose pyranoside (8) | 450 | 449 | 287 (100); | Fr5 |
| 32.14 | Quercetin (9) | 302 | 301 | 271 (100); 255 (40); 179 (30) | Fr5 |
| 33.00 | Eriodictyol hexoside (10) | 450 | 449 | 287 (100);431 (20) | Fr5; Fr6 |
| 33.20 | Quercetin hexoside (11) | 464 | 463 | 301 (100); 300 (75); 271 (5); 179 (5) | Fr5; Fr6 |
| 33.20 | Catechin gallate (12) | 442 | 441 | 289 (100); | Fr5 |
| 34.40 | Quercetin monoglucoronide (13) | 478 | 477 | 301 (100) | Fr5 |
| 35.25 | Kaempferol hexoside (14) | 448 | 447 | 285 | Fr5 |
| 35.25 | Quercetin galloyl hexoside isomer (15) | 616 | 615 | 301 (100); 463 (25) | Fr5 |
| 35.25 | Methyl gallate (16) | 184 | 183 | 168 (78); 124 (20) | Fr5, Fr6 |
| 35.66 | Kaempferol hexoside (17) | 448 | 447 | 285 | Fr6 |
| 36.70 | Quercetin pentoside (18) | 434 | 433 | 300 (100); 301 (30); 271 (20); 179 (5) | Fr6 |
| 37.70 | Pentagalloylglucose isomer (19) | 940 | 939 | 769 (100); 787 (15); 617 (10); 447 (5) | Fr5; Fr6 |
| 40.16 | Hexagalloyl glucose isomer (20) | 1092 | 1091 | - | Fr5; Fr6 |
| 42.10 | Apigenin galloyl glucoside (21) | 584 | 583 | 431 (100), 269 (60) | Fr6 |
| 43.10 | Eptagalloyl glucose (22) | 1244 | 1243 | - | Fr5 |
| 46.10 | Luteolin 2′′-O-deoxyosyl-6-C-(6-deoxy-pento-hexosulosyl) (23) | 576 | 575 | 531 (35); 411 (25), 429 (50) | Fr6 |
| 48.08 | Kaempferol (24) | 286 | 285 | 241 (100); 175 (40); 199 (30); 242 (25); 161 (10) | Fr6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources 2021, 10, 45. https://doi.org/10.3390/resources10050045
Cardullo N, Leanza M, Muccilli V, Tringali C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources. 2021; 10(5):45. https://doi.org/10.3390/resources10050045
Chicago/Turabian StyleCardullo, Nunzio, Melania Leanza, Vera Muccilli, and Corrado Tringali. 2021. "Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants" Resources 10, no. 5: 45. https://doi.org/10.3390/resources10050045
APA StyleCardullo, N., Leanza, M., Muccilli, V., & Tringali, C. (2021). Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources, 10(5), 45. https://doi.org/10.3390/resources10050045






