Protein Hydrolysates from Flaxseed Oil Cake as a Media Supplement in CHO Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Flaxseed Protein Isolates and Hydrolysates
2.3. Adherent Cell Culture and Media
2.4. Suspension Cell Culture and Media
2.5. Cell Growth, Metabolite, and Recombinant IgG Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Hydrolysates
3.2. Adherent CHO Cell Culture in DMEM
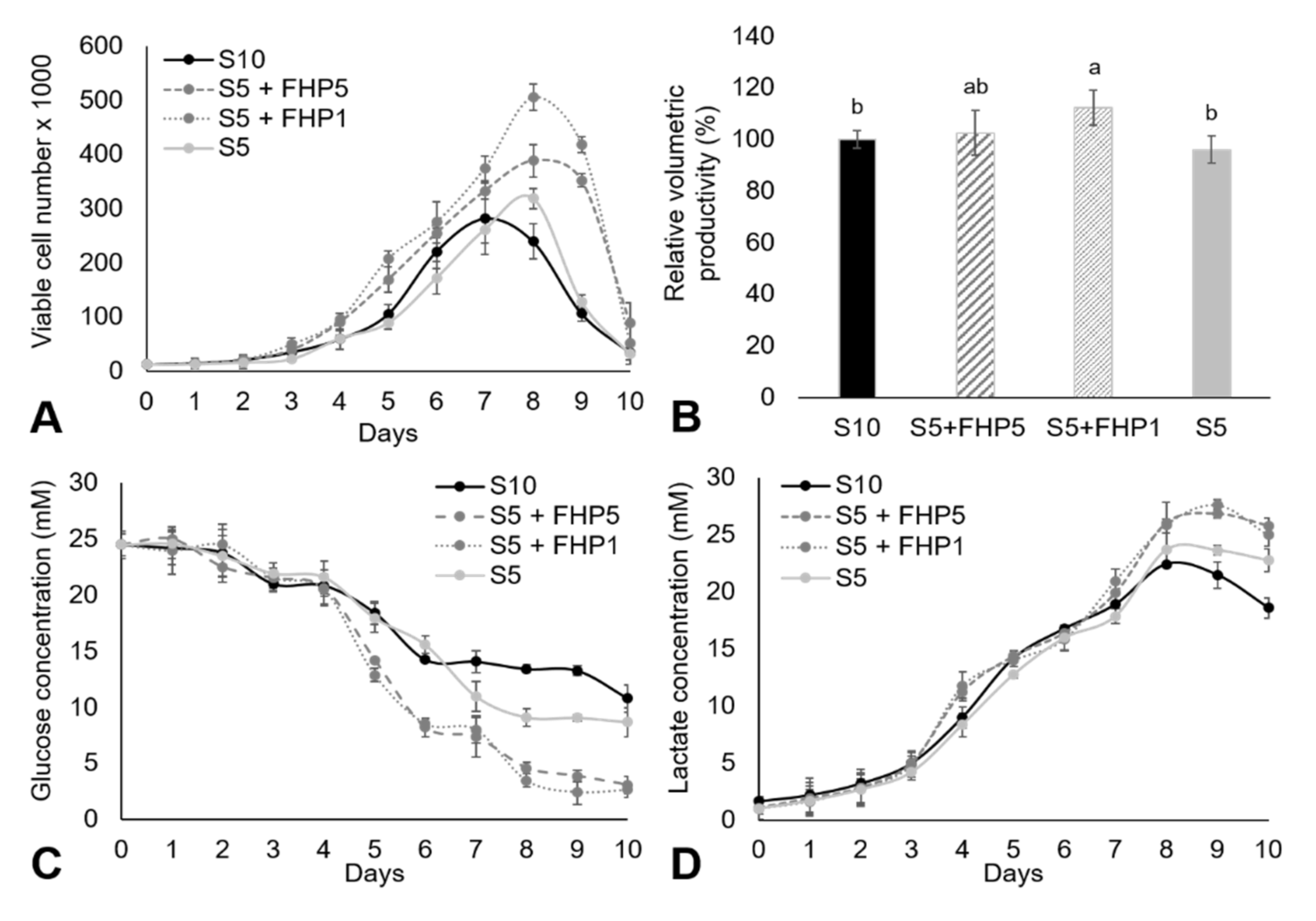
3.3. Suspension Cell Culture in CDM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sinacore, M.S.; Drapeau, D.; Adamson, S.R. Adaptation of Mammalian Cells to Growth in Serum-Free Media. Mol. Biotechnol. 2000, 15, 249–258. [Google Scholar] [CrossRef]
- Taylor, W.; Dworkin, R.; Pumper, R.; Evans, V. Biological efficacy of several commercially available peptones for mammalian cells in culture. Exp. Cell Res. 1972, 74, 275–279. [Google Scholar] [CrossRef]
- Girón-Calle, J.; Vioque, J.; Pedroche, J.; Alaiz, M.; Yust, M.M.; Megías, C.; Millan, F. Chickpea protein hydrolysate as a substitute for serum in cell culture. Cytotechnology 2008, 57, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Babcock, J.F.; Antosh, A. Partial Replacement of Chemically Defined CHO Media with Plant-Derived Protein Hydrolysates. In Proceedings of the 21st Annual Meeting of the European Society for Animal Cell Technology (ESACT), Dublin, Ireland, 7–10 June 2009; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Sunil, L.; Appaiah, P.; Kumar, P.K.P.; Krishna, A.G.G. Preparation of food supplements from oilseed cakes. J. Food Sci. Technol. 2014, 52, 2998–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasupuleti, V.K.; Holmes, C.; Demain, A.L. Applications of Protein Hydrolysates in Biotechnology. In Protein Hydrolysates in Biotechnology; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Butler, M. Serum-free media: Standardizing cell culture system. Pharm. Bioprocess. 2013, 1, 315–318. [Google Scholar] [CrossRef]
- Ling, W.L.W.; Bai, Y.; Cheng, C.; Padawer, I.; Wu, C. Development and manufacturability assessment of chemically-defined medium for the production of protein therapeutics in CHO cells. Biotechnol. Prog. 2015, 31, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Franěk, F.; Hohenwarter, O.; Katinger, H. Plant Protein Hydrolysates: Preparation of Defined Peptide Fractions Promoting Growth and Production in Animal Cells Cultures. Biotechnol. Prog. 2000, 16, 688–692. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Rubilar, M.; Jara, C.; Verdugo, M.; Sineiro, J.; Shene, C. Flaxseed and flaxseed cake as a source of compounds for food industry. J. Soil Sci. Plant Nutr. 2010, 10, 454–463. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, S.; Panjagari, N.R.; Raman, R.K. Flaxseed (Linum usitatissimum). In Oilseeds: Health Attributes and Food Applications; Springer: Singapore, 2021. [Google Scholar]
- Zhai, S.S.; Zhou, T.; Li, M.M.; Zhu, Y.W.; Feng, P.S.; Zhang, X.F.; Ye, H.; Wang, W.C.; Yang, L.; Li, M.C. Fermentation of flaxseed cake increases its nutritional value and utilization in ducklings. Poult. Sci. 2019, 98, 5636–5647. [Google Scholar] [CrossRef]
- Stodolak, B.; Janiszewska, A.S.; Wywrocka-Gurgul, A.; Wikiera, A. Solid-State Fermented Flaxseed Oil Cake of Improved Antioxidant Capacity as Potential Food Additive. J. Food Process. Preserv. 2016, 41, e12855. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Antioxidant Properties of Flaxseed Protein Hydrolysates: Influence of Hydrolytic Enzyme Concentration and Peptide Size. J. Am. Oil Chem. Soc. 2018, 95, 1105–1118. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.; Wang, B.; Adhikari, B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016, 197, 212–220. [Google Scholar] [CrossRef]
- Ho, S.C.; Nian, R.; Woen, S.; Chng, J.; Zhang, P.; Yang, Y. Impact of hydrolysates on monoclonal antibody productivity, purification and quality in Chinese hamster ovary cells. J. Biosci. Bioeng. 2016, 122, 499–506. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska-Cagnazzo, A.; Kulczyk, A. Use of Different Proteases to Obtain Flaxseed Protein Hydrolysates with Antioxidant Activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef] [Green Version]
- Udenigwe, C.C.; Lu, Y.-L.; Han, C.-H.; Hou, W.-C.; Aluko, R.E. Flaxseed protein-derived peptide fractions: Antioxidant properties and inhibition of lipopolysaccharide-induced nitric oxide production in murine macrophages. Food Chem. 2009, 116, 277–284. [Google Scholar] [CrossRef]
- Logarušić, M.; Radošević, K.; Bis, A.; Panić, M.; Slivac, I.; Srček, V.G. Biological Potential of Flaxseed Protein Hydrolysates Obtained by Different Proteases. Plant Foods Hum. Nutr. 2020, 75, 518–524. [Google Scholar] [CrossRef]
- Gupta, A.J.; Wierenga, P.A.; Gruppen, H.; Boots, J.-W. Influence of protein and carbohydrate contents of soy protein hydrolysates on cell density and IgG production in animal cell cultures. Biotechnol. Prog. 2015, 31, 1396–1405. [Google Scholar] [CrossRef]
- Spearman, M.; Lodewyks, C.; Richmond, M.; Butler, M. The bioactivity and fractionation of peptide hydrolysates in cultures of CHO cells. Biotechnol. Prog. 2014, 30, 584–593. [Google Scholar] [CrossRef]
- Kim, Y.; Chaudhry, M.A.; Kennard, M.L.; Jardon, M.A.; Braasch, K.; Dionne, B.; Butler, M.; Piret, J.M. Fed-batch CHO cell t-PA production and feed glutamine replacement to reduce ammonia production. Biotechnol. Prog. 2012, 29, 165–175. [Google Scholar] [CrossRef]
- Ballez, J.S.; Mols, J.; Burteau, C.; Agathos, S.N.; Schneider, Y.J. Plant protein hydrolysates support CHO-320 cells proliferation and recombinant IFN-γ production in suspension and inside microcarriers in protein-free media. Cytotechnology 2004, 44, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Mols, J.; Peeters-Joris, C.; Agathos, S.; Schneider, Y.-J. Origin of rice protein hydrolysates added to protein-free media alters secretion and extracellular proteolysis of recombinant interferon-γ as well as CHO-320 cell growth. Biotechnol. Lett. 2004, 26, 1043–1046. [Google Scholar] [CrossRef]
- Chabanon, G.; da Costa, L.A.; Farges, B.; Harscoat, C.; Chenu, S.; Goergen, J.-L.; Marc, A.; Marc, I.; Chevalot, I. Influence of the rapeseed protein hydrolysis process on CHO cell growth. Bioresour. Technol. 2008, 99, 7143–7151. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, L.; Cai, T.; Chen, Q.; Ma, Q.; Yang, J.; Meng, C.; Hong, J. Purification and identification of two novel antioxidant peptides from perilla (Perilla frutescens L. Britton) seed protein hydrolysates. PLoS ONE 2018, 13, e0200021. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.J.; Hageman, J.A.; Wierenga, P.A.; Boots, J.-W.; Gruppen, H. Chemometric analysis of soy protein hydrolysates used in animal cell culture for IgG production—An untargeted metabolomics approach. Process. Biochem. 2014, 49, 309–317. [Google Scholar] [CrossRef]
- Peričin, D.; Radulović-Popović, L.; Vaštag, Ž.; Mađarev-Popović, S.; Trivić, S. Enzymatic hydrolysis of protein isolate from hull-less pumpkin oil cake: Application of response surface methodology. Food Chem. 2009, 115, 753–757. [Google Scholar] [CrossRef]
- Tavarini, S.; de Leo, M.; Matteo, R.; Lazzeri, L.; Braca, A.; Angelini, L. Flaxseed and Camelina Meals as Potential Sources of Health-Beneficial Compounds. Plants 2021, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M. Methodology for Determining Degree of Hydrolysis of Proteins in Hydrolysates: A Review. J. AOAC Int. 2010, 93, 1515–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tehrani, M.H.H.; Batal, R.; Kamalinejad, M.; Mahbubi, A. Extraction and purification of flaxseed proteins and studying their antibacterial activities. J. Plant Sci. 2014, 2, 70–76. [Google Scholar]
- Tang, C.-H.; Wang, X.-S.; Yang, X.-Q. Enzymatic hydrolysis of hemp (Cannabis sativa L.) protein isolate by various proteases and antioxidant properties of the resulting hydrolysates. Food Chem. 2009, 114, 1484–1490. [Google Scholar] [CrossRef]
- Zurdo, J.; Arnell, A.; Obrezanova, O.; Smith, N.; de la Cuesta, R.G.; Gallagher, T.R.A.; Michael, R.; Stallwood, Y.; Ekblad, C.; Abrahmsén, L.; et al. Early Implementation of QbD in Biopharmaceutical Development: A Practical Example. BioMed Res. Int. 2015, 2015, 1–19. [Google Scholar] [CrossRef] [Green Version]
- McGillicuddy, N.; Floris, P.; Albrecht, S.; Bones, J. Examining the sources of variability in cell culture media used for biopharmaceutical production. Biotechnol. Lett. 2017, 40, 5–21. [Google Scholar] [CrossRef]
- Hamdi, A.; Széliová, D.; Ruckerbauer, D.E.; Rocha, I.; Borth, N.; Zanghellini, J. Key Challenges in Designing CHO Chassis Platforms. Processes 2020, 8, 643. [Google Scholar] [CrossRef]
- Kim, M.M.; Audet, J. On-demand serum-free media formulations for human hematopoietic cell expansion using a high dimensional search algorithm. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Kishishita, S.; Katayama, S.; Kodaira, K.; Takagi, Y.; Matsuda, H.; Okamoto, H.; Takuma, S.; Hirashima, C.; Aoyagi, H. Optimization of chemically defined feed media for monoclonal antibody production in Chinese hamster ovary cells. J. Biosci. Bioeng. 2015, 120, 78–84. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logarušić, M.; Gaurina Srček, V.; Berljavac, S.; Leboš Pavunc, A.; Radošević, K.; Slivac, I. Protein Hydrolysates from Flaxseed Oil Cake as a Media Supplement in CHO Cell Culture. Resources 2021, 10, 59. https://doi.org/10.3390/resources10060059
Logarušić M, Gaurina Srček V, Berljavac S, Leboš Pavunc A, Radošević K, Slivac I. Protein Hydrolysates from Flaxseed Oil Cake as a Media Supplement in CHO Cell Culture. Resources. 2021; 10(6):59. https://doi.org/10.3390/resources10060059
Chicago/Turabian StyleLogarušić, Marijan, Višnja Gaurina Srček, Sara Berljavac, Andreja Leboš Pavunc, Kristina Radošević, and Igor Slivac. 2021. "Protein Hydrolysates from Flaxseed Oil Cake as a Media Supplement in CHO Cell Culture" Resources 10, no. 6: 59. https://doi.org/10.3390/resources10060059
APA StyleLogarušić, M., Gaurina Srček, V., Berljavac, S., Leboš Pavunc, A., Radošević, K., & Slivac, I. (2021). Protein Hydrolysates from Flaxseed Oil Cake as a Media Supplement in CHO Cell Culture. Resources, 10(6), 59. https://doi.org/10.3390/resources10060059





