Oil/Water Separation Using Waste-Derived Functional Materials with Special Wetting Behavior
Abstract
:1. Introduction
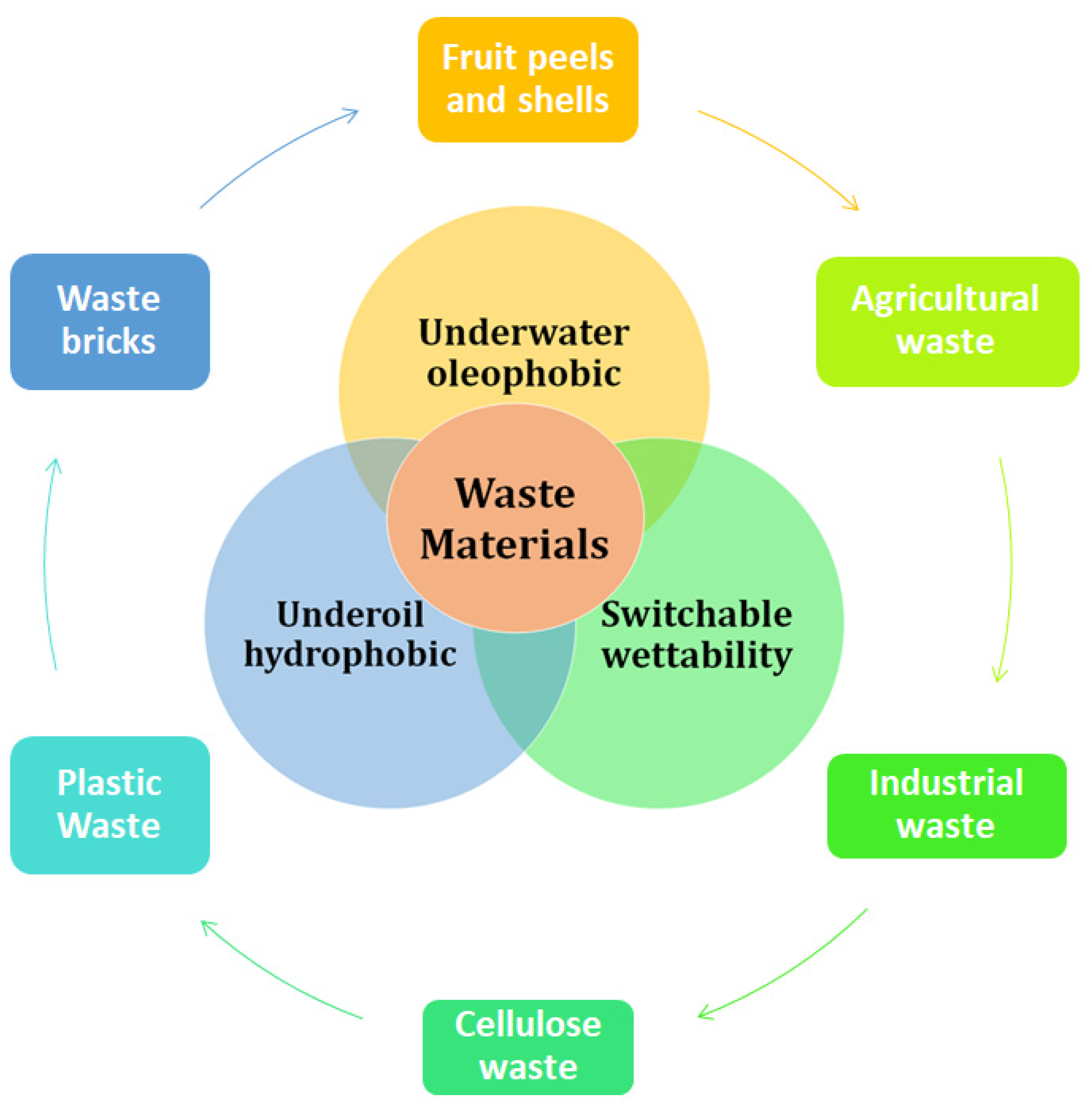
2. Waste Materials with Special Wettability for Oil/Water Separation
2.1. Underwater Superoleophobic-Superhydrophilic Surfaces
2.2. Underoil Superhydrophobic-Superoleophilic Surfaces
3. Summary and Future Work
- (1)
- Most of the studies are still limited to the lab scale only, and are very difficult to apply at the industrial scale. Therefore, it is highly essential to examine their applicability in real case problems with the oil of different viscosities under natural environmental conditions.
- (2)
- The matrix of oily contaminated wastewater is more complex. Therefore, design and development of multi-functional materials should be focused on performing real field applications.
- (3)
- Generally, special wettability waste materials have weak mechanical properties and a very small service life. However, longevity is one of the essential factors during its practical application to treat real field contaminated water at a large scale. Therefore, further research must be focused on the combination of theoretical and experimental investigations to achieve industrialization and large-scale oil/water separation in the real field practical application of special wettability waste materials with a continuous mode of operation.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Q.; Lu, T.; Deng, Y.; Zhang, Y.; Ma, W.; Xiong, R.; Huang, C. Bio-based materials with special wettability for oil-water separation. Sep. Purif. Technol. 2022, 297, 121445. [Google Scholar] [CrossRef]
- Qiao, A.; Huang, R.; Penkova, A.; Qi, W.; He, Z.; Su, R. Superhydrophobic, elastic and anisotropic cellulose nanofiber aerogels for highly effective oil/water separation. Sep. Purif. Technol. 2022, 295, 121266. [Google Scholar] [CrossRef]
- Hu, D.D.; Li, Y.D.; Weng, Y.; Peng, H.Q.; Zeng, J.B. Fabrication of sustainable and durable superwetting cotton fabrics with plant polyphenol for on-demand oil/water separation. Ind. Crops Prod. 2022, 186, 115264. [Google Scholar] [CrossRef]
- Yin, C.; Qi, X.; Zhao, S.; Han, X.; Guo, C.; Wu, X. Natural silk fibers incorporated aramid nanofibers sponges for efficient oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129323. [Google Scholar] [CrossRef]
- Singh, A.K.; Mishra, S.; Singh, J.K. Underwater superoleophobic biomaterial based on waste potato peels for simultaneous separation of oil/water mixtures and dye adsorption. Cellulose 2019, 26, 5497–5511. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, J.K. An efficient use of waste PE for hydrophobic surface coating and its application on cotton fibers for oil-water separator. Prog. Org. Coat. 2019, 131, 301–310. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, J.K. Fabrication of durable superhydrophobic coatings on cotton fabrics with photocatalytic activity by fluorine-free chemical modification for dual-functional water purification. New J. Chem. 2017, 41, 4618–4628. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, J.K. Fabrication of zirconia based durable superhydrophobic-superoleophilic fabrics using non fluorinated materials for oil-water separation and water purification. RSC Adv. 2016, 6, 103632–103640. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Mir, S.; Naderifar, A.; Rahidi, A.M.; Alaei, M. Recent advances in oil/water separation using nanomaterial-based filtration methods for crude oil processing-a review. J. Pet. Sci. Eng. 2022, 215, 110617. [Google Scholar] [CrossRef]
- Xiang, M.; Jiang, M.; Zhang, Y.; Liu, Y.; Shen, F.; Yang, G.; He, Y.; Wang, L.; Zhang, X.; Deng, S. Fabrication of a novel superhydrophobic and superoleophilic surface by one-step electrodeposition method for continuous oil/water separation. Appl. Surf. Sci. 2018, 434, 1015–1020. [Google Scholar] [CrossRef]
- Han, Z.; Du, C.; Shang, Z. Experiment study on the treatment effect of the hydrocarbon degradation microorganism to treat the oily wastewater. IOP Conf. Ser. Earth Environ. Sci. 2020, 467, 012161. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, J.; Hao, H.; Dutta, P.K. High-flux, efficient and reusable zeolite/stainless steel meshes for oil/water separation. Microporous Mesoporous Mater. 2022, 336, 111870. [Google Scholar] [CrossRef]
- Liu, J.; Aday, X.; Wang, X.; Li, Z.; Liu, J. On demand oil/water separation enabled by microporous ultra-thin aluminum foil with asymmetric wettability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129334. [Google Scholar] [CrossRef]
- Luo, X.; He, Z.; Gong, H.; He, L. Recent advances in oil-water separation materials with special wettability modified by graphene and its derivatives: A review. Chem. Eng. Process. Process Intensif. 2022, 170, 108678. [Google Scholar] [CrossRef]
- Chen, P.; Yin, D.; Song, P.; Liu, Y.; Cai, L.; Wang, H.; Zhang, L. Demulsification and oil recovery from oil-in-water cutting fluid wastewater using electrochemical micromembrane technology. J. Clean. Prod. 2020, 244, 118698. [Google Scholar] [CrossRef]
- Said, A.; Al Abdulgader, H.; Alsaeed, D.; Drmosh, Q.A.; Baroud, T.N.; Saleh, T.A. Hydrophobic tungsten oxide-based mesh modified with hexadecanoic branches for efficient oil/water separation. J. Water Process Eng. 2022, 49, 102931. [Google Scholar] [CrossRef]
- Yang, S.; Sha, S.; Lu, H.; Wu, J.; Ma, J.; Wang, D.; Hou, C.; Sheng, Z. Graphene oxide and reduced graphene oxide coated cotton fabrics with opposite wettability for continuous oil/water separation. Sep. Purif. Technol. 2021, 259, 118095. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, C.; Dai, M.; Lin, D.; Ali, I.; Alhewairini, S.S.; Zheng, X.; Chen, G.; Li, J.; Naz, I. Recent development of super-wettable materials and their applications in oil-water separation. J. Clean. Prod. 2020, 266, 121624. [Google Scholar] [CrossRef]
- Singh, A.K. Ocimum sanctum mediated phytosynthesis of metallic nanoparticles: A review. Bioresour. Technol. Rep. 2022, 19, 101118. [Google Scholar] [CrossRef]
- Singh, A.K. A review on plant extract-based route for synthesis of cobalt nanoparticles: Photocatalytic, electrochemical sensing and antibacterial applications. Curr. Res. Green Sustain. Chem. 2022, 5, 100270. [Google Scholar] [CrossRef]
- Singh, A.K. Flower extract-mediated green synthesis of bimetallic Cu[sbnd]Zn oxide nanoparticles and its antimicrobial efficacy in hydrocolloid films. Bioresour. Technol. Rep. 2022, 18, 101034. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, K.P. Optimization of phosphate removal from aqueous solution using activated carbon supported zero-valent iron nanoparticles: Application of RSM approach. Appl. Water Sci. 2018, 8, 226. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, K.P. Evaluation of phosphate removal capacity of Fe3O4–ZVINPs from aqueous solution: Optimization using response surface analysis. Res. Chem. Intermed. 2016, 42, 7397–7415. [Google Scholar] [CrossRef]
- Mousa, H.M.; Hamdy, M.; Yassin, M.A.; El-Sayed Seleman, M.M.; Abdel-Jaber, G.T. Characterization of nanofiber composite membrane for high water flux and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129655. [Google Scholar] [CrossRef]
- Singh, A.K.; Ketan, K.; Singh, J.K. Simple and green fabrication of recyclable magnetic highly hydrophobic sorbents derived from waste orange peels for removal of oil and organic solvents from water surface. J. Environ. Chem. Eng. 2017, 5, 5250–5259. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Dastageer, M.A. Polyimide based super-wettable membranes/materials for high performance oil/water mixture and emulsion separation: A review. Adv. Colloid Interface Sci. 2021, 297, 102525. [Google Scholar] [CrossRef]
- Paul, M.; Upadhaya, D.; Dhar Purkayastha, D.; Krishna, M.G. ZnO/WO3.H2O micro-nanostructures coated mesh for efficient separation of oil-water mixture. Appl. Surf. Sci. 2022, 583, 152476. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Zhao, Q.; Li, Y.; Zhang, B. Ultrahigh throughput and efficient separation of oil/water mixtures using superhydrophilic multi-scale CuBTC-coated meshes. Sep. Purif. Technol. 2021, 279, 119802. [Google Scholar] [CrossRef]
- Kabiri, B.; Norouzbeigi, R.; Velayi, E. Efficient oil/water separation using grass-like nano-cobalt oxide bioinspired dual-structured coated mesh filters. Surf. Interfaces 2022, 30, 101825. [Google Scholar] [CrossRef]
- Yin, X.; Yu, S.; Wang, B.; Wang, L.; Wang, J.; Liu, E.; Li, H.; Chen, Z. A durable Ni3S2coated mesh with reversible transition between superhydrophobicity and underwater superoleophobicity for efficient oil-water separation. J. Environ. Chem. Eng. 2022, 10, 107890. [Google Scholar] [CrossRef]
- Liu, H.; Yang, L.; Dou, B.; Lan, J.; Shang, J.; Lin, S. Zwitterionic hydrogel-coated cotton fabrics with underwater superoleophobic, self-healing and anti-fouling performances for oil-water separation. Sep. Purif. Technol. 2021, 279, 119789. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, S.; Pal, P.; Das, A.; Maity, J. Fabrication of AgNPs/Silane coated mechanical and washing durable hydrophobic cotton textile for self-cleaning and oil-water separation application. J. Indian Chem. Soc. 2022, 99, 100283. [Google Scholar] [CrossRef]
- Salhi, B.; Baig, N.; Abdulazeez, I.; Al-Ahmed, A.; Aljundi, I.H. High flux polyaniline-coated ceramic membrane for effective separation of emulsified oil-in-water. Ceram. Int. 2022, 48, 25246–25253. [Google Scholar] [CrossRef]
- Peng, K.; Huang, Y.; Peng, N.; Chang, C. Antibacterial nanocellulose membranes coated with silver nanoparticles for oil/water emulsions separation. Carbohydr. Polym. 2022, 278, 118929. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, X.; Qiu, L.; Yang, F. 2D nanoneedle-like ZnO/SiO2 Janus membrane with asymmetric wettability for highly efficient separation of various oil/water mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129352. [Google Scholar] [CrossRef]
- Jafari, B.; Rezaei, E.; Abbasi, M.; Hashemifard, S.A.; Khosravi, A.; Sillanpää, M. Application of Mullite-Zeolite-Alumina microfiltration membranes coated by SiO2 nanoparticles for separation of oil-in-water emulsions. J. Eur. Ceram. Soc. 2022, 42, 6005–6014. [Google Scholar] [CrossRef]
- Baig, U.; Gondal, M.A.; Dastageer, M.A. Oil-water separation using surface engineered superhydrophobic and superoleophilic membrane for the production of clean water. J. Water Process Eng. 2022, 45, 102473. [Google Scholar] [CrossRef]
- Sadler, E.; Crick, C.R. Suction or gravity-fed oil-water separation using PDMS-coated glass filters. Sustain. Mater. Technol. 2021, 29, e00321. [Google Scholar] [CrossRef]
- Zheng, H.; Lehtinen, M.J.; Liu, G. Hydrophobic modification of sintered glass filters for the separation of organic solvents and gasoline from water as well as emulsified water. J. Environ. Chem. Eng. 2021, 9, 106449. [Google Scholar] [CrossRef]
- Gong, L.; Zhu, H.; Wu, W.; Lin, D.; Yang, K. A durable superhydrophobic porous polymer coated sponge for efficient separation of immiscible oil/water mixtures and oil-in-water emulsions. J. Hazard. Mater. 2022, 425, 127980. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Wang, Q.M.; Lü, Q.F.; Wu, J. L-lysine functionalized Ti3C2Tx coated polyurethane sponge for high-throughput oil–water separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128396. [Google Scholar] [CrossRef]
- Venkatesan, N.; Yuvaraj, P.; Fathima, N.N. Fabrication of non-fluorinated superhydrophobic and flame retardant porous material for efficient oil/water separation. Mater. Chem. Phys. 2022, 286, 126190. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Mousa, H.M.; Fahmy, H.S.; Abouzeid, R.; Abdel-Jaber, G.T.; Ali, W.Y. Polyvinylidene fluoride-cellulose nanocrystals hybrid nanofiber membrane for energy harvesting and oil-water separation applications. Mater. Lett. 2022, 306, 130965. [Google Scholar] [CrossRef]
- Mousa, H.M.; Alfadhel, H.; Ateia, M.; Abdel-Jaber, G.T.; A, G.A. Polysulfone-iron acetate/polyamide nanocomposite membrane for oil-water separation. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100314. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, J.K. Fabrication of durable super-repellent surfaces on cotton fabric with liquids of varying surface tension: Low surface energy and high roughness. Appl. Surf. Sci. 2017, 416, 639–648. [Google Scholar] [CrossRef]
- Mousa, H.M.; Alfadhel, H.; Nasr, E.A. Engineering and characterization of antibacterial coaxial nanofiber membranes for oil/water separation. Polymers 2020, 12, 2597. [Google Scholar] [CrossRef]
- Ni, T.; You, Y.; Xie, Z.; Kong, L.; Newman, B.; Henderson, L.; Zhao, S. Waste-derived carbon fiber membrane with hierarchical structures for enhanced oil-in-water emulsion separation: Performance and mechanisms. J. Memb. Sci. 2022, 653, 120543. [Google Scholar] [CrossRef]
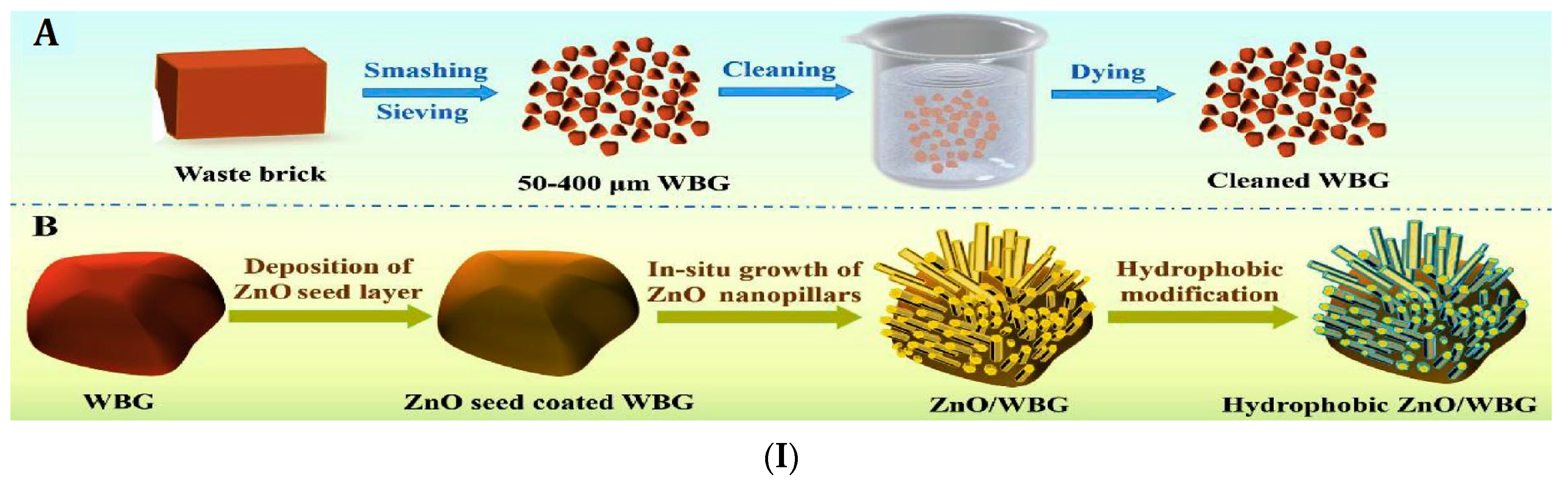
- Li, Z.; Zhang, T.; Wang, M.; Qiu, F.; Yue, X.; Yang, D. Hierarchical structurized waste brick with opposite wettability for on-demand oil/water separation. Chemosphere 2020, 251, 126348. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sirous, F.; Hussain, C.M. Sawdust, a versatile, inexpensive, readily available bio-waste: From mother earth to valuable materials for sustainable remediation technologies. Adv. Colloid Interface Sci. 2021, 295, 102492. [Google Scholar] [CrossRef] [PubMed]
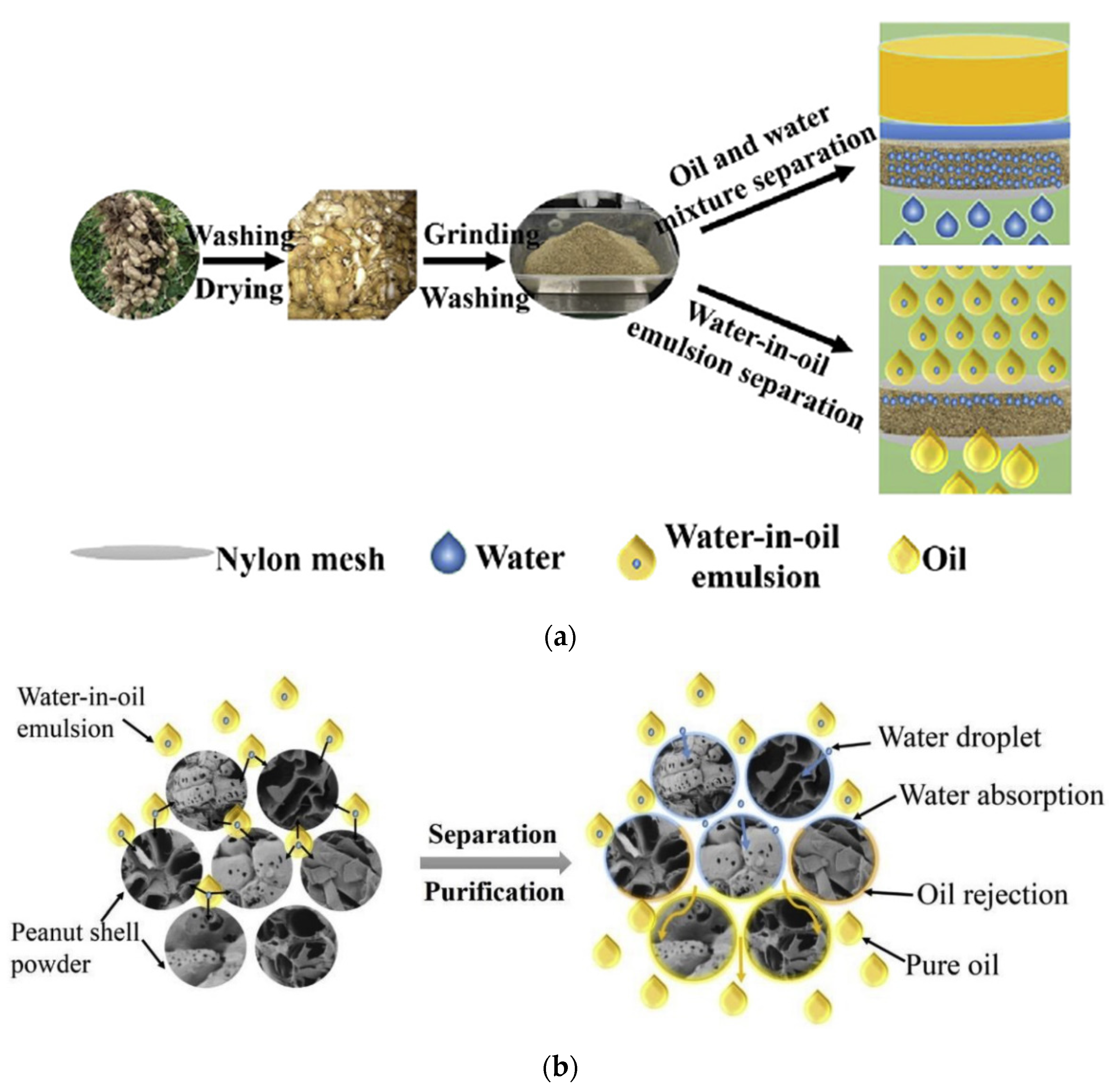
- Dai, Y.; Jing, Z.; Qiu, Z.; Zhu, Y.; Qiu, F.; Pan, J.; Zhang, T.; Li, C. Multifunctional biomass carbon fiber aerogel based on resource utilization of agricultural waste-peanut shells for fast and efficient oil–water/emulsion separation. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2022, 283, 115819. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakajima, L. Sustainable development goals for advanced materials provided by industrial wastes and biomass sources. Curr. Opin. Green Sustain. Chem. 2021, 28, 100439. [Google Scholar] [CrossRef]
- Soni, A.; Das, P.K.; Hashmi, A.W.; Yusuf, M.; Kamyab, H.; Chelliapan, S. Challenges and opportunities of utilizing municipal solid waste as alternative building materials for sustainable development goals: A review. Sustain. Chem. Pharm. 2022, 27, 100706. [Google Scholar] [CrossRef]
- Elsheekh, K.M.; Kamel, R.R.; Elsherif, D.M.; Shalaby, A.M. Achieving sustainable development goals from the perspective of solid waste management plans. J. Eng. Appl. Sci. 2021, 68, 9. [Google Scholar] [CrossRef]
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A.G. The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Zhao, B.; Ren, L.; Du, Y.; Wang, J. Eco-friendly separation layers based on waste peanut shell for gravity-driven water-in-oil emulsion separation. J. Clean. Prod. 2020, 255, 120184. [Google Scholar] [CrossRef]
- Li, J.; Xu, C.; Zhang, Y.; Tang, X.; Qi, W.; Wang, Q. Gravity-directed separation of both immiscible and emulsified oil/water mixtures utilizing coconut shell layer. J. Colloid Interface Sci. 2018, 511, 233–242. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Li, D.; Tang, X.; Feng, H.; Qi, W.; Wang, Q. Multifunctional walnut shell layer used for oil/water mixtures separation and dyes adsorption. Appl. Surf. Sci. 2017, 419, 869–874. [Google Scholar] [CrossRef]
- Abdel-Salam, M.O.; Younis, S.A.; Moustafa, Y.M.; Al-Sabagh, A.M.; Khalil, M.M.H. Microwave—Assisted production of hydrophilic carbon-based magnetic nanocomposites from saw-dust for elevating oil from oil field waste water. J. Clean. Prod. 2020, 249, 119355. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Y.; Song, T.; Bao, M.; Li, Y.; Lu, J.; Li, Y. Preparation of superhydrophobic magnetic sawdust for effective oil/water separation. J. Clean. Prod. 2020, 253, 120058. [Google Scholar] [CrossRef]
- Liu, W.; Cui, M.; Shen, Y.; Zhu, G.; Luo, L.; Li, M.; Li, J. Waste cigarette filter as nanofibrous membranes for on-demand immiscible oil/water mixtures and emulsions separation. J. Colloid Interface Sci. 2019, 549, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Qiu, F.; Yue, X.; Chen, Y.; Xu, J.; Zhang, T. Aramid nanofiber aerogel membrane extract from waste plastic for efficient separation of surfactant-stabilized oil-in-water emulsions. J. Environ. Chem. Eng. 2021, 9, 106137. [Google Scholar] [CrossRef]
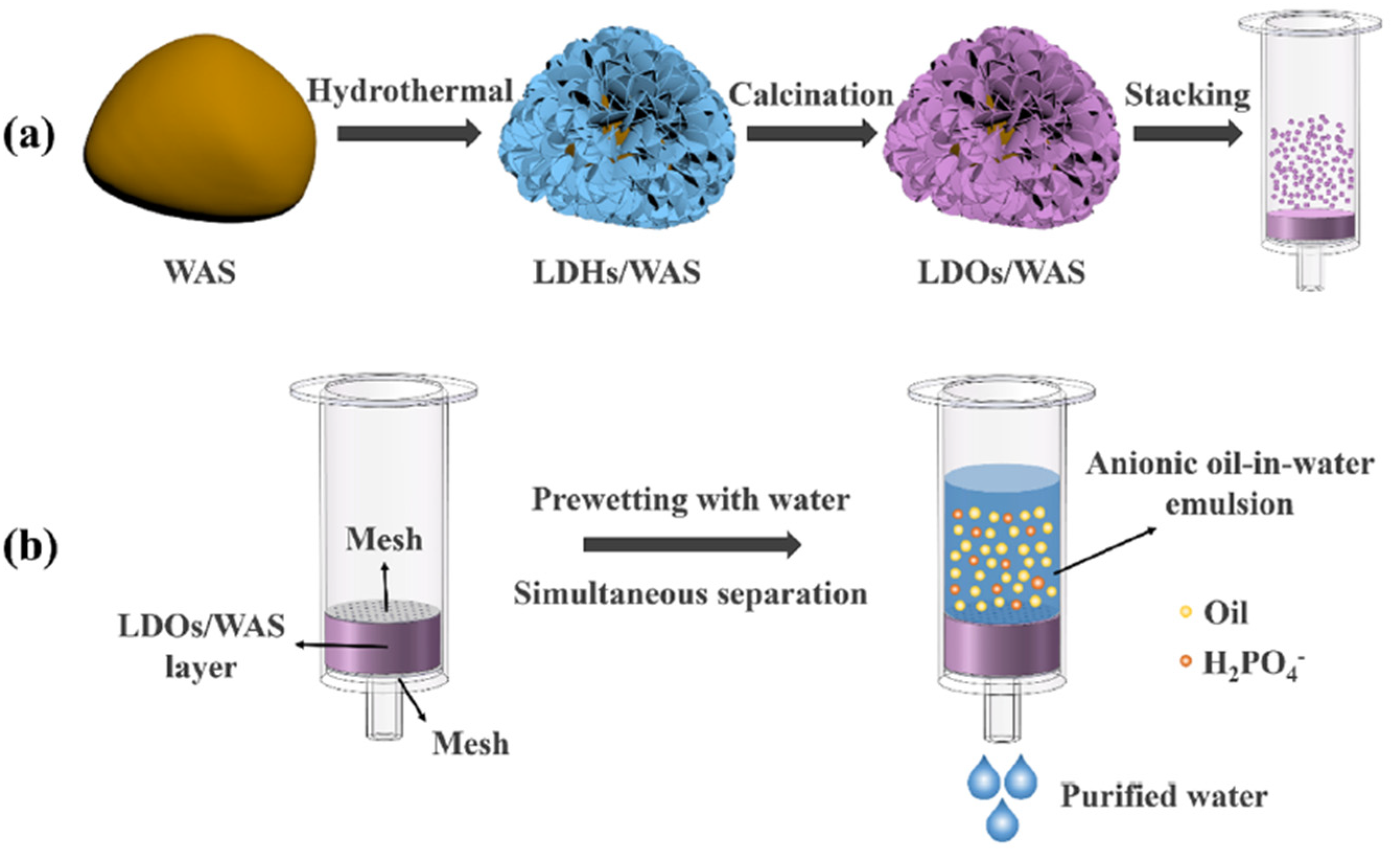
- Tian, Q.; Qiu, F.; Li, Z.; Xiong, Q.; Zhao, B.; Zhang, T. Structured sludge derived multifunctional layer for simultaneous separation of oil/water emulsions and anions contaminants. J. Hazard. Mater. 2022, 432, 128651. [Google Scholar] [CrossRef]
- Wu, M.; Mu, P.; Li, B.; Wang, Q.; Yang, Y.; Li, J. Pine powders-coated PVDF multifunctional membrane for highly efficient switchable oil/water emulsions separation and dyes adsorption. Sep. Purif. Technol. 2020, 248, 117028. [Google Scholar] [CrossRef]
- Sun, C.; Chen, K.; Wiafe Biney, B.; Wang, K.; Liu, H.; Guo, A.; Xia, W. Switchable wettability of grain-stacked filter layers from polyurethane plastic waste for oil/water separation. J. Colloid Interface Sci. 2022, 610, 970–981. [Google Scholar] [CrossRef]
- Wang, W.; Yang, D.; Mou, L.; Wu, M.; Wang, Y.; Tan, F.; Yang, F. Remodeling of waste corn stalks into renewable, compressible and hydrophobic biomass-based aerogel for efficient and selective oil/organic solvent absorption. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128940. [Google Scholar] [CrossRef]
- Yu, M.; Mishra, D.; Cui, Z.; Wang, X.; Lu, Q. Recycling papermill waste lignin into recyclable and flowerlike composites for effective oil/water separation. Compos. Part B Eng. 2021, 216, 108884. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Guo, J.; Chen, T.; Liu, H. Superhydrophobic polypyrrole-coated cigarette filters for effective oil/water separation. Appl. Sci. 2020, 10, 1985. [Google Scholar] [CrossRef]
- Singh, A.K. Surface engineering using PDMS and functionalized nanoparticles for superhydrophobic coatings: Selective liquid repellence and tackling COVID-19. Prog. Org. Coat. 2022, 171, 107061. [Google Scholar] [CrossRef]
- Lipika; Singh, A.K. Polydimethylsiloxane based sustainable hydrophobic/oleophilic coatings for oil/water separation: A review. Clean. Mater. 2022, 6, 100136. [Google Scholar] [CrossRef]
- Akhlamadi, G.; Goharshadi, E.K. Sustainable and superhydrophobic cellulose nanocrystal-based aerogel derived from waste tissue paper as a sorbent for efficient oil/water separation. Process Saf. Environ. Prot. 2021, 154, 155–167. [Google Scholar] [CrossRef]
- Azad, P.; Raut, S.; Vaish, R. Candle soot-coated egg carton material for oil water separation and detergent adsorption. Bull. Mater. Sci. 2020, 43, 7. [Google Scholar] [CrossRef]
- Chen, X.; Du, G.; Pizzi, A.; Xi, X. Superhydrophobic and Superoleophilic Fiber from Waste Bamboo Processing Residues for Oil/water Selective Separation. J. Wood Chem. Technol. 2020, 40, 58–72. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Zhao, X.; Du, R.; Xu, S.; Wang, Y.Z. Recycling waste epoxy resin as hydrophobic coating of melamine foam for high-efficiency oil absorption. Appl. Surf. Sci. 2020, 529, 147151. [Google Scholar] [CrossRef]
- Dai, G.; Zhang, Z.; Du, W.; Li, Z.; Gao, W.; Li, L. Conversion of skin collagen fibrous material waste to an oil sorbent with pH-responsive switchable wettability for high-efficiency separation of oil/water emulsions. J. Clean. Prod. 2019, 226, 18–27. [Google Scholar] [CrossRef]
- Yu, C.; Lin, W.; Jiang, J.; Jing, Z.; Hong, P.; Li, Y. Preparation of a porous superhydrophobic foam from waste plastic and its application for oil spill cleanup. RSC Adv. 2019, 9, 37759–37767. [Google Scholar] [CrossRef]
- Sow, P.K.; Ishita; Singhal, R. Sustainable approach to recycle waste polystyrene to high-value submicron fibers using solution blow spinning and application towards oil-water separation. J. Environ. Chem. Eng. 2020, 8, 102786. [Google Scholar] [CrossRef]
- Liu, X.; Tian, F.; Zhao, X.; Du, R.; Xu, S.; Wang, Y.Z. Multiple functional materials from crushing waste thermosetting resins. Mater. Horiz. 2021, 8, 234–243. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Yunos, M.Z.; Ismail, A.F.; Salleh, W.N.W. Facile fabrication of superhydrophobic and superoleophilic green ceramic hollow fiber membrane derived from waste sugarcane bagasse ash for oil/water separation. Arab. J. Chem. 2020, 13, 3558–3570. [Google Scholar] [CrossRef]
- Ao, C.; Jiang, L.; Wang, Q.; Xue, X.; Gai, J.; Zhang, W.; Lu, C. One-pot superhydrophilic surface modification of waste polyurethane foams for high-efficiency oil/water separation. J. Environ. Manag. 2022, 315, 115140. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qian, W.; Chen, Y.; Xu, P.; Li, J.; Yang, J. A new treasure in industrial solid waste—Coal fly ash for effective oil/water separation. J. Taiwan Inst. Chem. Eng. 2021, 118, 196–203. [Google Scholar] [CrossRef]
- Lu, J.; Li, F.; Miao, G.; Miao, X.; Ren, G.; Wang, B.; Song, Y.; Li, X.; Zhu, X. Superhydrophilic/superoleophobic shell powder coating as a versatile platform for both oil/water and oil/oil separation. J. Memb. Sci. 2021, 637, 119624. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, H.; Xia, B.; Guo, K. The underwater superoleophobic natural pomelo peel fibers powders coatings for efficiently oil/water separation. J. Nat. Fibers 2019, 16, 1177–1188. [Google Scholar] [CrossRef]
- Wei, B.; Luo, X.; Song, X.; Guo, H.; Dai, L.; Zhang, H.; Wang, G. Quartz sand filter media with special wettability for continuous and efficient oil/water separation and dye adsorption. Processes 2020, 8, 1083. [Google Scholar] [CrossRef]
- Li, L.; Zhu, J.; Zeng, Z. New Approach for Recycling Office Waste Paper: An Efficient and Recyclable Material for Oily Wastewater Treatment. ACS Appl. Mater. Interfaces 2020, 12, 55894–55902. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, J.; Yue, X.; Qiu, F.; Yang, D.; Zhang, T. Study on the application of waste bricks in emulsified oil-water separation. J. Clean. Prod. 2020, 251, 119609. [Google Scholar] [CrossRef]


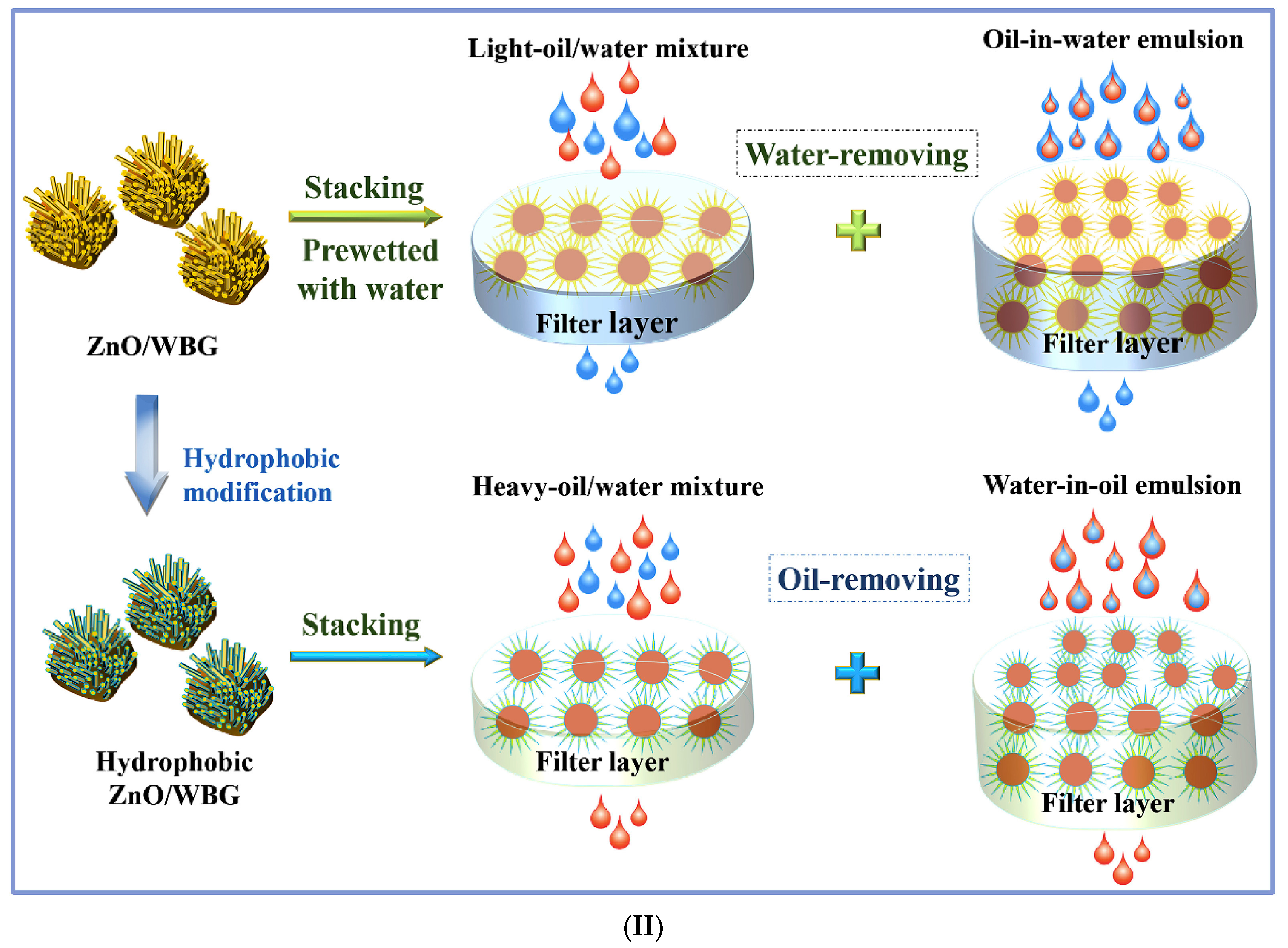



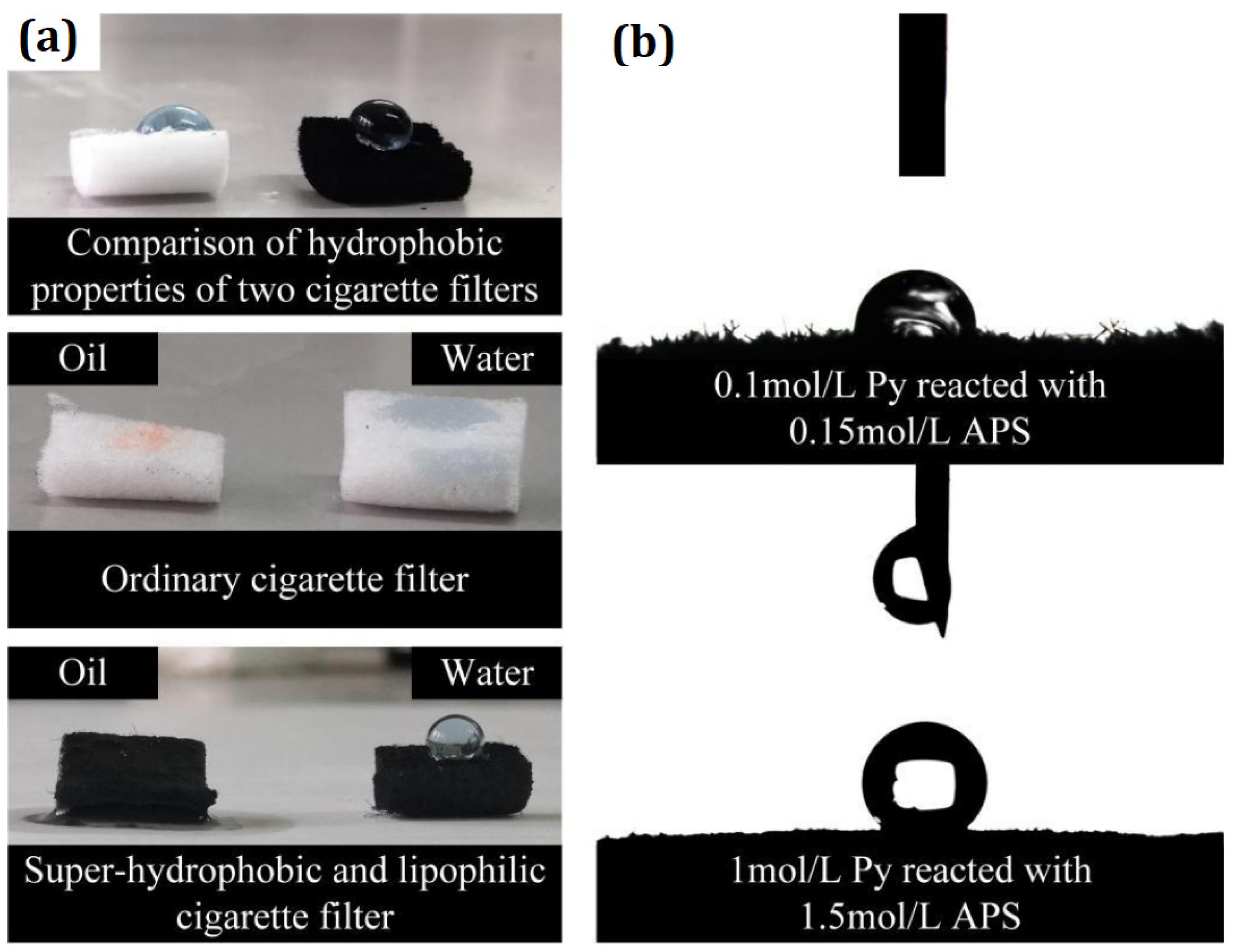
| Mechanism | Separation Process | Waste Material | Sources of Waste Materials | Other Material for Surface Modification | Coating Method | Contact Angle | Separation Efficacy | Flux/Sorption Capacity | Separated Oils | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrophobic /oleophilic | Sorption | Waste tissue paper | Cellulose waste | Polyvinyl alcohol, sodium chlorite, sodium hydroxide, tetraethyl orthosilicate | Freeze-drying | WCA 154.93° ± 4.14 | - | 69−168 g g−1 Sorption capacity | Chloroform, motor oil, acetone, DMF, olive oil and toluene | The modified aerogel exhibits sorption efficay greater than 92% even after repeating 20 cycles and 89% up 50 cycles of repeation process. | [72] |
| Egg carton material | Cellulose waste | Acetone, candle soot | Dip-coating | WCA = 142.2°, OCA = 43.1° | - | Absorption: 3 g g−1 | Petrol, diesel, refined oil, coconut, engine and mustard oil | The maximum and minium sorption capacity of the modified surfaces were 3.1 and 1.6 g g−1 for mustard oil and petrol, respectively. | [73] | ||
| Waste bamboo | Agricultural waste | Methyl trimethoxy silane, Anhydrous Alcohol | Chemical vapor deposition and delignification | WCA = 153°, OCA = 0° | - | Absorption: 18.8 g g−1 | Silicone oil, diesel oil, chloroform, paraffin oil and toluene | The highest oil absorption capacities of the developed surfaces was >40–55%. | [74] | ||
| Epoxy resins | Industrial waste | Epoxy oligomer, 4, 4-diaminodiphenyl methane, N-methyl kelopyrrolidide | Dip-coating | WCA = 146.5° | - | Absorption: 116 g g−1. | Soybean oil, colza oil, pump oil, n-hexane, chloroform and silicone oil | The selective oil absorption ability of developed surface stabilized was approximately 53 g g−1 with respect to gasoline even after the 10 successive repetition of separation cycles. | [75] | ||
| Skin collagen fiber waste | Industrial waste | Methacrylic acid, glycidyl Methacrylate, sodium dodecyl sulphate, dodecyl Mercaptan | In situ free radical polymerization | WCA 145° | 99.93 ± 0.03% | -- | Soybean oil and motor oil | The developed modified surfcace exhbited high separation efficiency (>99.93%) even after 10 cycles and 3 cycles for surfactant free oil in water emulsion and surfactant-stabilized oil-in-water emulsion respectively. | [76] | ||
| Polystyrene foam | Plastic waste | Styrene, Tetraethyl orthosilicate, Ammonia solution and hexadecyltrimethoxysilane | Pickering emulsion (HIPPE) technique | - | - | 20.4–58.1 g g−1 Absorption capacity | Dichloromethane, chloroform, acetone, hexane, dichloromethane, acetic ether, methanol, ethanol, toluene, peanut oil, diesel, pump oil and crude oil | The fabricated foam adsorb oil and revealed the adsorption capacity above 90% even after the 10 consecutive cycles. | [77] | ||
| Filtration | Waste polystyrene | Plastic waste | Ethyl acetate, craft polystyrene | Blow spinning | WCA 138° OCA 0° | 97% | - | Diesel oil | After repeating consecutive two cycles the separation efficacy of the modified material was >90%. | [78] | |
| Thermosetting resins | Plastic waste | - | Simple Mechanical crushing | WCA 117° | 97% | 15 987 L m−2 h−1 s Separation flux | Toluene, chloroform, n-hexane and gasoline | The prepared surface can easily separate the oil/water mixtures as well as emulsion with the droplet size more than 50 nm. | [79] | ||
| Sugarcane bagasse ash | Agricultural waste | Methyl triethoxysilane, tetraethoxysilane | Sol–gel process | WCA 163.9° | 99.9% | Oil flux: 137.2 L m−2 h−1 s | Crude oil | The separation efficacy of the modified surfaces was examined in consideration of process variables such as grafting time (30 to 60 min), grafting cycle (1–4 cycles), and calcination temperature (400–600 °C) to separation effieciency. | [80] | ||
| Underwater super-oleophobic/ hydrophilic | Filtration | Polyurethane foams | Plastic waste | Ferric chloride, dopamine, ammonium hydroxide, polydopamine | Polymerization | Underwater OCA 145.7 ± 2.8 | 98.7% | Water flux higher than 57,796 L h−1 m−2 | Hexane, cyclohexane, liquid paraffin, pump oil and petroleum ether | The oil/water separation effucacy of the developed surface of modfied foam was >97% even after the 100 cycles of the recycling test. | [81] |
| Carbon fibers | Industrial waste | Cellulose filter papers, tannic acid, tris(hydroxymethyl)- Aminomethane (Tris), (3-Aminopropyl) triethoxysilane, sodium Dodecyl sulphate, sodium hydroxide, hydrochloric Acid, dichloromethane (DCM) | Pyrolysis method | Underwater OCA 157.2° | 99.8% | - | Dichloromethane (DCM), canola oil, n-hexane, kerosene and silicone oils | The developed surface has high roughness 2.1 times higher than the raw material as well as effective surface area was 1.6 times higher than the control due to development of micro–nanostructured sructured on the surface. | [49] | ||
| Coal fly ash | Industrial waste | Coal fly ash, distilled water | - | Underwater OCA 155 ± 2° | 99.9% | - | Kerosene, diesel and hexane | Oil/water separation efficacy was directly proportional to the thickness of the separating membrane. However separating flux was decreases from 1050 L m−2 h−1 to 513 L m−2 h−1 with the increase of thickness. | [82] | ||
| Natural shell | Raw materials discarded on the beach | Perfluorooctanoic acid, (3-aminopropyl) triethoxysilane and bis (3-(trimethoxy silyl) propyl) amine, Spray-Mount™ Super 75 | Dip-coating method | Underwater OCA 154° | 99.3% | - | Chloroform, olive oil, decane and hexadecane | The developed surface was capable to separate both oil/water and oil/oil mixtures of different polarity. | [83] | ||
| Pomelo Peel fibers | Fruit waste | Anhydrous ethanol, waterborne polyurethane | Spraying method | Underwater OCA | 97.0% | - | Kerosene, motor oil and soybean oil, hexane, liquid paraffin and chloroform | The modified mesh exhbited high separation efficiency (>97.0%) in oil/water mixtures of different pHs. | [84] | ||
| Coconut shell waste | Fruit waste | Quartz sand, waterborne polyurethane | Dip-coating method | Underwater OCA 151.2° | 99.92% | - | Hexane, dichloromethane, trichloromethane, anhydrous ethanol and petroleum ether | The developed material exhibits the permeability coefficient of organic solvents such as cyclohexane, petroleum ether, dichloromethane, and trichloromethane were higher than 10 m/h than water 9.37 m/h. | [85] | ||
| Waste cigarette filter | Cellulose waste | Trichloromethane, acetone and N, N-dimethylformamide | Electrospinning approach | Underwater OCA | 99.9% | Water flux was about 1000 L m−2 h−1 | Kerosene, diesel, petroleum ether, hexane and trichloromethane | The prepared surface exhibits underwater superoleophobicity and underoil super hydrophobicity. In addition, separation efficiency was >99% even after the 10 repeating cycles of sepration process. | [62] | ||
| Waste Paper | Cellulose waste | - | - | Underwater OCA 151 ± 2.5° | 99% | - | Soyabean oil, cyclohexane and hexane | The modified waste papers exhbited very flux of oil/water mixture sepration revealed water flux, i.e., 1126, 1837, 3246, 1273, and 1145 L·m−2·h−1 for stickers, notebook, lens paper, envelopes, and receipts, respectively. | [86] | ||
| Bricks Powder | Waste bricks | Distilled water and ethanol | Physical refining process | Underwater OCA 150° | 98.4% | Flux 4384 L m−2 h−1 | Hexane, methylbenzene, trichloromethane and absolute ethyl alcohol and soyabean oil | The fabricated waste bricks granules (100–400 µm) have superhydrophilicity and superoleophilicity in air and under-liquid amphiphobic properties. | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.K. Oil/Water Separation Using Waste-Derived Functional Materials with Special Wetting Behavior. Resources 2022, 11, 83. https://doi.org/10.3390/resources11100083
Singh AK. Oil/Water Separation Using Waste-Derived Functional Materials with Special Wetting Behavior. Resources. 2022; 11(10):83. https://doi.org/10.3390/resources11100083
Chicago/Turabian StyleSingh, Arun K. 2022. "Oil/Water Separation Using Waste-Derived Functional Materials with Special Wetting Behavior" Resources 11, no. 10: 83. https://doi.org/10.3390/resources11100083
APA StyleSingh, A. K. (2022). Oil/Water Separation Using Waste-Derived Functional Materials with Special Wetting Behavior. Resources, 11(10), 83. https://doi.org/10.3390/resources11100083







