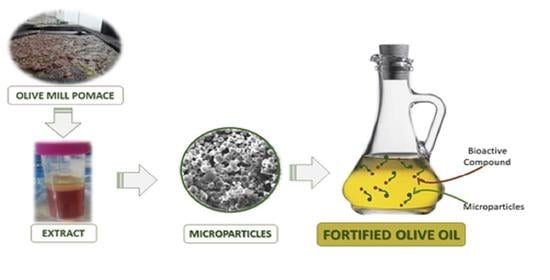
Olive Mill Pomace Extract Loaded Ethylcellulose Microparticles as a Delivery System to Improve Olive Oils Oxidative Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Encapsulation of Phenolic Compounds from Olive Mill Pomace
2.3. Characterization of Olive Mill Pomace Extract-Loaded Ethylcellulose Microparticles
2.4. Schaal Oven Storage Stability Test
2.5. Determination of Oxidative Stability Indices
2.5.1. Determination of Peroxide Value (PV)
2.5.2. Determination of p-Anisidine Value (p-AV)
2.5.3. Determination of the Total Oxidation (TOTOX) Value
2.5.4. Determination of Free Fatty Acids (FFA) Content
2.5.5. Changes on the K232 and K270 Extinction Coefficients
2.5.6. Evaluation of the Total Antioxidant Activity and the Total Phenolic Content on Olive Oil Samples
2.6. Statistical Analysis
3. Results and Discussion
3.1. Olive Mill Pomace Extract and Microparticles Characterization
3.2. Changes in the Peroxide Values (PVs)
3.3. Changes on the p-Anisidine Values (p-AVs)
3.4. Changes in the Total Oxidation (TOTOX) Values
3.5. Changes in the Free Fatty Acids (FFAs) Content
3.6. Changes on the K232 and K270 Extinction Coefficients
3.7. Changes in the Total Antioxidant Activity (TAA) and the Total Phenolic Content (TPC)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [PubMed]
- Donner, M.; Radić, I.; Erraach, Y.; El Hadad-Gauthier, F. Implementation of Circular Business Models for Olive Oil Waste and By-Product Valorization. Resources 2022, 11, 68. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Deriving valorization of phenolic compounds from olive oil by-products for food applications through microencapsulation approaches: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 61, 920–945. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of olive oil phenols in neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moral, R.E.E. Influence of Olive Oil: Its Components on Breast Cancer: Molecular, Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef]
- Modesti, M. E-Nose and Olfactory Assessment: Teamwork or a Challenge to the Last Data? The Case of Virgin Olive Oil Stability and Shelf Life. Appl. Sci. 2021, 11, 8453. [Google Scholar] [CrossRef]
- Méndez, L.; Sacchi, R.; Medina, I.; Aubourg, S.P. Nutritional and Preservative Properties of Polyphenol-Rich Olive Oil: Effect on Seafood Processing and Storage. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Bravo-Diaz, C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 455–477. [Google Scholar]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef] [Green Version]
- Wanasundara, P.K.J.P.D.; Shahidi, F. Antioxidants: Science, Technology, and Applications. Bailey’s Ind. Oil Fat Prod. 2005, 6, 1–61. [Google Scholar]
- Silveira Alexandre, A.C.; Corrêa Albergaria, F.; dos Santos Ferraz e Silva, L.M.; Carneiro Fernandes, L.A.; de Sousa Gomes, M.E.; Pimenta, C.J. Effect of natural and synthetic antioxidants on oxidation and storage stability of mechanically separated tilapia meat. LWT 2022, 154, 112679. [Google Scholar]
- Augustyniak, A.; Bartosz, G.; Čipak, A.; Duburs, G.; Horáková, L.U.; Łuczaj, W.; Majekova, M.; Odysseos, A.D.; Rackova, L.; Skrzydlewska, E.; et al. Natural and synthetic antioxidants: An updated overview. Free. Radic. Res. 2010, 44, 1216–1262. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Tavares, L.; Santos, L. Response Surface Modeling and Optimization of the Extraction of Phenolic Antioxidants from Olive Mill Pomace. Molecules 2022, 27, 8620. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Tavares, L.; Santos, L. Extraction and encapsulation of bioactive compounds from olive mill pomace: Influence of loading content on the physicochemical and structural properties of microparticles. J. Food Meas. Charact. 2022, 16, 3077–3094. [Google Scholar] [CrossRef]
- Paulo, F.; Tavares, L.; Santos, L. In vitro digestion, bioaccessibility, and release kinetics studies of encapsulated bioactive compounds obtained from olive mill pomace. J. Food Meas. Charact. 2022, 16, 4880–4895. [Google Scholar] [CrossRef]
- Tavares, L.; Santos, L.; Noreña, C.P.Z. Bioactive compounds of garlic: A comprehensive review of encapsulation technologies, characterization of the encapsulated garlic compounds and their industrial applicability. Trends Food Sci. Technol. 2021, 114, 232–244. [Google Scholar]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- Gaitzsch, F.; Gäbler, A.; Kraume, M. Analysis of droplet expulsion in stagnant single water-in-oil-in-water double emulsion globules. Chem. Eng. Sci. 2011, 66, 4663–4669. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; De la Torre, R. Potential Role of Olive Oil Phenolic Compounds in the Prevention of Neurodegenerative Diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [Green Version]
- European Commission, Additives of the European Parliament and of the Council on food additives by establishing a Union list of food additives approved for use in food additives, food enzymes, food flavourings and nutrients. Off. J. Eur. Union 2011, 1130, 178–204.
- Ahmadi, P.; Jahanban-Esfahlan, A.; Ahmadi, A.; Tabibiazar, M.; Mohammadifar, M. Development of Ethyl Cellulose-based Formulations: A Perspective on the Novel Technical Methods. Food Rev. Int. 2022, 38, 685–732. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Inclusion of hydroxytyrosol in ethyl cellulose microparticles: In vitro release studies under digestion conditions. Food Hydrocoll. 2018, 84, 104–116. [Google Scholar] [CrossRef]
- Khwaldia, K.; Attour, N.; Matthes, J.; Beck, L.; Schmid, M. Olive byproducts and their bioactive compounds as a valuable source for food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1218–1253. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Castaño, Á.L.; Lozano, M.; González-Gómez, D. Influence of the microencapsulation on the quality parameters and shelf-life of extra-virgin olive oil encapsulated in the presence of BHT and different capsule wall components. Food Res. Int. 2012, 45, 256–261. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Ind. Crops Prod. 2016, 80, 141–147. [Google Scholar] [CrossRef]
- Michotte, D.; Rogez, H.; Chirinos, R.; Mignolet, E.; Campos, D.; Larondelle, Y. Linseed oil stabilisation with pure natural phenolic compounds. Food Chem. 2011, 129, 1228–1231. [Google Scholar]
- Sun-Waterhouse, D.; Zhou, J.; Miskelly, G.M.; Wibisono, R.; Wadhwa, S.S. Stability of encapsulated olive oil in the presence of caffeic acid. Food Chem. 2011, 126, 1049–1056. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils. Food Chem. 2002, 77, 503–510. [Google Scholar] [CrossRef]
- Wai, W.T.; Saad, B.; Lim, B.P. Determination of TOTOX value in palm oleins using a FI-potentiometric analyzer. Food Chem. 2009, 113, 285–290. [Google Scholar] [CrossRef]
- Maggio, R.M.; Kaufman, T.S.; Carlo, M.D.; Cerretani, L.; Bendini, A.; Cichelli, A.; Compagnone, D. Monitoring of fatty acid composition in virgin olive oil by Fourier transformed infrared spectroscopy coupled with partial least squares. Food Chem. 2009, 114, 1549–1554. [Google Scholar] [CrossRef]
- Pokorny, J.; Yanishlieva, N.; Gordon, M.H. Antioxidants in Food: Practical Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.P.; Pereira, J.A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- European Commission, Commission Regulation No 2568/91. Off. J. Eur. Union 2019, 53, 1–83.
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.F.; Ramalhosa, M.J.; Alves, R.C.; Dias, A.; Soares, C.M.D.; Oliva-Teles, M.T.; Delerue-Matos, C. Total antioxidant capacity of plant infusions: Assessment using electrochemical DNA-based biosensor and spectrophotometric methods. Food Control 2016, 68, 153–161. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods Enzymol; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Malheiro, R.; Rodrigues, N.; Manzke, G.; Bento, A.; Pereira, J.A.; Casal, S. The use of olive leaves and tea extracts as effective antioxidants against the oxidation of soybean oil under microwave heating. Ind. Crops Prod. 2013, 44, 37–43. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Asensio, C.M.; Nepote, V.; Grosso, N.R. Chemical stability of extra-virgin olive oil added with oregano essential oil. J. Food Sci. 2011, 76, S445–S450. [Google Scholar] [CrossRef]
- Frega, N.; Mozzon, M.; Lercker, G. Effects of free fatty acids on oxidative stability of vegetable oil. J. Am. Oil Chem. Soc. 1999, 76, 325–329. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M.I. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007, 100, 246–254. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Pro-Oxidant Effects of β-Carotene During Thermal Oxidation of Edible Oils. J. Am. Oil Chem. Soc. 2013, 90, 881–889. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa de Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant effects of a hydroxytyrosol-based pharmaceutical formulation on body composition, metabolic state, and gene expression: A randomized double-blinded, placebo-controlled crossover trial. Oxid. Med. Cell. Longev. 2017, 2017, 2473495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Alonso, S.; Fregapane, G.; Salvador, M.D.; Gordon, M.H. Changes in phenolic composition and antioxidant activity of virgin olive oil during frying. J. Agric. Food Chem. 2003, 51, 667–672. [Google Scholar] [CrossRef]
- Ocakoglu, D.; Tokatli, F.; Ozen, B.; Korel, F. Distribution of simple phenols, phenolic acids and flavonoids in Turkish monovarietal extra virgin olive oils for two harvest years. Food Chem. 2009, 113, 401–410. [Google Scholar] [CrossRef]
- Gargouri, B.; Zribi, A.; Bouaziz, M. Effect of containers on the quality of Chemlali olive oil during storage. J. Food Sci. Technol. 2015, 52, 1948–1959. [Google Scholar] [CrossRef]
- Samaniego Sánchez, C.; Troncoso González, A.M.; García-Parrilla, M.C.; Quesada Granados, J.J.; López García de la Serrana, H.; López Martínez, M.C. Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Anal. Chim. Acta 2007, 593, 103–107. [Google Scholar] [CrossRef]
 ) and incorporating BHA (
) and incorporating BHA ( ), BHT (
), BHT ( ), BHA + BHT (
), BHA + BHT ( ), extract (
), extract ( ) and microparticles (
) and microparticles ( ) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—Blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—Blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
 ) and incorporating BHA (
) and incorporating BHA ( ), BHT (
), BHT ( ), BHA + BHT (
), BHA + BHT ( ), extract (
), extract ( ) and microparticles (
) and microparticles ( ) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—Blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—Blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
 ) and incorporating BHA (
) and incorporating BHA ( ), BHT (
), BHT ( ), BHA + BHT (
), BHA + BHT ( ), extract (
), extract ( ) and microparticles (
) and microparticles ( ) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage. (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage. (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
 ) and incorporating BHA (
) and incorporating BHA ( ), BHT (
), BHT ( ), BHA + BHT (
), BHA + BHT ( ), extract (
), extract ( ) and microparticles (
) and microparticles ( ) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage. (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage. (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
 ) and incorporating BHA (
) and incorporating BHA ( ), BHT (
), BHT ( ), BHA + BHT (
), BHA + BHT ( ), extract (
), extract ( ) and microparticles (
) and microparticles ( ) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—Blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—Blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
 ) and incorporating BHA (
) and incorporating BHA ( ), BHT (
), BHT ( ), BHA + BHT (
), BHA + BHT ( ), extract (
), extract ( ) and microparticles (
) and microparticles ( ) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—Blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; EVOO—Extra-Virgin Olive Oil; MPs—Microparticles; ROO—Blend of refined and virgin olive oils; VOO—Virgin Olive Oil).
 ) and incorporating BHA (
) and incorporating BHA ( ), BHT (
), BHT ( ), BHA + BHT (
), BHA + BHT ( ), extract (
), extract ( ) and microparticles (
) and microparticles ( ) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage. (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; MPs—Microparticles).
) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage. (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; MPs—Microparticles).
 ) and incorporating BHA (
) and incorporating BHA ( ), BHT (
), BHT ( ), BHA + BHT (
), BHA + BHT ( ), extract (
), extract ( ) and microparticles (
) and microparticles ( ) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage. (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; MPs—Microparticles).
) extra-virgin olive oil (A), virgin olive oil (B), and a blend of refined and virgin oil (C) during storage. (BHA—Butylated Hydroxyanisole; BHT—Butylated Hydroxytoluene; MPs—Microparticles).

| Antioxidant Activity a | |
| IC50 mg/gOMP | 223.9 ± 1.2 |
| mg/L extract | 74.6 ± 0.4 |
| Phenols Content a | |
| mgGAE/gOMP | 50.5 ± 1.5 |
| mgGAE/L extract | 16.8 ± 0.5 |
| Bioactive Compounds | Encapsulation Efficiency (EE) (% w/w) | Theoretical Loading Content (TLC) (% w/w) | Actual Loading Content (ALC) (% w/w) |
|---|---|---|---|
| Antioxidants (AO) | 92.6 ± 2.1 | 18.9 ± 2.7 | 17.5 ± 1.6 |
| Phenolic Compounds (PC) | 96.0 ± 0.3 | 5.0 ± 0.4 | 4.8 ± 0.9 |
| Olive Oil Sample | Storage Time (Days) | Peroxide Value (mEq/kg) * | |||||
|---|---|---|---|---|---|---|---|
| Blank | BHA | BHT | BHA + BHT | OMP Extract | MPs | ||
| EVOO | 0 | 11.5 ± 0.2 e | 11.3 ± 0.2 e | 11.5 ± 0.3 d | 11.2 ± 0.4 e | 11.6 ± 0.1 d | 11.5 ± 0.2 e |
| 6 | 15.5 ± 0.1 d | 13.4 ± 0.2 d | 14.5 ± 0.2 c | 13.9 ± 0.2 d | 13.1 ± 0.1 c | 12.1 ± 0.1 d | |
| 12 | 17.7 ± 0.1 c | 15.8 ± 0.2 c | 15.7 ± 0.1 b | 15.4 ± 0.2 c | 14.9 ± 0.2 b | 15.8 ± 0.2 c | |
| 18 | 18.6 ± 0.1 b | 17.8 ± 0.1 b | 18.6 ± 0.2 a | 17.9 ± 0.5 b | 17.7 ± 0.3 a | 16.6 ± 0.1 b | |
| 24 | 20.4 ± 0.6 a | 19.5 ± 0.3 a | 19.4 ± 0.6 a | 19.1 ± 0.3 a | 18.0 ± 0.2 a | 17.9 ± 0.6 a | |
| VOO | 0 | 11.4 ± 0.1 e | 11.4 ± 0.1 e | 11.4 ± 0.1 d | 11.3 ± 0.1 e | 11.4 ± 0.1 e | 11.5 ± 0.1 e |
| 6 | 13.5 ± 0.1 d | 12.2 ± 0.2 d | 15.5 ± 0.1 c | 13.5 ± 0.3 d | 12.4 ± 0.2 d | 12.3 ± 0.2 d | |
| 12 | 15.9 ± 0.1 c | 13.3 ± 0.3 c | 16.9 ± 0.2 b | 15.4 ± 0.6 c | 14.6 ± 0.2 c | 13.3 ± 0.1 c | |
| 18 | 19.7 ± 0.2 b | 15.4 ± 0.2 b | 18.7 ± 0.2 a | 16.7 ± 0.3 b | 16.1 ± 0.1 b | 14.1 ± 0.2 b | |
| 24 | 21.7 ± 0.5 a | 18.6 ± 0.4 a | 19.5 ± 0.5 a | 19.1 ± 0.2 a | 18.3 ± 0.4 a | 16.2 ± 0.5 a | |
| ROO | 0 | 11.7 ± 0.1 e | 11.6 ± 0.2 c | 11.8 ± 0.1 d | 11.6 ± 0.2 c | 11.4 ± 0.1 d | 11.4 ± 0.1 c |
| 6 | 12.6 ± 0.1 d | 12.1 ± 0.1 b | 12.3 ± 0.3 c | 12.3 ± 0.1 b | 12.3 ± 0.2 c | 12.2 ± 0.1 b | |
| 12 | 23.4 ± 0.2 c | 13.5 ± 0.5 a | 13.8 ± 0.2 b | 12.7 ± 0.6 b | 12.5 ± 0.1 c | 12.4 ± 0.2 b | |
| 18 | 14.7 ± 0.1 b | 13.8 ± 0.3 a | 14.6 ± 0.1 a | 14.3 ± 0.1 a | 13.6 ± 0.1 b | 12.5 ± 0.6 b | |
| 24 | 15.8 ± 0.2 a | 14.2 ± 0.7 a | 14.8 ± 0.2 a | 14.9 ± 0.4 a | 14.8 ± 0.2 a | 13.4 ± 0.4 a | |
| Olive Oil Sample | Storage Time (Days) | TOTOX Value a | |||||
|---|---|---|---|---|---|---|---|
| Blank | BHA | BHT | BHA + BHT | OMP Extract | MPs | ||
| EVOO | 0 | 17.7 ± 0.4 e | 17.6 ± 0.4 e | 18.0 ± 0.6 e | 18.0 ± 0.6 e | 17.8 ± 0.2 e | 18.0 ± 0.5 d |
| 6 | 24.0 ± 0.3 d | 21.8 ± 0.4 d | 24.6 ± 0.4 d | 23.3 ± 0.3 d | 22.0 ± 0.2 d | 21.0 ± 1.2 c | |
| 12 | 30.4 ± 0.4 c | 25.9 ± 0.4 c | 27.1 ± 0.3 c | 26.2 ± 0.8 c | 25.2 ± 0.3 c | 25.6 ± 1.4 b | |
| 18 | 35.4 ± 0.2 b | 31.5 ± 0.3 b | 33.7 ± 0.4 b | 32.3 ± 0.8 b | 30.3 ± 0.5 b | 27.9 ± 1.2 b | |
| 24 | 43.5 ± 0.9 a | 38.2 ± 0.6 a | 36.9 ± 1.2 a | 36.8 ± 0.7 a | 33.5 ± 0.4 a | 31.0 ± 1.2 a | |
| VOO | 0 | 16.7 ± 0.2 e | 16.9 ± 0.2 e | 17.3 ± 0.2 f | 17.1 ± 0.4 e | 17.0 ± 0.2 e | 16.7 ± 0.2 e |
| 6 | 20.9 ± 0.3 d | 20.3 ± 0.4 d | 24.3 ± 0.2 d | 23.2 ± 0.4 d | 20.7 ± 0.3 d | 19.7 ± 0.3 d | |
| 12 | 32.0 ± 0.4 c | 23.0 ± 0.5 c | 27.1 ± 0.4 c | 28.0 ± 1.5 c | 24.6 ± 0.3 c | 22.1 ± 0.2 c | |
| 18 | 37.2 ± 0.4 b | 28.5 ± 0.4 b | 32.1 ± 0.4 b | 32.4 ± 0.6 b | 29.3 ± 0.3 b | 25.3 ± 0.2 b | |
| 24 | 43.9 ± 0.9 a | 36.5 ± 0.6 a | 37.2 ± 0.1 a | 36.1 ± 0.8 a | 33.6 ± 0.5 a | 30.2 ± 0.4 a | |
| ROO | 0 | 18.2 ± 0.3 d | 18.2 ± 0.4 e | 18.7 ± 0.2 e | 19.3 ± 0.3 e | 17.7 ± 0.2 e | 18.2 ± 0.1 e |
| 6 | 23.7 ± 0.2 c | 21.4 ± 0.2 d | 21.6 ± 0.5 d | 22.2 ± 0.2 d | 20.8 ± 0.4 d | 19.7 ± 0.2 d | |
| 12 | 38.8 ± 0.6 a | 24.7 ± 0.7 c | 24.4 ± 0.4 c | 24.0 ± 1.0 c | 21.6 ± 0.2 c | 22.8 ± 0.2 c | |
| 18 | 33.3 ± 0.4 b | 28.6 ± 0.5 b | 29.3 ±0.2 b | 29.4 ± 0.2 b | 27.4 ± 0.2 b | 24.5 ± 0.7 b | |
| 24 | 37.0 ± 0.9 a | 33.9 ± 1.4 a | 34.3 ± 0.4 a | 34.7 ± 0.4 a | 31.9 ± 0.4 a | 29.8 ± 0.6 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulo, F.; Tavares, L.; Santos, L. Olive Mill Pomace Extract Loaded Ethylcellulose Microparticles as a Delivery System to Improve Olive Oils Oxidative Stability. Resources 2023, 12, 6. https://doi.org/10.3390/resources12010006
Paulo F, Tavares L, Santos L. Olive Mill Pomace Extract Loaded Ethylcellulose Microparticles as a Delivery System to Improve Olive Oils Oxidative Stability. Resources. 2023; 12(1):6. https://doi.org/10.3390/resources12010006
Chicago/Turabian StylePaulo, Filipa, Loleny Tavares, and Lúcia Santos. 2023. "Olive Mill Pomace Extract Loaded Ethylcellulose Microparticles as a Delivery System to Improve Olive Oils Oxidative Stability" Resources 12, no. 1: 6. https://doi.org/10.3390/resources12010006