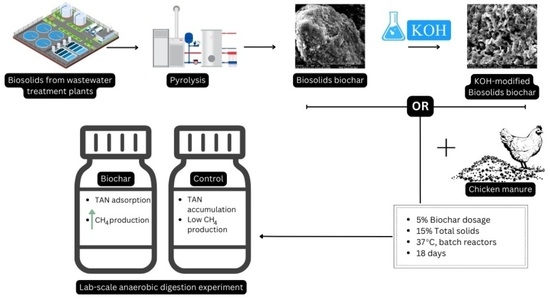
Biosolids-Derived Biochar Improves Biomethane Production in the Anaerobic Digestion of Chicken Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Collection and Preparation
2.2. Chicken Manure and Sludge Characterisation
2.3. Biochar Treatment and Characterisation
2.4. Experimental Setup and Gas Sampling
2.5. Biomethane Determination
2.6. Post-Digestion Chemical Analysis
2.7. Biochar Extraction
2.8. DNA Extraction
2.9. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.10. 16S rRNA Amplicon Sequencing for Bacterial Phyla and Archaeal Family Analysis
2.11. Data Analysis
3. Results and Discussion
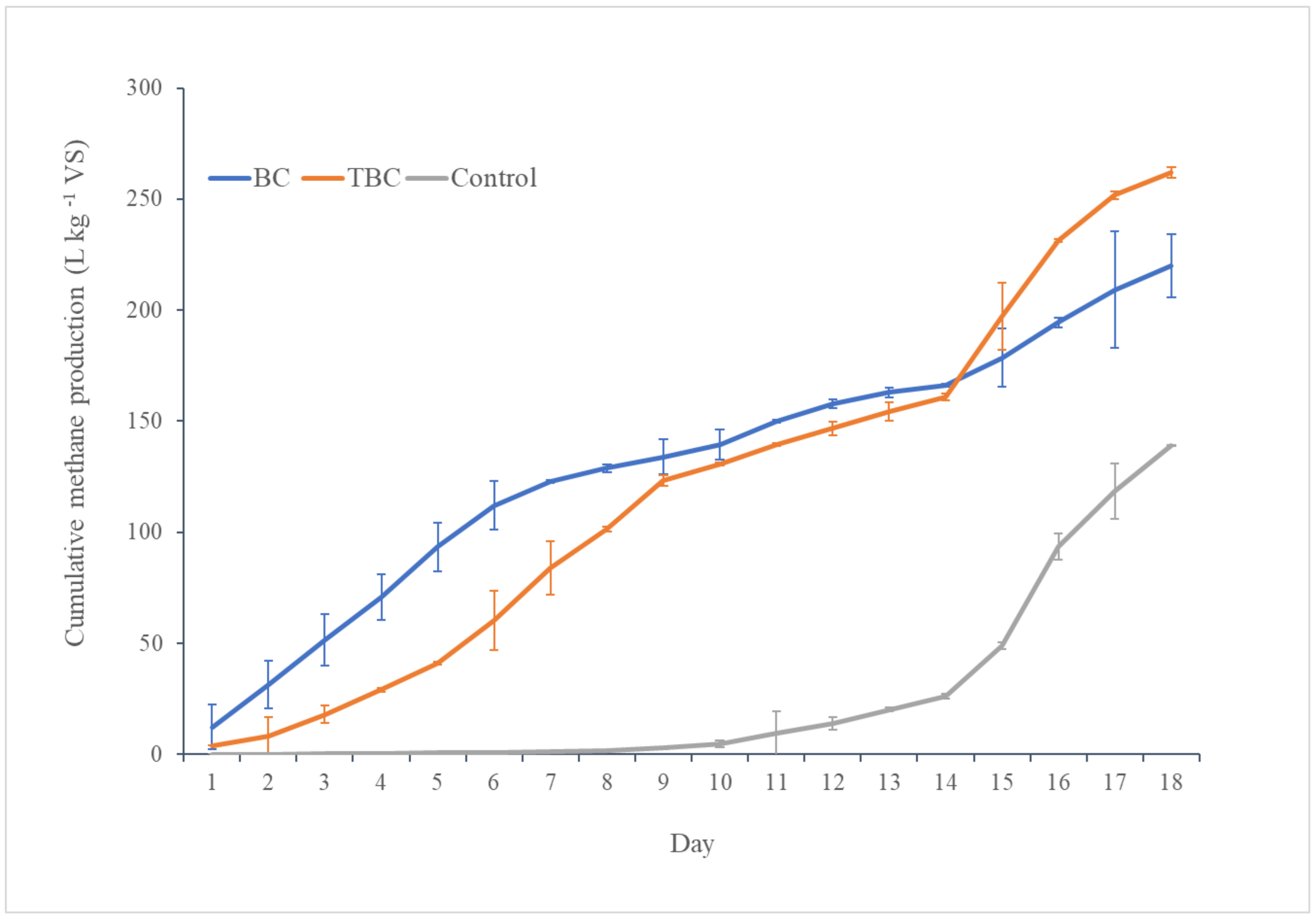
3.1. Effect of Biochar on Biomethane Production
3.2. Effect of Biochar on Total Ammonia Nitrogen Reduction
3.3. Effect of Biochar on Digestates Properties
3.4. Changes in Biochar Characteristics following Modification
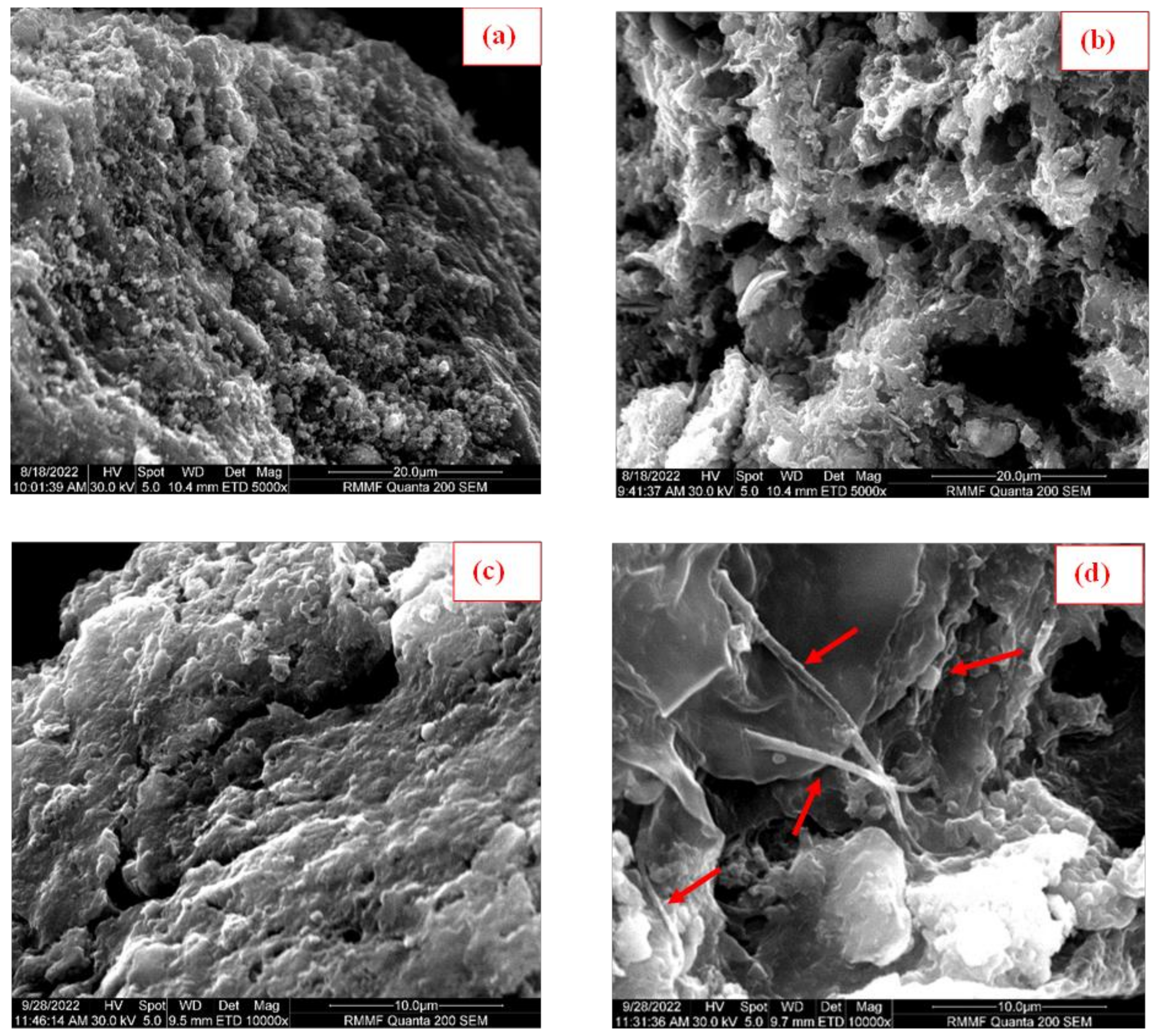
3.5. Changes in Biochar Surface Morphology and Brunauer-Emmett-Teller Surface Area Analysis
3.6. Effect of Biochar on Microbial Communities
3.6.1. Quantitative Analysis of the Microbial Communities
3.6.2. Changes in Bacterial Phyla Induced by the Addition of Biochar
3.6.3. Changes in the Archaeal Population Induced by the Addition of Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic digestion of chicken manure: Mitigating process inhibition at high ammonia concentrations by selenium supplementation. Biomass Bioenergy 2018, 108, 439–446. [Google Scholar] [CrossRef]
- Jurgutis, L.; Slepetiene, A.; Volungevicius, J.; Amaleviciute-Volunge, K. Biogas production from chicken manure at different organic loading rates in a mesophilic full scale anaerobic digestion plant. Biomass Bioenergy 2020, 141, 105693. [Google Scholar] [CrossRef]
- Chen, B.; Shao, Y.; Shi, M.; Ji, L.; He, Q.; Yan, S. Anaerobic digestion of chicken manure coupled with ammonia recovery by vacuum-assisted gas-permeable membrane process. Biochem. Eng. J. 2021, 175, 108135. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Osman, A.I.; Ai, P.; Zhou, Z.; Meng, F.; Rooney, D.W. Bioenergy production from chicken manure: A review. Environ. Chem. Lett. 2023, 21, 2707–2727. [Google Scholar] [CrossRef]
- Ngo, T.; Shahsavari, E.; Shah, K.; Surapaneni, A.; Ball, A.S. Improving bioenergy production in anaerobic digestion systems utilising chicken manure via pyrolysed biochar additives: A review. Fuel 2022, 316, 123374. [Google Scholar] [CrossRef]
- Shi, X.; Lin, J.; Zuo, J.; Li, P.; Li, X.; Guo, X. Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. J. Environ. Sci. 2017, 55, 49–57. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Bujoczek, G.; Oleszkiewicz, J.; Sparling, R.; Cenkowski, S. High Solid Anaerobic Digestion of Chicken Manure. J. Agric. Eng. Res. 2000, 76, 51–60. [Google Scholar] [CrossRef]
- Bi, S.; Qiao, W.; Xiong, L.; Mahdy, A.; Wandera, S.M.; Yin, D.; Dong, R. Improved high solid anaerobic digestion of chicken manure by moderate in situ ammonia stripping and its relation to metabolic pathway. Renew. Energy 2020, 146, 2380–2389. [Google Scholar] [CrossRef]
- Ma, J.; Amjad Bashir, M.; Pan, J.; Qiu, L.; Liu, H.; Zhai, L.; Rehim, A. Enhancing performance and stability of anaerobic digestion of chicken manure using thermally modified bentonite. J. Clean. Prod. 2018, 183, 11–19. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Qiao, W.; Mahdy, A.; Xiong, L.; Yin, D.; Fan, R.; Dach, J.; Dong, R. Enhanced methanogenic performance and metabolic pathway of high solid anaerobic digestion of chicken manure by Fe2+ and Ni2+ supplementation. Waste Manag. 2019, 94, 10–17. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, P.; Zhang, H.; Yuan, W. Biochar production and applications in agro and forestry systems: A review. Sci. Total Environ. 2020, 723, 137775. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Show, P.L.; Zhang, X.; Cao, Y.; Vasseghian, Y. Green catalyst derived from zero-valent iron onto porous biochar for removal of Rhodamine B from aqueous solution in a Fenton-like process. Carbon Lett. 2023. [Google Scholar] [CrossRef]
- Premarathna, K.; Biswas, J.K.; Kumar, M.; Varjani, S.; Mickan, B.; Show, P.L.; Lau, S.Y.; Novo, L.A.; Vithanage, M. Biofilters and bioretention systems: The role of biochar in the blue-green city concept for stormwater management. Environ. Sci. Water Res. Technol. 2023. [Google Scholar] [CrossRef]
- Ma, J.; Chen, F.; Xue, S.; Pan, J.; Khoshnevisan, B.; Yang, Y.; Liu, H.; Qiu, L. Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresour. Technol. 2021, 325, 124697. [Google Scholar] [CrossRef]
- Wang, S.; Ai, S.; Nzediegwu, C.; Kwak, J.-H.; Islam, M.S.; Li, Y.; Chang, S.X. Carboxyl and hydroxyl groups enhance ammonium adsorption capacity of iron (III) chloride and hydrochloric acid modified biochars. Bioresour. Technol. 2020, 309, 123390. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Wang, G.; Chu, Y.; Zhu, J.; Sheng, L.; Liu, G.; Xing, Y.; Fu, P.; Li, Q.; Chen, R. Multi-faceted influences of biochar addition on swine manure digestion under tetracycline antibiotic pressure. Bioresour. Technol. 2022, 346, 126352. [Google Scholar] [CrossRef]
- Wang, S.; Shi, F.; Li, P.; Yang, F.; Pei, Z.; Yu, Q.; Zuo, X.; Liu, J. Effects of rice straw biochar on methanogenic bacteria and metabolic function in anaerobic digestion. Sci. Rep. 2022, 12, 6971. [Google Scholar] [CrossRef]
- He, X.; Han, L.; Fu, B.; Du, S.; Liu, Y.; Huang, G. Effect and microbial reaction mechanism of rice straw biochar on pore methane production during mainstream large-scale aerobic composting in China. J. Clean. Prod. 2019, 215, 1223–1232. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, M.; Meng, X.; Zhou, J.L.; Zhang, H.; Shen, X. The role of biochar on alleviating ammonia toxicity in anaerobic digestion of nitrogen-rich wastes: A review. Bioresour. Technol. 2022, 351, 126924. [Google Scholar] [CrossRef] [PubMed]
- Roman-Perez, C.C.; Hernandez-Ramirez, G.; Kryzanowski, L.; Puurveen, D.; Lohstraeter, G. Greenhouse gas emissions, nitrogen dynamics and barley productivity as impacted by biosolids applications. Agric. Ecosyst. Environ. 2021, 320, 107577. [Google Scholar] [CrossRef]
- Australian Water Association. Australian Biosolids Statistics. Available online: https://www.biosolids.com.au/info/what-are-biosolids (accessed on 30 November 2022).
- Hakeem, I.G.; Halder, P.; Marzbali, M.H.; Patel, S.; Rathnayake, N.; Surapaneni, A.; Short, G.; Paz-Ferreiro, J.; Shah, K. Mild sulphuric acid pre-treatment for metals removal from biosolids and the fate of metals in the treated biosolids derived biochar. J. Environ. Chem. Eng. 2022, 10, 107378. [Google Scholar] [CrossRef]
- Thoma, E.D.; Wright, R.S.; George, I.; Krause, M.; Presezzi, D.; Villa, V.; Preston, W.; Deshmukh, P.; Kauppi, P.; Zemek, P.G. Pyrolysis processing of PFAS-impacted biosolids, a pilot study. J. Air Waste Manag. Assoc. 2022, 72, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kundu, S.; Halder, P.; Ratnnayake, N.; Marzbali, M.H.; Aktar, S.; Selezneva, E.; Paz-Ferreiro, J.; Surapaneni, A.; de Figueiredo, C.C.; et al. A critical literature review on biosolids to biochar: An alternative biosolids management option. Rev. Environ. Sci. Bio/Technol. 2020, 19, 807–841. [Google Scholar] [CrossRef]
- Song, X.; Xue, X.; Chen, D.; He, P.; Dai, X. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Stylianou, M.; Christou, A.; Dalias, P.; Polycarpou, P.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Physicochemical and structural characterization of biochar derived from the pyrolysis of biosolids, cattle manure and spent coffee grounds. J. Energy Inst. 2020, 93, 2063–2073. [Google Scholar] [CrossRef]
- Ngo, T.; Khudur, L.S.; Hakeem, I.G.; Shah, K.; Surapaneni, A.; Ball, A.S. Wood Biochar Enhances the Valorisation of the Anaerobic Digestion of Chicken Manure. Clean Technol. 2022, 4, 420–439. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef]
- Ma, J.; Pan, J.; Qiu, L.; Wang, Q.; Zhang, Z. Biochar triggering multipath methanogenesis and subdued propionic acid accumulation during semi-continuous anaerobic digestion. Bioresour. Technol. 2019, 293, 122026. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Sun, C.; Liu, R.; Yellezuome, D.; Zhu, X.; Bai, R.; Liu, M.; Sun, M. Anaerobic co-digestion of corn stover and chicken manure using continuous stirred tank reactor: The effect of biochar addition and urea pretreatment. Bioresour. Technol. 2021, 319, 124197. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Vu, T.M.; Trinh, V.T.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Kalpit, S.H.A.H.; Kundu, K.S.; Patel, R.S.; Winter, D.J. A Method and System for Pyrolysis. U.S. Patent 20220411698A1, 29 December 2022. [Google Scholar]
- Adhikari, S.; Gascó, G.; Méndez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of pyrolysis parameters on phosphorus fractions of biosolids derived biochar. Sci. Total Environ. 2019, 695, 133846. [Google Scholar] [CrossRef]
- Yang, Y.; Meehan, B.; Shah, K.; Surapaneni, A.; Hughes, J.; Fouché, L.; Paz-Ferreiro, J. Physicochemical Properties of Biochars Produced from Biosolids in Victoria, Australia. Int. J. Environ. Res. Public Health 2018, 15, 1459. [Google Scholar] [CrossRef]
- Kassongo, J.; Shahsavari, E.; Ball, A.S. Renewable energy from the solid-state anaerobic digestion of grape marc and cheese whey at high treatment capacity. Biomass Bioenergy 2020, 143, 105880. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J.; Zitomer, D.H. Biochar from Pyrolysis of Biosolids for Nutrient Adsorption and Turfgrass Cultivation. Water Env. Res 2015, 87, 2098–2106. [Google Scholar] [CrossRef]
- EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; EPA: Washington, DC, USA, 1996. [Google Scholar]
- Shapovalov, Y.; Zhadan, S.; Bochmann, G.; Salyuk, A.; Nykyforov, V. Dry Anaerobic Digestion of Chicken Manure: A Review. Appl. Sci. 2020, 10, 7825. [Google Scholar] [CrossRef]
- Kizito, S.; Jjagwe, J.; Mdondo, S.W.; Nagawa, C.B.; Bah, H.; Tumutegyereize, P. Synergetic effects of biochar addition on mesophilic and high total solids anaerobic digestion of chicken manure. J. Environ. Manag. 2022, 315, 115192. [Google Scholar] [CrossRef]
- Shahsavari, E.; Aburto-Medina, A.; Taha, M.; Ball, A.S. A quantitative PCR approach for quantification of functional genes involved in the degradation of polycyclic aromatic hydrocarbons in contaminated soils. MethodsX 2016, 3, 205–211. [Google Scholar] [CrossRef]
- Krohn, C.; Jin, J.; Wood, J.L.; Hayden, H.L.; Kitching, M.; Ryan, J.; Fabijański, P.; Franks, A.E.; Tang, C. Highly decomposed organic carbon mediates the assembly of soil communities with traits for the biodegradation of chlorinated pollutants. J. Hazard. Mater. 2021, 404, 124077. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Z.B.; Siddique, M.N.I.; Nayeem, A.; Adyel, T.M.; Ismail, S.B.; Ibrahim, M.Z. Biochar application as sustainable precursors for enhanced anaerobic digestion: A systematic review. J. Environ. Chem. Eng. 2021, 9, 105489. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Synergistic enhancement of biohydrogen production from grass fermentation using biochar combined with zero-valent iron nanoparticles. Fuel 2019, 251, 420–427. [Google Scholar] [CrossRef]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent achievements in enhancing anaerobic digestion with carbon- based functional materials. Bioresour. Technol. 2018, 266, 555–567. [Google Scholar] [CrossRef]
- Masebinu, S.; Akinlabi, E.; Muzenda, E.; Aboyade, A. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Rambabu, N.; Azargohar, R.; Dalai, A.K.; Adjaye, J. Evaluation and comparison of enrichment efficiency of physical/chemical activations and functionalized activated carbons derived from fluid petroleum coke for environmental applications. Fuel Process. Technol. 2013, 106, 501–510. [Google Scholar] [CrossRef]
- Khalil, A.; Sergeevich, N.; Borisova, V. Removal of ammonium from fish farms by biochar obtained from rice straw: Isotherm and kinetic studies for ammonium adsorption. Adsorpt. Sci. Technol. 2018, 36, 1294–1309. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, C.; Watson, J.; Sharma, B.K.; Si, B.; Zhang, Y. Adsorption or direct interspecies electron transfer? A comprehensive investigation of the role of biochar in anaerobic digestion of hydrothermal liquefaction aqueous phase. Chem. Eng. J. 2022, 435, 135078. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Wang, D.; Chen, F.; Li, X.; Zeng, G.; Yang, Q. Potential impact of salinity on methane production from food waste anaerobic digestion. Waste Manag. 2017, 67, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Ni, B.; Wang, Q.; Wang, D.; Yang, Q.; Sun, Y.; Zeng, G.; Li, X. Combined Effect of Free Nitrous Acid Pretreatment and Sodium Dodecylbenzene Sulfonate on Short-Chain Fatty Acid Production from Waste Activated Sludge. Sci. Rep. 2016, 6, 21622. [Google Scholar] [CrossRef]
- Aramrueang, N.; Zhang, R.; Liu, X. Application of biochar and alkalis for recovery of sour anaerobic digesters. J. Environ. Manag. 2022, 307, 114538. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Molaey, R.; Sürmeli, R.Ö.; Sahinkaya, E.; Çalli, B. Biogas production from chicken manure: Co-digestion with spent poppy straw. Int. Biodeterior. Biodegrad. 2017, 119, 205–210. [Google Scholar] [CrossRef]
- Chiappero, M.; Fiore, S.; Berruti, F. Impact of biochar on anaerobic digestion: Meta-analysis and economic evaluation. J. Environ. Chem. Eng. 2022, 10, 108870. [Google Scholar] [CrossRef]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- Huang, H.; Tang, J.; Gao, K.; He, R.; Zhao, H.; Werner, D. Characterization of KOH modified biochars from different pyrolysis temperatures and enhanced adsorption of antibiotics. RSC Adv. 2017, 7, 14640–14648. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Fan, S. Preparation of KOH and H3PO4 Modified Biochar and Its Application in Methylene Blue Removal from Aqueous Solution. Processes 2019, 7, 891. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Lü, F.; Liu, Y.; Shao, L.; He, P. Powdered biochar doubled microbial growth in anaerobic digestion of oil. Appl. Energy 2019, 247, 605–614. [Google Scholar] [CrossRef]
- Sawayama, S.; Tada, C.; Tsukahara, K.; Yagishita, T. Effect of ammonium addition on methanogenic community in a fluidized bed anaerobic digestion. J. Biosci. Bioeng. 2004, 97, 65–70. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Ziganshin, A.M. Anaerobic Digestion of Chicken Manure in the Presence of Magnetite, Granular Activated Carbon, and Biochar: Operation of Anaerobic Reactors and Microbial Community Structure. Microorganisms 2022, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Perman, E.; Schnürer, A.; Björn, A.; Moestedt, J. Serial anaerobic digestion improves protein degradation and biogas production from mixed food waste. Biomass Bioenergy 2022, 161, 106478. [Google Scholar] [CrossRef]
- Su, H.; Liu, L.; Wang, S.; Wang, Q.; Jiang, Y.; Hou, X.; Tan, T. Semi-continuous anaerobic digestion for biogas production: Influence of ammonium acetate supplement and structure of the microbial community. Biotechnol. Biofuels 2015, 8, 13. [Google Scholar] [CrossRef]
- Niu, Q.; Takemura, Y.; Kubota, K.; Li, Y.-Y. Comparing mesophilic and thermophilic anaerobic digestion of chicken manure: Microbial community dynamics and process resilience. Waste Manag. 2015, 43, 114–122. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Z.; Ran, W.; Yuan, H.; Li, X. Performance and microbial community dynamics in anaerobic co-digestion of chicken manure and corn stover with different modification methods and trace element supplementation strategy. Bioresour. Technol. 2021, 325, 124713. [Google Scholar] [CrossRef]
- Li, D.; Sun, M.; Xu, J.; Gong, T.; Ye, M.; Xiao, Y.; Yang, T. Effect of biochar derived from biogas residue on methane production during dry anaerobic fermentation of kitchen waste. Waste Manag. 2022, 149, 70–78. [Google Scholar] [CrossRef]
- Kurade, M.B.; Saha, S.; Kim, J.R.; Roh, H.-S.; Jeon, B.-H. Microbial community acclimatization for enhancement in the methane productivity of anaerobic co-digestion of fats, oil, and grease. Bioresour. Technol. 2020, 296, 122294. [Google Scholar] [CrossRef]
- Zheng, Z.; Cai, Y.; Zhang, Y.; Zhao, Y.; Gao, Y.; Cui, Z.; Hu, Y.; Wang, X. The effects of C/N (10–25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res. 2021, 188, 116466. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Probst, M.; Franke-Whittle, I.H.; Ebner, C.; Podmirseg, S.M.; Etemadi-Shalamzari, M.; Hupfauf, S.; Insam, H. Microbiota in anaerobic digestion of sewage sludge with and without co-substrates. Water Environ. J. 2019, 33, 214–222. [Google Scholar] [CrossRef]
- Shakeri Yekta, S.; Liu, T.; Axelsson Bjerg, M.; Šafarič, L.; Karlsson, A.; Björn, A.; Schnürer, A. Sulfide level in municipal sludge digesters affects microbial community response to long-chain fatty acid loads. Biotechnol. Biofuels 2019, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Liu, J.; Xu, Q.; Han, Z.; Xu, X.; Zhu, L. Tolerance of Aceticlastic Methanogenesis Enhanced by Magnetite under the Condition of Ammonia Stress. ACS Sustain. Chem. Eng. 2020, 8, 1417–1426. [Google Scholar] [CrossRef]



| Characteristics | Unit | Chicken Manure | Sludge | Chicken Manure + Sludge |
|---|---|---|---|---|
| pH | _ | 8.2 ± 0.0 | 7.5 ± 0.1 | 7.5 ± 0.1 |
| Salinity | % | 27.0 ± 0.0 | 4.5 ± 0.7 | 12.3 ± 0.6 |
| Electrical Conductivity | mS cm−1 | 54.5 ± 0.8 | 8.4 ± 0.4 | 24.7 ± 1.2 |
| Moisture Content | % | 19.3 ± 1.6 | 98.3 ± 0.1 | 85.4 ± 0.8 |
| Total Solids | % | 80.6 ± 1.5 | 2.2 ± 0.0 | 14.6 ± 0.8 |
| Volatile Solids | % | 54.0 ± 1.7 | 1.7 ± 0.1 | 10.6 ± 1.0 |
| Total Ammonia Nitrogen | mg L−1 | 3850.0 ± 353.0 | 1200 ± 0.0 | 2722.3 ± 195.4 |
| Soluble Chemical Oxygen Demand | g L−1 | 37.7 ± 3.3 | 1.5 ± 0.2 | 14.9 ± 0.8 |
| Digesters | TAN at Day 18 (mg L−1) | Change in TAN (mg L−1) | TAN Reduction (%) |
|---|---|---|---|
| No Biochar (Control) | 2950 ± 195.4 | +227.7 | 0% |
| Biosolids Biochar (BC) | 2200 ± 0.0 | −522.3 | 25% |
| Alkali Biochar (KOH-BC) | 1900 ± 0.0 | −822.3 | 35.5% |
| Characteristics | Chicken Manure, Sludge, and No Biochar (Control) | Chicken Manure, Sludge, and Biochar (BC) | Chicken Manure, Sludge, and Alkali Biochar (KOH-BC) | |||
|---|---|---|---|---|---|---|
| Value | Change | Value | Change | Value | Change | |
| pH | 8.1 ± 0.0 | N.A. | 8.0 ± 0.0 | N.A. | 8.0 ± 0.0 | N.A. |
| Electrical Conductivity (mS cm−1) | 23.4 ± 0.7 | −5.4% | 16.2 ± 1.1 | −34.6% | 31.9 ± 0.2 | +28.8% |
| Salinity (%) | 11.5 ± 0.7 | −6.7% | 8.5 ± 0.7 | −31.1% | 12.5 ± 5.0 | +1.4% |
| Soluble chemical oxygen demand (g L−1) | 20.9 ± 3.7 | +40.8% | 9.0 ± 0.0 | −39.4% | 8.9 ± 0.8 | −40.4% |
| Characteristics | Unit | BC | KOH-BC |
|---|---|---|---|
| pH | NA | 9.7 | 9.3 |
| Electrical Conductivity | mS cm−1 | 3.9 | 13.6 |
| BET surface area | m2 g−1 | 15.5 | 18.8 |
| ICP-MS metals concentration | mg kg−1 | ||
| K | 7662.9 | 11,629.5 | |
| Na | 5350.9 | 5173.4 | |
| Mg | 7894.7 | 8359.1 | |
| Al | 17,941.1 | 18,362.8 | |
| Ca | 90,346.4 | 95,502.0 | |
| V | 17.7 | 18.4 | |
| Cr | 26.3 | 28.0 | |
| Mn | 379.1 | 401.9 | |
| Fe | 22,902.6 | 24,109.0 | |
| Co | 4.1 | 4.4 | |
| Ni | 24.2 | 25.9 | |
| Cu | 996.5 | 1070.3 | |
| Zn | 1497.2 | 1547.8 | |
| As | 4.7 | 5.1 | |
| Mo | 5.0 | 5.6 | |
| Cd | 1.0 | 0.9 | |
| Sb | 0.6 | 0.7 | |
| Ba | 265.4 | 280.9 | |
| Pb | 29.6 | 30.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.; Ngo, T.; Khudur, L.S.; Krohn, C.; Dike, C.C.; Hakeem, I.G.; Shah, K.; Surapaneni, A.; Ball, A.S. Biosolids-Derived Biochar Improves Biomethane Production in the Anaerobic Digestion of Chicken Manure. Resources 2023, 12, 123. https://doi.org/10.3390/resources12100123
Hassan S, Ngo T, Khudur LS, Krohn C, Dike CC, Hakeem IG, Shah K, Surapaneni A, Ball AS. Biosolids-Derived Biochar Improves Biomethane Production in the Anaerobic Digestion of Chicken Manure. Resources. 2023; 12(10):123. https://doi.org/10.3390/resources12100123
Chicago/Turabian StyleHassan, Soulayma, Tien Ngo, Leadin S. Khudur, Christian Krohn, Charles Chinyere Dike, Ibrahim Gbolahan Hakeem, Kalpit Shah, Aravind Surapaneni, and Andrew S. Ball. 2023. "Biosolids-Derived Biochar Improves Biomethane Production in the Anaerobic Digestion of Chicken Manure" Resources 12, no. 10: 123. https://doi.org/10.3390/resources12100123
APA StyleHassan, S., Ngo, T., Khudur, L. S., Krohn, C., Dike, C. C., Hakeem, I. G., Shah, K., Surapaneni, A., & Ball, A. S. (2023). Biosolids-Derived Biochar Improves Biomethane Production in the Anaerobic Digestion of Chicken Manure. Resources, 12(10), 123. https://doi.org/10.3390/resources12100123