Abstract
The global impact of water and soil contamination has become a serious issue that affects the world and all living beings. In this sense, multiple treatment alternatives have been developed at different scales to improve quality. Among them, biochar has become a suitable alternative for environmental remediation due to its high efficiency and low cost, and the raw material used for its production comes from residual biomass. A biochar is a carbonaceous material with interesting physicochemical properties (e.g., high surface area, porosity, and functional surface groups), which can be prepared by different synthesis methods using agricultural wastes (branches of banana rachis, cocoa shells, cane bagasse, among others) as feedstock. This state-of-the-art review is based on a general description of biochar for environmental remediation. Biochar’s production, synthesis, and multiple uses have also been analyzed. In addition, this work shows some alternatives used to improve the biochar properties and thus its efficiency for several applications, like removing heavy metals, oil, dyes, and other toxic pollutants. Physical and chemical modifications, precursors, dopants, and promoting agents (e.g., Fe and N species) have been discussed. Finally, the primary uses of biochar and the corresponding mechanism to improve water and soil quality (via adsorption, heterogeneous photocatalysis, and advanced oxidation processes) have been described, both at laboratory and medium and large scales. Considering all the advantages, synthesis methods, and applications, biochar is a promising alternative with a high potential to mitigate environmental problems by improving water and soil quality, reducing greenhouse gas emissions, and promoting the circular economy through residual biomass, generating value-added products for several uses.
1. Introduction
Biochar is an important, interesting, low-cost material with various agricultural, industrial, and scientific applications. Biochar is a name given to vegetable-derived charcoal, which can be used as an agent to improve soil and water quality [1,2,3]. This carbon-rich substance can be produced by the carbonization of biomass residues (e.g., wood, dung, manure, or leaves) in thermal conversion processes, such as pyrolysis, torrefaction, and hydrothermal carbonization (HTC) [4,5,6]. Among them, pyrolysis is the most common process to obtain biochar under anaerobic conditions and high temperatures [7,8,9]. In addition, heat, syngas, liquid fuels, and pyroligneous acid (wood vinegar) are also generated during this process [10,11,12].
The HTC is a novel technology that produces carbonaceous materials, e.g., biochar. This process has received much attention due to its eco-friendly, cost-effective, and straightforward approach [8,13,14]. During the HTC, the raw material is treated at high pressures and temperatures to produce various biochar-based materials with a high calorific value, low humidity, and high combustion performance [15,16], known as hydrochar [17]. In turn, the hydrochar can be used to generate energy in fuel cells [15], for gas storage [18], as a soil amendment [19], as a catalyst [20], and as an adsorbent to retain pollutants in water, such as heavy metals [14,21] and dyes [14,22].
The term biochar has been used in recent times; however, the origins of its concept are ancient. The Amazon basin has zones up to 2 m deep of terra Preta, a mixture of very fertile dark-colored soil with high carbon content, ceramic fragments, and organic debris, that has supported the agricultural needs of the Amazonian people for centuries [23,24,25]. The name biochar is related to a carbonaceous material used for environmental rehabilitation, especially for soil improvement and water treatment. In addition, biochar has potentially significant implications for climate change mitigation, for example, in capturing CO2 from air and industrial sources [26,27]. Likewise, using the gases generated during the pyrolysis process, biochar production may be integrated with other processes, such as bioenergy generation [6,28].
The use of biochar for agricultural and environmental purposes has been thoroughly researched and evaluated. This substance benefits agriculture and the environment in various ways, and its soil persistence and nutrient retention capabilities make it an excellent soil additive for enhancing crop yields. Biochar can be applied to mitigate soil contamination by immobilizing heavy metals and organic pollutants [29,30,31,32]. Heavy metals in soils are extremely damaging contaminants that hinder soil qualities necessary for successful crop performance [25,33,34]. Heavy metals are not biodegradable and remain in polluted soil and water for long periods [35,36]. Soil contamination by heavy metals (e.g., Cd, Cr, Hg, Pb, Cu, Zn, As, Co, Ni, and Se) and persistent organic pollutants (POPs, such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), polychlorinated dibenzo-dioxin (PCDD), and polychlorinated dibenzofurans) is a global issue that threatens human life and health [37]. Removing heavy metals from polluted soils can be expensive and time-consuming for agriculture [33,38]. However, biochar can stabilize Cd, Cu, Ni, Pb, and Zn in soil and reduce their bioavailability through enhanced sorption (based on electrostatic attraction, ion exchange, and surface complexation) and chemical precipitation (incurred by raising the soil pH and adding of carbonate and phosphate ash) [29,39,40,41]. Thus, the physicochemical properties of biochar can be considered to aid in the adsorption of heavy metals and organic contaminants in soils, which is highly beneficial for environmental mitigation [34,42,43,44].
On the other hand, biochar can be used as a cleaning agent for polluted water, whether in any industrial or agricultural field [41,45,46,47]. Due to the importance of hydrogeological resources, it is critical to care for and protect them, along with technical and scientific efforts to mitigate the negative environmental impact of the world. The number of contaminating compounds introduced into aquatic ecosystems is rising because of the varied uses of water to fulfill various human activities [48,49]. Various components, such as pesticides, pathogens, heavy metals, detergents, dyes, medications, and personal care and hygiene products, alter the water quality [33,48,50,51]. One of the major water pollutants is oil activity, which, due to the release of oil into the environment, involves operations related to the exploitation and transport of hydrocarbons, resulting in the gradual deterioration of the environment [52,53,54,55], directly affecting the water and, as a result, the affectation of the ground. When hydrocarbons combine with water, they form an impenetrable barrier that limits the growth of biological activity, causing direct harm to fauna and soils that rely on this already polluted water [56,57].
Biomass conversion favors the production of a carbonaceous material (i.e., biochar) with interesting physicochemical properties, which allow it to be used as an adsorbent for both organic and inorganic compounds present in water [49,58,59]. Among these properties are a high porosity, surface area, and various surface functional groups, such as carboxyls (–COOH), phenols, and hydroxyls (–OH) [21,36,59,60]. All the properties have allowed the use of biochar for wastewater treatment to be increased, considering that the adsorption process with biochar has significant advantages compared to other treatment methods, like the low cost, easy operation, and easy maintenance [61,62,63,64]. Thus, biochar has become a substance that meets the requirement of improving the quality of wastewater generated in industries or any other field, taking advantage of its obtaining from agricultural waste resources.
There is a high amount of organic and inorganic waste in the world. The most common organic wastes are categorized as plastic wastes (e.g., bottles, plastics) or residual biomass according to their origin. In many countries, especially in South America, a considerable amount of agricultural residues (e.g., banana husk, cocoa, coffee, rice husk, corn, or oil palm) is generated daily, which have not been used for any application. Furthermore, most of them, when discarded, generate environmental damage, such as the emission of toxic gases into the atmosphere and water pollution [65,66]. In this sense, using a substance produced by residual biomass (i.e., biochar) that helps reduce environmental pollution is highly beneficial. Considering this fact, agricultural waste might be used as raw materials to mitigate the damage caused by contaminants worldwide at different scales. Thus, using carbonaceous materials produced by agricultural wastes would be an economical and low-cost alternative, which can also generate an added value to the residual biomass that is rejected or wasted.
Unlike other works, this review article focuses on several lines of interest, starting with an overview of biochar synthesis methods, e.g., pyrolysis and hydrothermal carbonization. Likewise, several types of biomass used as raw material to obtain this carbonaceous material have been listed and analyzed. In addition to these topics, an exhaustive review has been conducted on some methods to modify the properties of biochar, considering both the physical and chemical methods. Moreover, several applications of the use of biochar have been widely studied, and how their efficiency can be correlated to physicochemical properties, along with different contaminant removal mechanisms (e.g., adsorption and advanced oxidation processes). Finally, a summary of the pros and cons of the use and applications of biochar for environmental remediation has been made, identifying that this carbonaceous material can still be improved, but the results are promising so that it can continue to be used and thus encourage the circular economy in the world.
2. Biochar: Feedstock, Synthesis Methods, and Properties
Biochar mainly comprises carbon (~60–90%), although it may also contain oxygen, hydrogen, and inorganic ash depending on the source biomass [67]. Biochar conversion is considered more environmentally benign than coal combustion, as biomass is carbon neutral [68]. Generally, biochar has a high surface area (above 100 m2/g), which depends on the raw material and the synthesis conditions [69]. As a result, biochar can be used in a variety of nonfuel applications, such as chemical adsorption (e.g., water treatment [21,70]) and carbon storage [68]. In addition, this carbonaceous material has also been used as a soil fertilizer [71,72].
2.1. Differences between Biochar, Activated Carbon and Charcoal
The carbon family involves interesting materials, such as biochar, charcoal, and activated carbon. These carbonaceous materials share the essence and origin, which is carbon. The most significant distinction is their synthesis methods, conditions, and applications. On the one hand, coal results from coalification, i.e., a geological process involving biomass conversion with water and sediments. Peat and lignite are intermediate stages of this process [73].
On the other hand, charcoal, biochar, and activated carbon are products of thermochemical processes, and they are defined as pyrogenic carbonaceous materials (PCM) [24]. Biochar and activated carbon are frequently used in agriculture for environmental remediation, such as filtering and purification. Meanwhile, charcoal is used for heating and cooking [74,75]. These materials’ physical and chemical properties are similar since they share the same carbonaceous origin. However, they have singular properties that distinguish them. Table 1 describes the marked difference between these carbonaceous materials and the main characteristics that define them and differ from each other.
Biochar is a member of the carbon family that, when mixed with other species, produces new hybridized nanomaterial biochar-based materials [76]. They have novel physicochemical properties and are highly effective for degrading water pollutants through adsorption, heterogeneous photocatalysis, and advanced oxidation processes. Braghiroli et al. [77] have reported a high sorption capacity of phenols and chemical intermediates with the use of activated biochar and other biochar-based materials for treating phenolic compounds, such as phenol, bisphenol A, p-nitrophenol, and pentachlorophenol, are toxic to health and the environment [78].

Table 1.
Description of the most influential carbonaceous materials.
Table 1.
Description of the most influential carbonaceous materials.
| Biochar | Charcoal | Activated Carbon | Ref. | |
|---|---|---|---|---|
| Definition | Carbonaceous material produced from organic matter, such as residual biomass, and it has environmental and energetic applications. | It is a porous black solid material made up of carbon in its amorphous state. | Carbonaceous material with a high surface area produced by the thermochemical conversion of organic matter, followed by an activation process to boost its adsorption capacity. | [73] |
| Feedstock | Agricultural residues: rice hulls, manure. Trees, shrubs, grasses, and wood. | Hardwood | Petroleum residues, agricultural residues, and biomass in general. | [6] |
| Characteristics | High adsorption and porosity | High burnability | High adsorption | [73] |
| Production | Pyrolysis, gasification, torrefaction, HTC. | Kiln-calcined Slow pyrolysis | There are two main processes: carbonization (pyrolysis, gasification, torrefaction, and HTC), followed by an activation process. | [6,73] |
| Cost | Low cost | Low cost | Expensive: high-temperature costs. | [6] |
| Illustrative image |  |  |  | |
| Uses | Filtration, water treatment, soil remediation. | Energetic use as fuel (cooking). | Water filtration, aesthetic uses, medical uses, water treatment. | [73,79,80,81] |
Compared to pure nano-photocatalysts, biochar-supported catalysts have larger surface areas, are more porous, have higher catalytic capacities, and are more stable [82]. Biochar may support hosting different catalytic nanoparticles because of its unique surface features, readily modifiable functional groups, chemical stability, and electrical conductivity [83,84].
2.2. Feedstock for Biochar Production
Biomass is living or once-living organic matter that can serve as a versatile renewable source for environmental and energy applications (e.g., electricity generation, heat provision) and for the production of many types of biofuels, compost, pharmaceutical products, other chemicals, and biomaterials, like biochar. Almost all organic materials (such as tree bark, nut shells, crop residues, and manure) can be used as feedstock for biochar using appropriate equipment [85,86,87]. Biomass as an initial resource can come from animal, vegetable, or human-generated waste, such as industrial or municipal waste (sewage) [66,88]. Biochar-based materials can have different characteristics and properties depending on the biomass used as feedstock, which, in turn, will allow the carbonaceous material to be used in specific applications. Table 2 shows the most common feedstock used to produce biochar, which can be any organic matter, from plant materials to industrial waste.

Table 2.
Commonly used feedstock for biochar production [89,90,91,92].
Biochar is commonly produced from vegetal residues called cellulosic biomass, such as firewood or rice residues. In recent years, other raw materials have been studied to produce biochar, such as algae, food waste, manure, and animal tissue [63,90,91], obtaining interesting results regarding its physical-chemical properties and applications. On the other hand, raw materials with a high biomass content, such as sewage and municipal solid waste (MSW), cannot be considered a suitable feedstock for biochar production since they may include contaminating components that can affect the biochar performance for soil or water treatments [93].
2.3. Synthesis Methods Used to Prepare Biochar
Biomass can be transformed using thermochemical conversion processes, like pyrolysis or HTC treatment, to produce biogas, liquid fuels (e.g., bio-oil), and solid materials, such as biochar [8,63]. Biomass valorization can be conducted through basic processes (e.g., pyrolysis, gasification, torrefaction, anaerobic digestion, or combustion), in which the organic matter can be transformed into heat, electricity, or by-products, like biochar-based materials [94]. Thermochemical conversion encompasses the degradation of biomass structure in either an oxygenic or anoxygenic atmosphere at high temperatures [95]. Biochar production begins from the initial conversion of biomass through thermochemical processes until a carbonaceous material with desired physicochemical properties is obtained. The operating principles, synthesis conditions, and their effect on biochar production for the most common thermochemical conversion processes are detailed below:
2.3.1. Pyrolysis
In the pyrolysis process, the biomass source, which was previously mentioned, is subjected to a thermal treatment to produce biochar and other by-products. Depending on the operating conditions, biogas, liquid bio-oil, and biochar can be generated during this process [96]. It is essential to note that biomass must be previously dried and ground to obtain a carbonaceous material of high quality and yield. The heating process is conducted at high temperatures (400–800 °C) without oxygen and allows biomass conversion into by-products for several applications, such as energy and environmental remediation. The by-products can be used as energy or residual heat to contribute to the pyrolysis or thermal treatment of the raw material. This thermal process releases the lowest percentage of carbon back into the atmosphere [58,71,97]. According to the literature, there are two types of pyrolysis: slow and fast pyrolysis, which depend on the temperature conditions and the heating rate [98]. The slow pyrolysis is conducted at temperatures ranging from 250 to 600 °C using heating rates of 1–10 °C/min [90,99]. While fast pyrolysis is based on the thermal conversion of biomass at temperatures above 600 °C, using heating rates higher than 50 °C/min [100]. The concentration and the physicochemical properties of the products formed (e.g., biogas, bio-oil, and biochar) can vary depending on the type of pyrolysis. Thus, during the slow pyrolysis of biomass, a large amount of biochar can be produced, generating low concentrations of gases and liquids with a high content of highly contaminant volatile organic compounds (VOCs) [101].
On the other hand, fast pyrolysis is mainly used to produce a high concentration of liquids (e.g., biofuel) with better physicochemical quality than those produced by slow pyrolysis, achieving a lower VOC content and a higher concentration of log-chain hydrocarbons [102]. Both types of pyrolysis can be used to produce biochar. However, the properties (e.g., carbon content, density, water retention capacity, functional groups, surface area) and applications of the carbonaceous materials will be different. Slow pyrolysis can be the best way to obtain water and soil remediation biochar. Meanwhile, fast pyrolysis can be the best route to produce biochar as fuel or precursor to other materials [101,103].
The pyrolysis of biomass modifies the size and arrangement of the carbonaceous structures, enhancing the physicochemical properties of the products obtained during the process [104]. In general, this impact becomes more robust at higher treatment temperatures. To obtain a higher biochar production yield, the temperature interval for pyrolysis should be around 400–800 °C [105,106]. Lua et al. [81] reported that by raising the pyrolysis temperature from 250 to 500 °C, the specific surface area can increase from 170 to 480 m2/g, which has been related to the increased evolution of volatile matter in pistachio nut shells, resulting in an improved pore growth at the biochar surface, reaching total pore volume values of 0.47 cm3/g (at 500 °C), which were much higher than those obtained at a pyrolysis temperature of 250 °C (0.193 cm3/g). This has been related to elevated temperatures supplying activation energy, which can favor conversion reactions, resulting in higher degrees of order in the carbonaceous structures [107].
2.3.2. Torrefaction
Similarly to pyrolysis, torrefaction is a thermochemical process based on biomass conversion into value-added products, e.g., biochar, biogas, and bio-oil [108]. However, this process differs from pyrolysis in operating conditions and formed product types. Torrefaction is a thermal process based on biomass dehydration, carbonization, and caramelization at relatively low temperatures (i.e., 200 to 300 °C) without oxygen [58]. Biochar is the only product generated during this process. However, the physicochemical properties (e.g., structural characteristics) of the carbonaceous material produced are inferior to those of pyrolysis [62,91].
2.3.3. Hydrothermal Carbonization
Hydrothermal carbonization (HTC) is a thermochemical technology for processing biomass with high moisture content in a hot compressed water system [14]. The main product of HTC is hydrochar, a type of biochar produced in this way. Apart from this carbonaceous material, aqueous (nutrient-rich) and gas phases (mainly CO2) can be produced depending on the operating conditions [109]. The carbon-rich hydrochar can be employed as fuel, coal substitute, gasification feedstock, soil additive for nutrient enrichment, or as an adsorbent or precursor of activated carbon [110]. The advantage of the HTC process is that biomass may be transformed into carbonaceous solids without an energy-intensive drying procedure or an anoxygenic atmosphere. Likewise, toxic chemical compounds and residual micropollutants are also avoided during HTC [111].
As mentioned below, HTC is a thermochemical process that uses heat to transform wet biomass feedstock into hydrochar. HTC is performed in a reactor at temperatures ranging from 120 to 300 °C under autogenous (self-generated) pressure or under pressure (2–6 MPa) with feedstock residence periods ranging from 0.5 to 8 h [112,113,114]. HTC offers a key advantage over other high-temperature thermochemical conversion processes (e.g., pyrolysis) because it is possible to use wet waste without a pre-drying process [19,115]. HTC may use a variety of feedstock, including aquatic biomass, agricultural waste, and industrial and animal waste [11]. Water is a favorable medium for heat transfer in HTC. However, there may be some mass transfer restrictions if the particle size variability in the feedstock is too large (above 2 cm) and the reaction time is too short (less than 30 min) [19]. As a result, particle size should be constant to provide uniform heat and mass transfer. On the other hand, the aqueous slurry needs to be centrifuged or filtered to separate the process water and particulates (wet cake). Biomass conversion processes mainly depend on the feedstock, the desired final product, and its corresponding use. Table 3 shows a summary of the typical thermochemical conversion processes, temperature conditions, and the products obtained in each of them.

Table 3.
Thermochemical conversion processes to obtain biochar used in different fields [9,116,117].
2.4. Methods of Biochar Activation and Modification
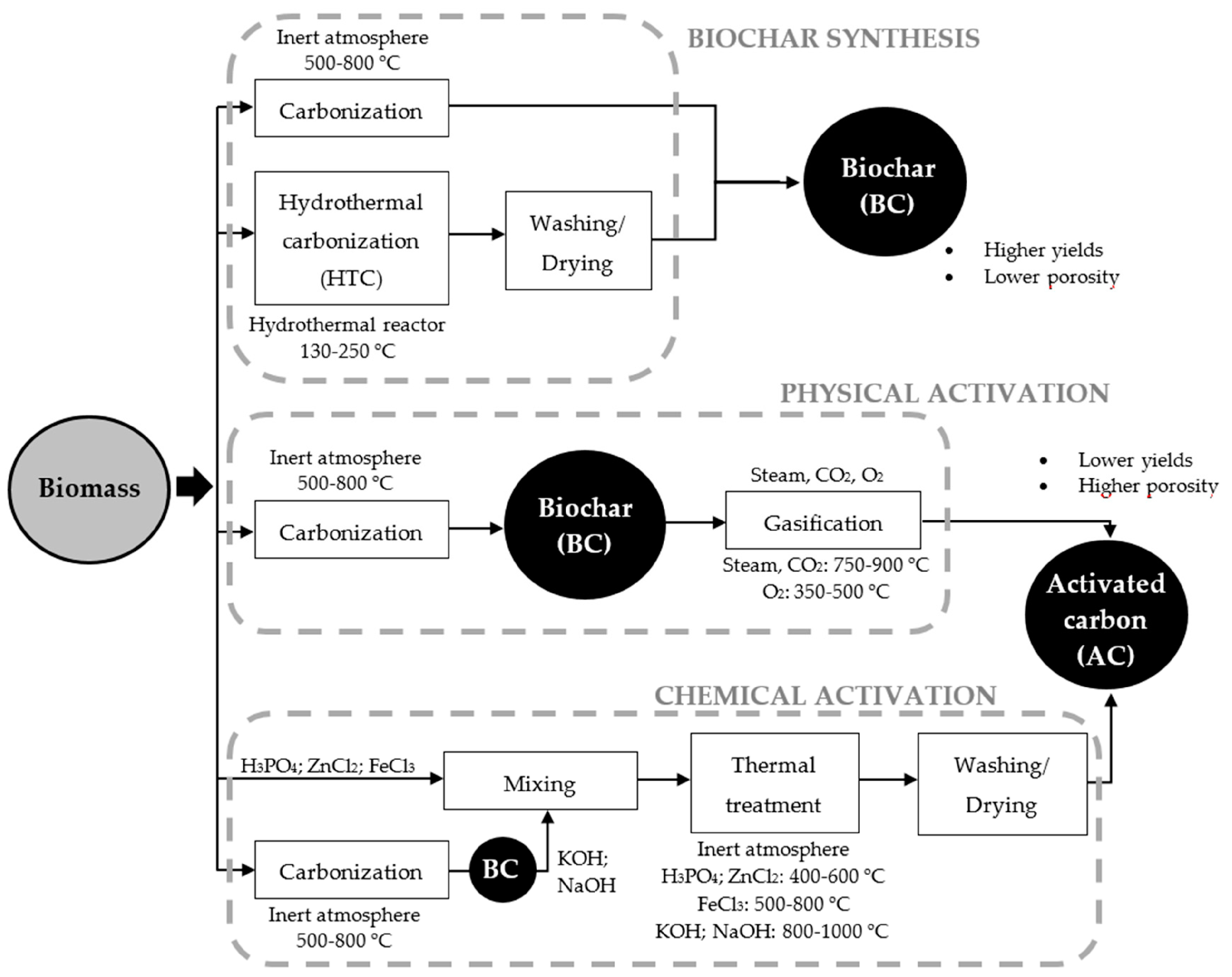
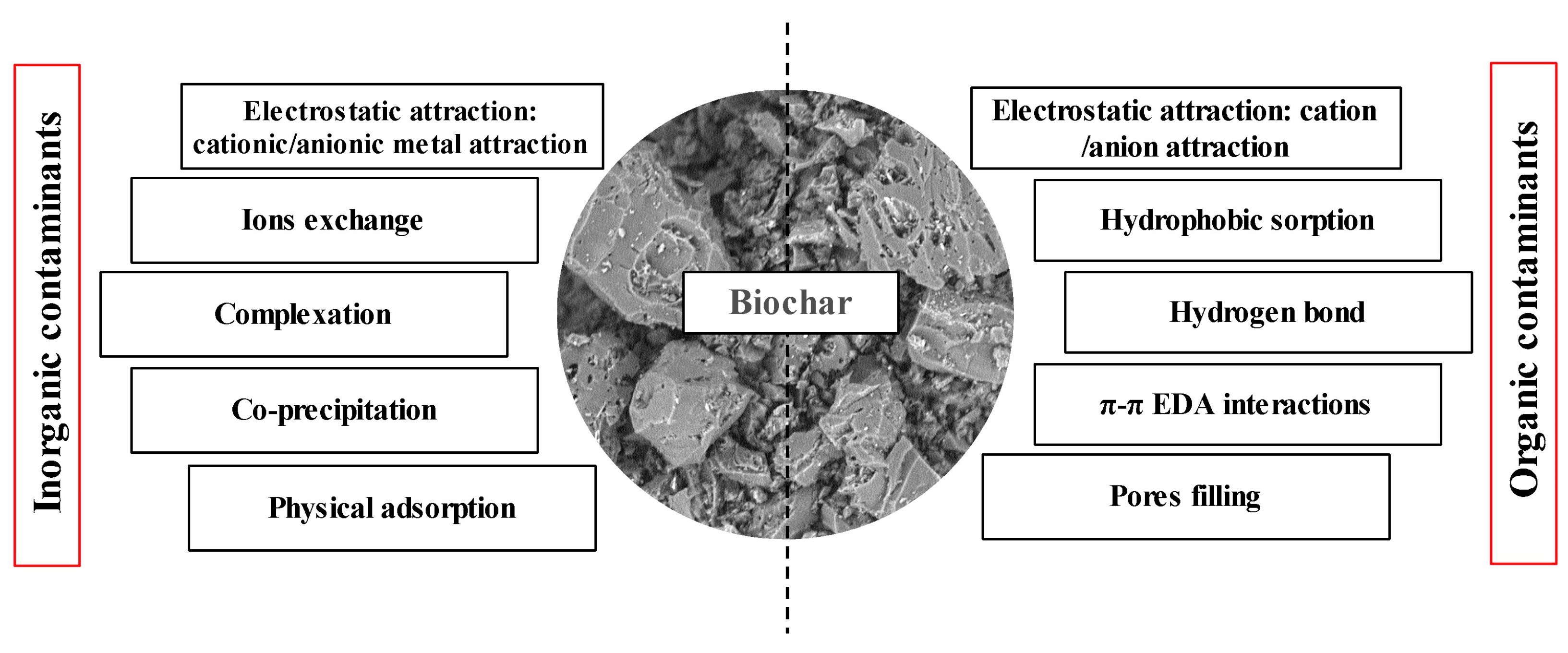
Physical and chemical activation methods can enhance biochar properties, such as impregnation or adding dopants or additives in the carbonaceous structure. Physical activation is accomplished by processing biochar with oxidizing agents, mostly steam or carbon dioxide, at temperatures ranging from 500 to 1000 °C. Water is a smaller molecule than carbon dioxide, which favors its penetration into the biochar pores [19,118], enhancing its morphological properties, like surface area and porosity [119]. Figure 1 shows some routes of physical and chemical activation of biochar, as well as the chemical compounds and thermal conditions used to enhance its physicochemical properties. Notably, the porosity, surface chemistry, and yields of carbon-based adsorbents produced significantly depend on the biomass composition of feedstock and the synthesis conditions [120].
Figure 1.
Activation routes of biochar and activated carbon. (Reprinted from [120]). © 2018 by the authors.
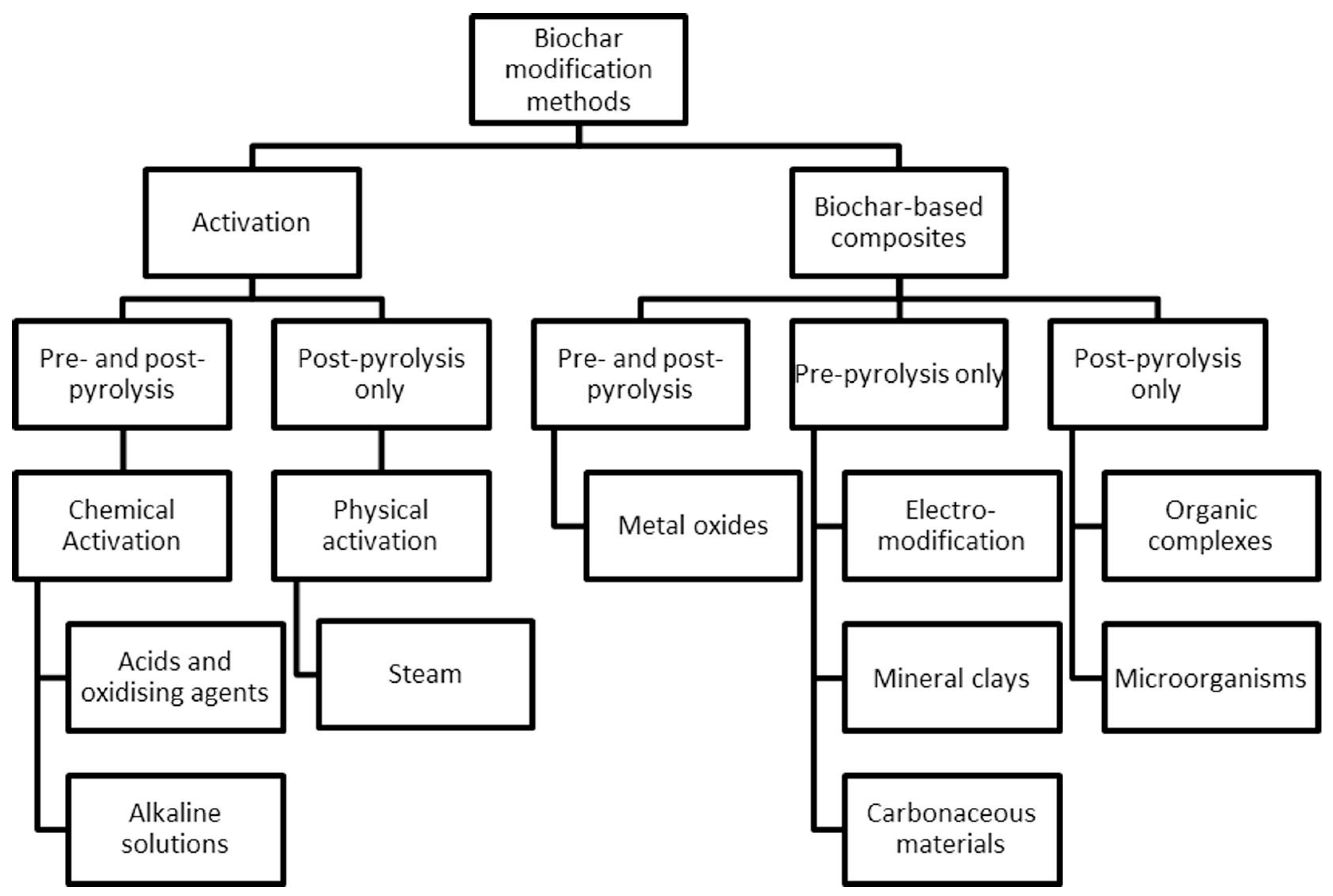
Many modification methods (e.g., chemical, physical, and biological routes) have been studied to improve the properties of biochar used for environmental purposes [63]. The widely used method has been the chemical alteration. Acid modification, alkalinity modification, oxidizing agent modification, metal salts, or oxidizing agent modification are the most common. In contrast, steam and gas purging have been the most common types of physical modification [19]. Figure 2 describes a categorization scheme of the modification methods most often reported in the literature [121]. The modification method that has attracted scientific attention for producing sorbents for water treatment involves embedding different elements into the biochar framework before, during, or after the thermochemical conversion. Physical activation of biochar using steam and chemical activation with acidic and alkaline solutions is usually performed after pyrolysis. However, remarkable results have been seen when chemical activation is performed before pyrolysis [122].

Figure 2.
Biochar modification methods to enhance properties. (Reprinted from [121], with permission from Elsevier). © 2017 Elsevier Ltd.
Interesting and novel physicochemical activation methods of biochar seek to improve functional stability, and these can be based on its modification with other species. In this sense, biochar-based composites can be prepared by impregnating or coating their surface with metal oxides, clays, carbonaceous structures (e.g., graphene oxide or carbon nanotubes), complex organic compounds, such as chitosan, among others [32,123,124].
2.4.1. Physical Activation
Physical activation enhances the surface pores of biochar and can also modify its chemical properties (e.g., surface functional groups, hydrophobicity, and polarity) [125]. Steam activation enhances the surface area and porosity of biochar [126]. Zhang et al. [127] have reported sludge-based pyrolysis to produce biochar, which was activated using a physical activator (CO2) to enhance its adsorption capacity of Pb2+ from an aqueous solution. The results revealed that the physical activation with CO2 enhanced the specific surface area by more than ten times, and its Pb2+ adsorption capacity increased from 7.6 mg/g to 22.4 mg/g [127]. The biochar activation with CO2 aided in the introduction of oxygen-containing functional groups. On the other hand, biochar activation with CH3COOK also enhanced the pore structure of sludge-based biochar, increasing its surface area more than ten times, from 81 m2/g to 908 m2/g, reaching a Pb2+ adsorption capacity of 47.6 mg/g [127].
During physical activation, biochar is exposed to a required amount of oxidizing agents, such as steam, ozone, carbon dioxide, or air, at temperatures typically above 500 °C [128]. These oxidizing chemicals enter the biochar structure and gasify the carbon atoms, opening and expanding previously inaccessible pores [129]. This type of activation can produce a biochar with larger surface areas and generate a large amount of surface oxygen functional groups, which frequently serve as active adsorption sites for pollutant removal [129].
Another physical process, like steam activation, is gas purging, in which gases (such as carbon dioxide) are mixed with the accessible amorphous carbon at the biochar surface in a restricted oxygen environment to produce carbon monoxide [130]. Moreover, carbon monoxide formation can increase the biochar surface area, improving its microporous structure and pore volume [27].
2.4.2. Chemical Activation
The most typical route for modifying the type and number of functional groups at the biochar surface is chemical activation, which involves doping a chemical agent into its structure. In this process, the raw material (i.e., biomass) is impregnated with a chemical agent, and the combination is subsequently thermally treated to obtain a biochar-doped material [129]. During the process, the chemical agent can act as an activator, which favors sample dehydration and prevents the generation of tar and volatile chemicals, thus increasing the yield of the carbonization process [131]. In addition, these activators can be used to increase the biochar-specific surface and pore volume and generate functional groups in its structure.
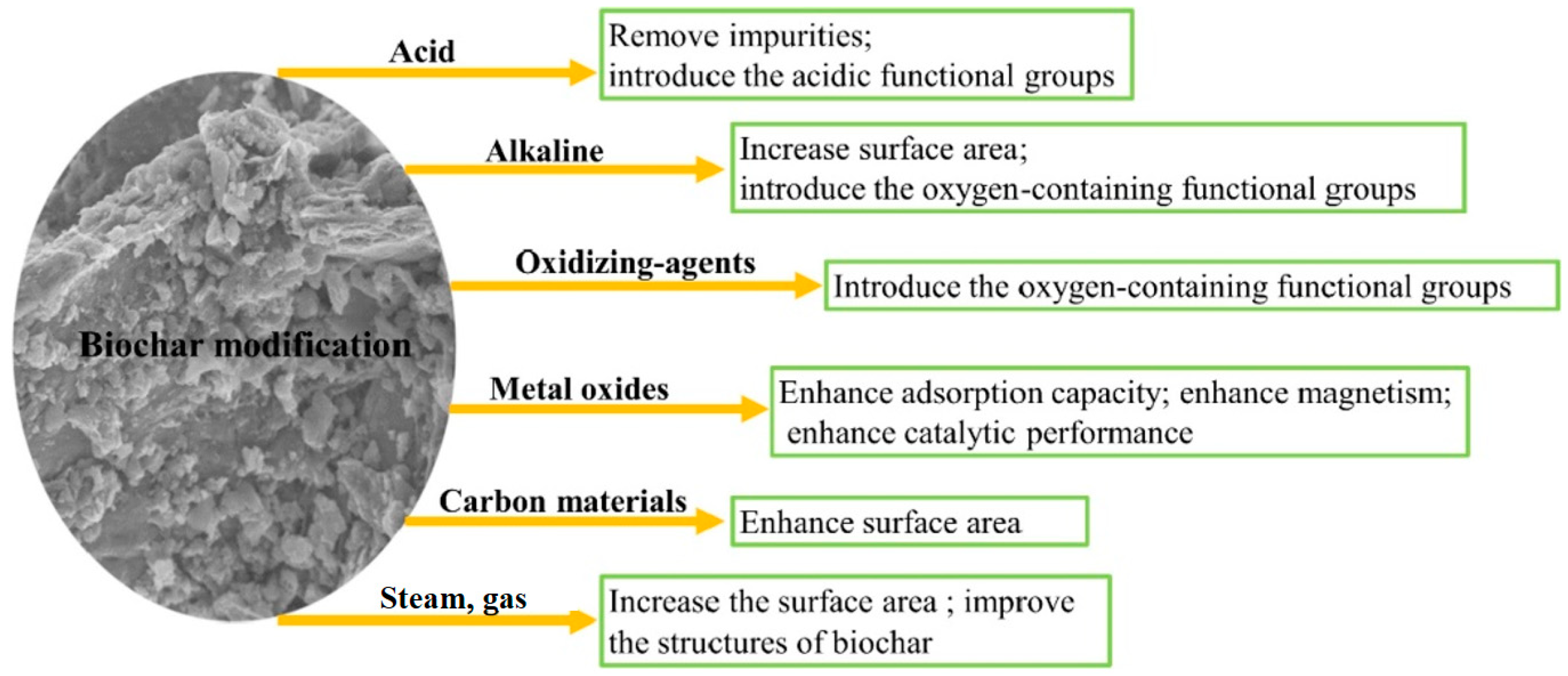
Depending on the final purpose of the carbonaceous material, acid or alkali activation can be employed. When soil amendment or water purification (heavy metal or colorant adsorption) is performed, acidic activation is preferred over alkali activation [132]. Alkali activation is more related to producing materials for energy storage [133,134] or electrochemical processes because of their high capacitances [132,133]. The impregnation of specific elements or promoters to increase biochar adsorption capacity has been widely reported for water purification. Figure 3 describes some biochar modification routes.

Figure 3.
Classification of biochar medication routes. (Reprinted from [19], with permission from Elsevier). © 2019 Elsevier Ltd.
As previously mentioned, the most common activators are alkalis (KOH, NaOH, and ZnCl2) [130] and acids (citric, nitric, sulfuric, and phosphoric) [119,135,136]. H3PO4 is commonly used as an activator because it can promote the bond breakage processes while maintaining the internal pore structure [137]. The distribution of chemical agents in the precursor before carbonization plays a vital role in the final product’s porosity improvement and functionality [138]. According to Fierro et al. [139], the effect of the added quantity of phosphoric acid for the activation of carbon derived from rice straw is essential to increase its yield until a certain quantity. When they used a H3PO4: biomass ratio equal to 1 (ranging from 0 to 1.6), the carbon yield increased by up to 10%.
Moreover, when the ratio was more significant than 1, an increase in the percentage of the carbonization yield was not observed. However, the specific surface area of the carbonaceous material increased from 520 to 786 m2/g. In this work, the volume of the pores was highly variable, and no tendency to deformation was observed. Likewise, Zakaria et al. [140] have reported that the effect of phosphoric acid to obtain mangrove-based activated carbon (with the H3PO4: precursor ratios of 3, 4, and 5) on its production yield and surface characteristics are also notable. They observed a gradual decrease in the yield of activated carbon (45–41%) as the ratio increased from 3 to 5. Other authors have also reported this fact [141,142,143,144]. Thus, it is noticeable that this trend is independent of the raw material. However, the carbon production yield depends on the raw material, as seen in Table 4.

Table 4.
Enhanced carbon production yield by activation with H3PO4.
According to the activated biochar definition [1,5,6,7,10], only rice straw, jackfruit peel waste, and rubber wood sawdust are considered activated biochar. The activation mechanism is related to the H3PO4:biomass ratio, temperature, and time [140,143,145,146]. Textural and morphology features are affected depending on time contact and temperature. Low activation time and temperature result in incomplete carbonization and a higher yield [146,147]. An appropriate H3PO4:biomass ratio, temperature, and activation time lead to improvement of the surface area and pore volume. However, beyond that, those properties can decrease, and it is because the increase in pore size leads to the collapse of the tiny pores [146].
H2SO4 and HNO3 have also been employed as activating agents of biochar. In general, the presence of H2SO4 during the biochar synthesis does not alter its structural properties (e.g., specific surface area and pore volume). However, this acid promotes the sulfonation reaction, generating polar functional groups (e.g., sulphonic groups –SO3H) at its surface [119,148,149], which, in turn, enhances its performance for several applications like ion and pollutant adsorption [148,150,151], biodiesel production [152,153] and other catalytic processes [119,154]. Likewise, HNO3-based species can modify the physicochemical properties of biochar-based materials and, thus, their performance in a specific application. Its presence promotes the generation of many types of surface functional groups through the oxidation and nitration of aromatic rings on the surface of biochar-based materials [132].
Moreover, HNO3 can remove partially combusted volatiles and impurities from the surface of biochar, enhancing its surface area and pore volume [155]. Güzel et al. [156] and Hadjittofi et al. [157] have demonstrated that nitric acid-activated biochar-adsorbents can effectively remove methylene blue and Cu2+ from aqueous solutions, respectively. In both cases, the activated carbonaceous materials exhibited higher adsorption capacities than non-activated biochar, attributed to the larger surface area, the lower point of zero charges, and more oxygen functional groups, like carboxylic, phenolic, and lactonic moieties.
On the other hand, using bases during the biochar activation can generate positive electrostatic charges on their surface, which generates a solid affinity for adsorbed negatively charged pollutants [119]. Among the bases used as biochar activators, KOH has been widely used because of the special features that it gives to biochar. Biochar properties (e.g., textural and morphological) can be improved using this chemical. The activation properties depend on the KOH: biochar ratio, temperature, and time [158,159,160]. Porosity development is associated with gasification (CO2 production) [161]. Different authors report different values of reached specific surface areas: 621 m2/g [161], 912.73 m2/g [159], and 2201 m2/g [160]. Their results differ due to the previously mentioned parameters and the synthesis process. Higher surfaces are obtained when the first raw material is converted to biochar followed by a post-chemical activation (KOH) [158,160] rather than direct one-pot pyrolysis and chemical activation [159,161]. Likewise, Trakal et al. [162] studied the effect of chemical activation on the removal efficiency of Cu from an aqueous solution using pure amorphous biochar and activated biochar (BCact). In this work, chemical activation with 2 M KOH substantially raised the total pore volume of biochar, obtaining values of 0.01 and 8.74 mL/g for amorphous biochar (surface area = 9.80 m2/g) and BCact (surface area = 11.6 m2/g), respectively. These results correlated with the Cu adsorption capacity, which was more significant for BCact (10.3 mg/g) than that obtained with amorphous biochar (8.77 mg/g).
2.5. Properties That Biochar Modification Processes Can Improve
Biochar modifications can enhance its structure and physicochemical properties (e.g., an increase in the surface area, the generation of oxygen-containing functional groups, and the increase in aromaticity, among others [163]), favoring its ability to adsorb contaminants, such as heavy metals [164]. It is due to generating active sites for specific uses, like in catalysis, water treatment, anaerobic digestion, soil remediation [93], supercapacitors, and fuel cell applications [118].
The pore size and surface functional groups of biochar are significant features that influence its efficiency as a pollutant adsorbent [165,166]. The surface functional groups in biochar are responsible for their strong metal adsorption ability [164]. Metal adsorption by biosorbents can occur via complexation between metals and different functional groups on the biosorbent surface or through electrostatic attractions between metal cations with negative charges and the functional groups at its surface [163]. According to Choudhary et al. [167], functional groups can act as adsorption sites for metal attraction and are located throughout the biochar matrix. In this sense, it is necessary to smash the biochar structure to expose a higher amount of functional groups and, therefore, to promote its efficiency for pollutant removal [167]. Considering this fact, heavy metal adsorption by biochar can occur at its surface (outer pores) as well as within the pore structure of the carbonaceous material (inner pores), depending on the type and amount of surface functional groups [164]. Likewise, the removal of other types of pollutants, like dyes [140,168,169], oil [170], pesticides [171], and pharmaceuticals [93,172], using biochar can occur through monolayer adsorption. During these treatment processes, the chemisorption predominates through the complexation, coordination, ion exchange, and chelation between pollutants and the carbonaceous materials surface, depending on the functional groups and the structural and other physicochemical properties of the biosorbents. These biochar properties depend on the raw material, synthesis method, activation routes, and the use of dopants, composites, and additives described below.
2.6. Dopants, Composites, and Additives Used to Improve Biochar Properties
Many attempts have been made to activate biochar without external doping agents, such as gas, steam, microwaves, acids, alkalis, and oxidants [118,129]. On the other hand, adding other materials to the biochar structure has been a novel strategy to produce composites with interesting properties, which can be used in several applications. Some of these strategies and applications of the carbonaceous materials are described in Table 5:

Table 5.
Some chemicals used to modify the properties and applications of biochar samples prepared by pyrolysis.
2.6.1. Dopants for Biochar
Adding a precursor or dopants can improve the physicochemical properties of biochar-based materials. Dopants promote the carbonaceous material’s reactivity, making it an interesting material for catalytic applications. Metallic and non-metallic dopants have been widely used. For example, the modification of biochar with transition metals, like iron, can enhance its specific surface area and the adsorption affinity. In contrast, modifications with non-metals and alkali/alkaline earth metals can decrease the property above [173]. Mašek et al. [44] have reported that potassium doping can increase the carbon-sequestration potential of biochar by 45%, making it an important strategy to prevent global warming.
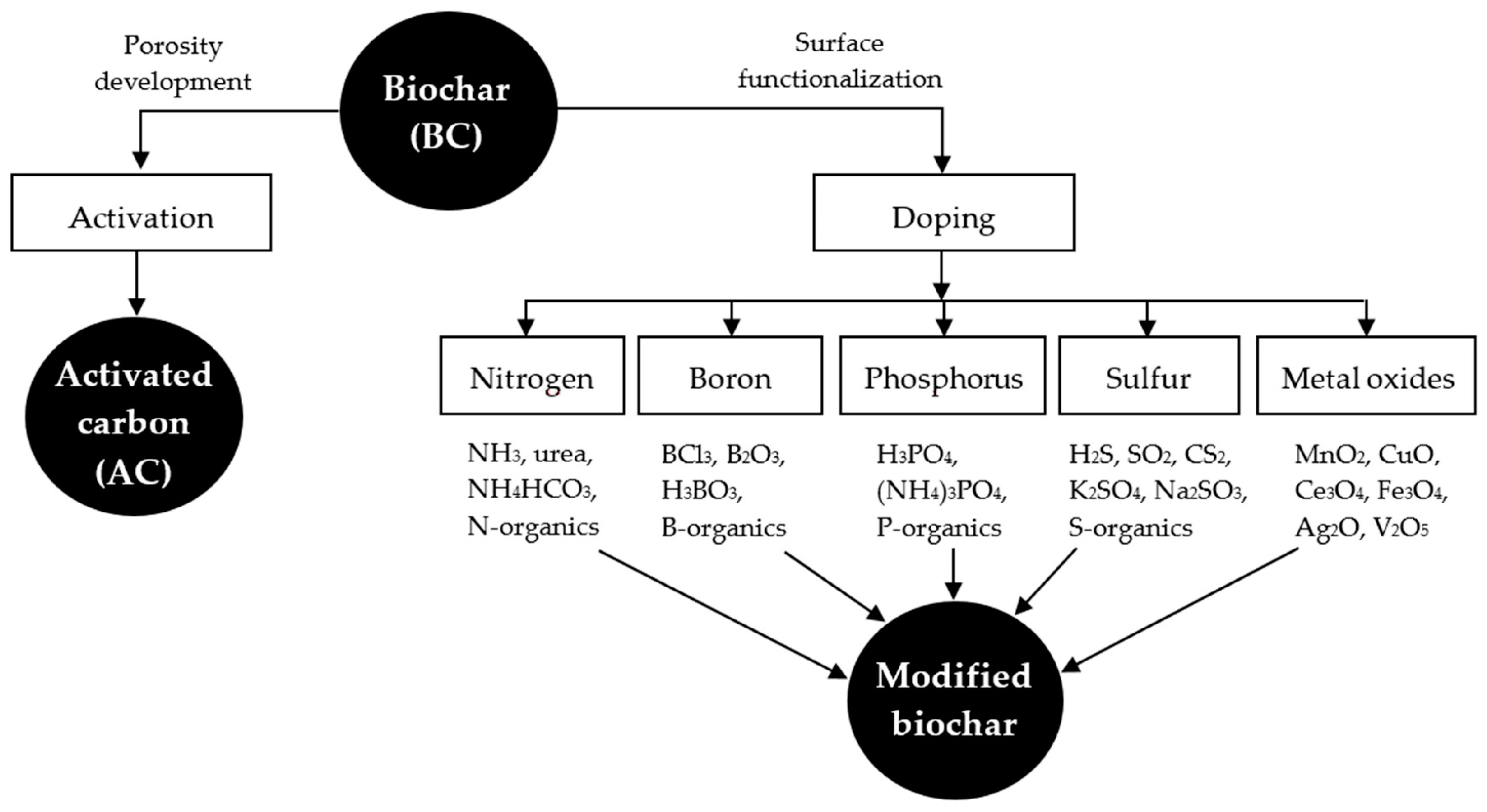
When dopants modify biochar, its functionality can be altered and could improve its performance for several environmental and energetic applications. Minerals, inorganic species, metals, and metal oxides have been the most common dopants to functionalize the biochar structure. Their presence in the carbonaceous matrix displays a significant improvement in adsorption performance, as well as in the selectivity of certain pollutants [174]. Jha [175] studied the effect of three chemical dopants on pollutant absorption using biochar-based adsorbents. These dopants were zinc oxide (ZnO), thiol (–SH), and manganese oxide (MnO2), which exhibited the highest pollutant removal. Other types of dopants have been used to promote the physicochemical properties of biochar and, therefore, its performance in a specific application. Figure 4 shows some dopants and precursors used to enhance the biochar surface.

Figure 4.
Elements and compounds used to improve the biochar structure. (Reprinted from [120]). © 2018 by the authors.
Doping techniques and procedures, such as impregnation, are the most common methods used for generating changes in the structure of biochar. Di Stasi et al. [176] produced activated biochar by wet impregnation using cerium nitrate hexahydrate or urea as dopant agents. The aqueous solutions were stirred at 80 °C until the water evaporated entirely. Subsequently, the samples were dried at 110 °C and then calcined in a reactor at 550 °C for 3 h in an inert environment (N2 atmosphere).
For water purification, well-developed porosity and hydrophobic surfaces are required to effectively enhance the adsorption capacity of organic or inorganic pollutants on biochar-based sorbents. The adsorption of inorganic or polar organic contaminants requires the presence of surface oxygen functional groups to improve the electrostatic attraction [60]. Unfortunately, sometimes biochar has a moderate to low surface area and a limited number of surface functional groups, which limits their performance [129]. For this reason, it is necessary to functionalize the biochar surface to improve its properties and, thus, its performance in a specific application. The surface chemistry of biochar can also be altered by doping heteroatoms such as N, P, S, and metal oxide from various sources [120]. Some modifications have been proposed to improve the adsorption capability of biochar-based materials, which are described below.
2.6.2. Iron-Doped Biochar
Among the dopants used during biochar synthesis, one of the most common and effective has been iron and its species, like iron oxide (Fe2O3) [177,178,179].
The presence of iron species in biochar can promote several properties and enhance its effectiveness in various applications. Iron species on the biochar are crucial in immobilization mechanisms and redox reactions [180,181,182]. They can enhance the biochar’s ability to retain essential plant nutrients, such as nitrogen and phosphorus, by forming complexes with nutrient ions, such as nitrates and phosphates [183]. In addition, iron can help buffer the pH of soils. It acts as a pH stabilizer, preventing extreme fluctuations in soil acidity or alkalinity [184]. Iron can reduce or oxidize various metals and organics. In the presence of iron, contaminants like arsenic or nitrate can undergo redox reactions that enhance their removal.
Table 6 presents diverse Fe-doped biochar samples from various feedstocks, detailing synthesis conditions and contaminant removal efficiencies. Remarkable examples include peanut hulls, which achieve 98% removal of Cr6+ through hydrothermal carbonization (HTC), and oak wood/bark biochar, which exhibits high removal rates (>98%) for Pb and Cd via pyrolysis and impregnation processes.

Table 6.
Fe-doped biochar samples and their synthesis conditions and removal efficiencies.
2.6.3. Nitrogen-Doped Biochar
Nitrogen (N) doping has attracted much attention as it can enhance the characteristics of carbon-based materials [192]. Because of the significant electronegativity of N, electron modulation can improve the surface polarity of biochar and generate unique electronegativities [193]. Moreover, N-doping into the biochar matrix can alter its electronic structure, enhancing its interaction with pollutants [194]. In addition, introducing N heteroatoms into the ordered sp2-hybridized graphite structure can modify the electrical charges of the original electron network due to the difference in electronegativity. Thus, unbalanced charged areas throughout the carbon structure can result in an electroactive state that may be used for various practical purposes. Likewise, it has been found that N-doping can improve the catalytic activity of nanocarbons, favor nanomaterial dispersion, and increase the detection limit of sensors [195].
The most common synthesis method for N-doped carbonaceous materials is the thermal decomposition of an inherent N-rich precursor [192]. It involves chemically pre- or post-treating biochar with ammonia, urea, melamine, or an N-containing organic polymer to add exogenous nitrogen into the carbon structure [196].
2.6.4. Phosphorus-Doped Biochar
Another way to enhance the biochar properties and performance in a specific application is by doping phosphorus species into the biochar structure. Including these phosphorous-based dopants aims to improve the pollutant removal capacity of biochar. Phosphoric acid (H3PO4) is a typical dopant that enhances biochar properties. Fan et al. [197], have prepared a series of novel N- and phosphorus-enriched biochar nanocomposites via co-pyrolysis with different ammonium polyphosphate (APP) weight ratios. They used the mixture of phosphorus and N dopants to improve the Pb2+ adsorption on the APP-doped biochar, observing that the Pb2+ removal efficiency of this last sorbent (723.6 mg/g) was significantly enhanced compared to that of the unmodified biochar (264.2 mg/g).
2.6.5. Composites for Biochar
Adding a composite material to the biochar structure can be an interesting enhancement strategy to improve its environmental remediation efficiency. Metal composites (e.g., Fe2O3 and iron sulfide), minerals (e.g., kaolinite), and layered double hydroxides (LDH) have been the typical composites used to promote the performance of the carbonaceous material during soil remediation (resulting in fertility improvements) and wastewater treatments [198]. LDHs are anionic clay minerals made up of positively charged metal hydroxide layers and anions in the interlayer gap to neutralize charge [199]. In pollutant adsorption, various LDH-biochar composites with divalent and trivalent metal cations (e.g., Mg-Al, Mg-Fe, Zn-Al, Ca-Al, and Ni-Fe) have been frequently used [200].
2.6.6. Other Additives for Biochar Modification
Other additives, such as phosphorus, zinc, and calcium species, can be added to biochar during its synthesis to improve its properties and broaden its applicability [201]. For example, adding calcium oxide to biochar followed by a heating process at 450 °C can generate a more stable carbonaceous material with fewer oxygen functional groups [202]. According to Li et al. [203], adding mineral additives to biochar promotes carbon retention and the stability of the solid in terms of carbon sequestration. They studied the use of kaolin, calcite (CaCO3), and calcium dihydrogen phosphate [Ca-(H2PO4)2] as additives in biochar obtained from rice straw biomass. These three minerals are frequently used to enhance soil quality and remediate soil and water pollution [204]. Likewise, adding these chemicals to biochar can improve the stability of the biochar-based material and, thus, its efficiency in removing pollutants [8].
3. Main Uses of Biochar
3.1. Biochar for Soil Remediation (Crop Improvement)
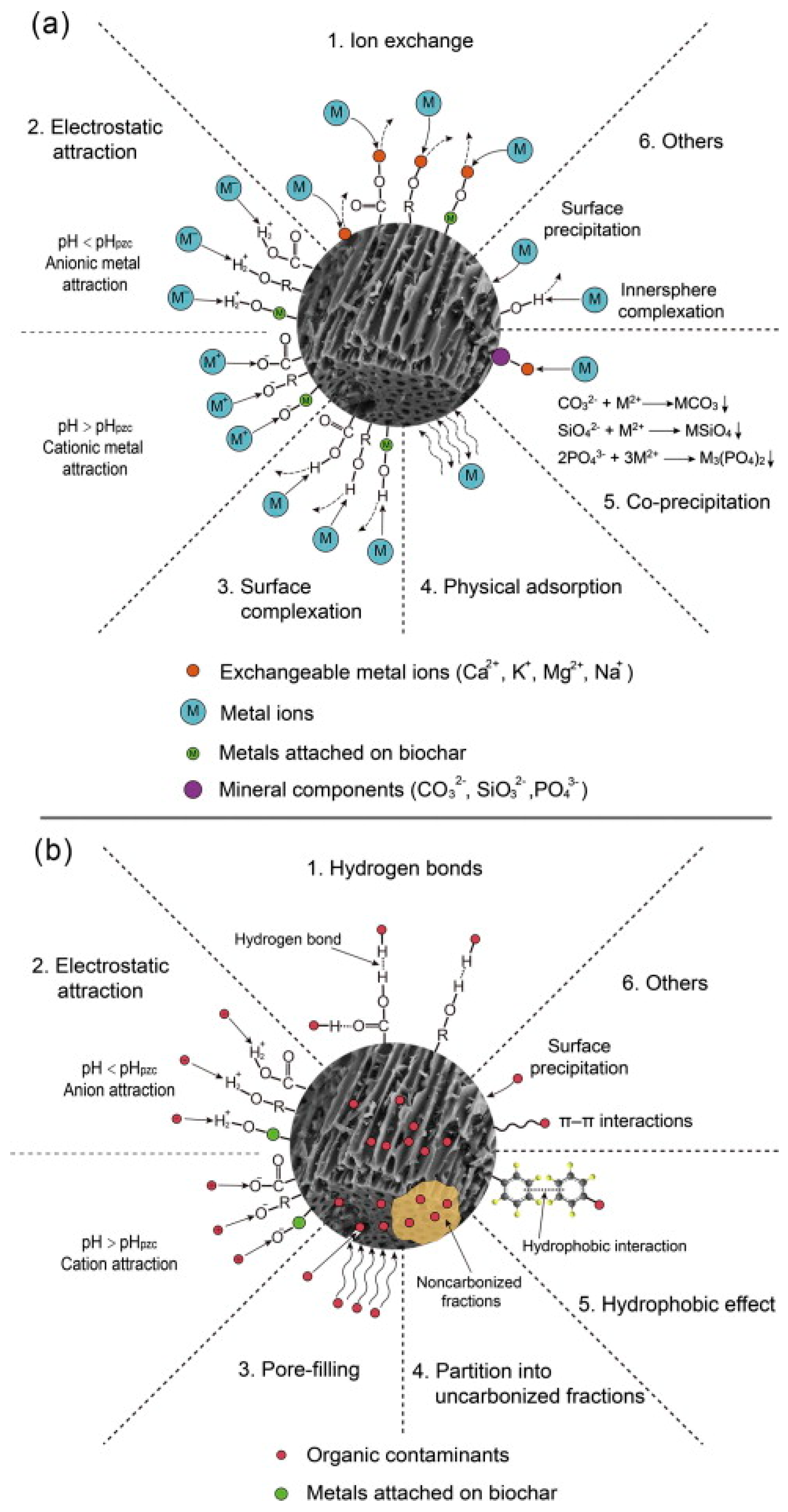
The presence of biochar can enhance soil characteristics and, at the same time, increase crop biomass and improve disease resistance. Biochar may improve soil fertility [205], soil quality (e.g., pH [40], cation exchange capacity (CEC), and water holding capacity [206]), and plant development [207,208]. Recently, biochar has been used to treat soil contaminated with heavy metals and organic contaminants [43]. Figure 5 shows the primary mechanisms for the remediation of contaminated soils containing heavy metals and organic pollutants using biochar. As seen here, precipitation, electrostatic interaction, and ion exchange are the most common mechanisms that describe soil remediation using biochar.
3.2. Biochar to Remove Pollutants in Water and Wastewater
Sources of water pollution can be classified in different ways. The most important sectors that generate wastewater or contribute to its pollution are domestic, agricultural, and industrial [209,210]. The word “contaminant,” according to the Safe Drinking Water Act, is defined as any physical (sediment or organic material suspended in the water), chemical (nitrogen, bleach, salts, pesticides, metals), biological (bacteria, viruses, protozoa, and parasites), or radiological (cesium, plutonium, and uranium) substance or species in water [211]. Some pollutants in drinking water may be dangerous or toxic at specific concentrations in drinking water, while others can be innocuous. The presence of pollutants does not always imply that the water is unsafe to drink. However, it is necessary to consider how to remove them. Table 7 shows the sub-classifications and various sources of water pollution.

Table 7.
Sources of wastewater generation [143].
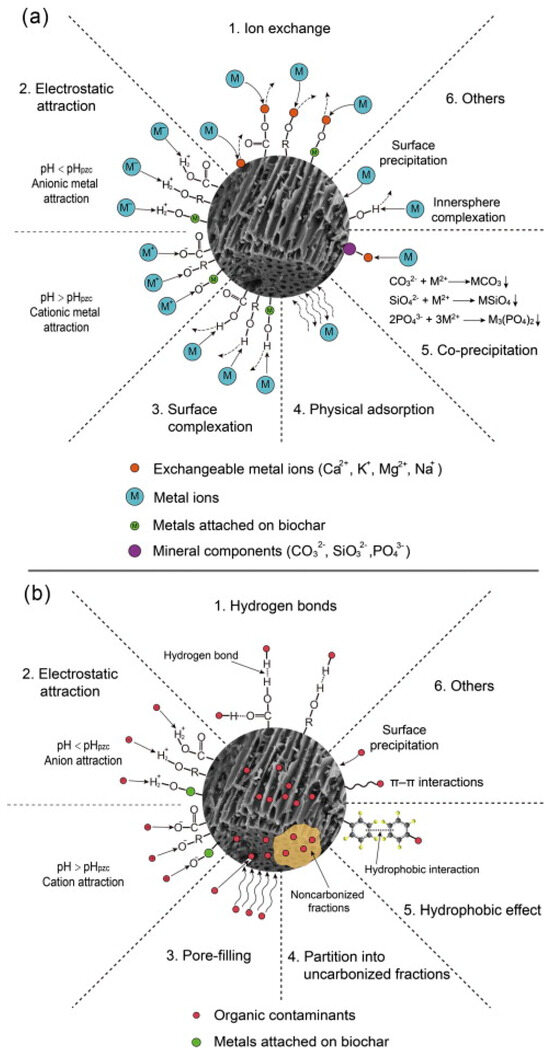
Figure 5.
Main mechanisms and description for remediation using biochar of contaminated soils with (a) heavy metals and (b) organic pollutants. (Reprinted from [212], with permission from Elsevier). © 2015 Elsevier Ltd.
Figure 5.
Main mechanisms and description for remediation using biochar of contaminated soils with (a) heavy metals and (b) organic pollutants. (Reprinted from [212], with permission from Elsevier). © 2015 Elsevier Ltd.
Biochar’s physical and chemical properties are influenced by the feedstock, synthesis method, and activation procedures, and its adsorption capacities are also influenced. This carbonaceous material can be used to remove a wide range of organic (agricultural), inorganic (toxic gases in the oil industry), and microbiological (pathogen) pollutants [213]. Table 8 describes some examples of types of contaminants in water that can be removed using biochar-based materials.

Table 8.
Frequent pollutants of water generated in industrial areas [145].
In addition to classifying the origin of wastewater, it is necessary to subdivide the types of contaminating agents in water and their effects on the hydric fluid. Table 9 describes the main contaminating agents, such as organic and inorganic agents, and their environmental effects.

Table 9.
Polluted water and its environmental effects [146,147,148,149].
Biochar treatment has a high potential for wastewater treatment [47,214]. Compared to conventional low-cost technologies (such as sand filtration, boiling, sun disinfection, and chlorination), water treatment using biochar has numerous potential advantages: (1) biochar is a low-cost and renewable adsorbent made from readily available biomaterials and skills, making it suitable for low-income communities; (2) existing methods primarily remove pathogens, whereas biochar removes chemical, biological, and physical contaminants; and (3) biochar preserves the organoleptic properties of water, whereas existing methods generate carcinogenic by-products (e.g., chlorination) and increase chemical contaminant concentrations (e.g., boiling) [213].
Biochar has been widely explored as an adsorbent for removing contaminants from wastewater due to its unique features, such as a large surface area, well-distributed pores, and a high abundance of surface functional groups [215]. The oxygenated functional groups (OFGs) in biochar are essential active sites for removing contaminants from the water via interfacial adsorption/redox reaction [214,216].
3.2.1. Mechanisms to Remove Pollutants from Water with Carbonaceous Materials
Low-cost biochar has emerged as the substitute for activated carbon for the removal of organic pollutants such as volatile organic compounds, aromatic dyes, hydrocarbons, agrochemicals, and others. Regarding inorganic contaminants, biochar has been successfully used for the removal of sulfides, ammonia, nitrates, phosphate, and heavy metals [92]. The application of biochar as an efficient contaminant remover depends on its remarkable characteristics, e.g., high specific surface area, cation exchange capacity, active functional groups, microporosity, and electrostatic interactions, among others. These properties govern the binding of polar compounds on the surface of biochar, which immobilizes the contaminants. Because of all this, biochar has been proposed in many reports as an efficient adsorbent to remove different types of organic and inorganic contaminants from water and soil in the near future. The adsorption of inorganic pollutants on biochar results from stoichiometric ionic exchange, electrostatic attraction, surface precipitation, surface sorption, and complexation [217]. In this sense, the adsorptive capabilities of biochar are influenced by various factors, including hydrophobicity, alkalinity, ion exchange capacity, and elemental compositions [218]. Surface functionality can also alter the biochar sorption capacity [219]. Rajapaksha et al. [220] have reported a mechanism of contaminant removal in water through the strong interaction between organic compounds and carbon membranes. A recent report [92] has summarized these processes to remove inorganic contaminants as a combined effect of several types of interactions, such as electrostatic interactions due to a high dependency on the point of zero charge, surface sorption because of the diffusion of the metal ions onto the pores of the sorbent, and chemical bonds with active functional groups. Also, via cation exchange as a result of the replacement of positive charges on the surface of biochar by metal ions, complexation takes place because of the oxygen functional group (for example, carboxyl and phenolic) with high efficiency of binding heavy metal ions. On the other hand, the removal of organic contaminants can also be connected with the combination of different interactions. These interactions are mainly hydrophobic interactions, pore filling, partitioning, electron donor and acceptor, and electrostatic interactions. The contaminants can be attracted to the carbonaceous membranes (e.g., graphene and biochar) through intermolecular forces, such as non-covalent bonds, hydrogen bonds, van der Waals forces, π–π stacking, and hydrophobic interactions. The mechanism of removal of contaminants by carbonaceous materials is illustrated in Figure 6.

Figure 6.
Mechanism of pollutant removal from water using granular carbonaceous materials (Reprinted from [221]). © 2020 by the authors.
3.2.2. Biochar Used at Medium and Large-Scale in Water Filtering Process
The global demand for safe and quality drinking water has become increasingly important due to the growing world population and anthropogenic activities. Water pollution by synthetic organic compounds, such as pesticides, medicines, and fuel components, is an increasing concern worldwide because these chemicals can bioaccumulate in the human body, causing cancer and other disorders. In recent years, many researchers have focused on the applications of biochar as a potential and efficient adsorbent to remove contaminants from aqueous solution. Due to its remarkable properties, numerous reports have been published confirming the many advantages of biochar for environmental uses, and it has been widely studied in removing both organic and inorganic contaminants [64]. As an efficient adsorbent, it has been used to immobilize heavy metal ions, even as a catalyst for the degradation of complex organic compounds. Nevertheless, the industrial application of these carbonaceous materials requires significant infrastructure expenditures [222,223]. Considering this fact, creating filters with carbonaceous materials at different scales becomes an excellent option to mitigate or reduce aquatic pollution at different scales. The use of biochar filters has been suggested as an option to replace both treatments of drinking water: the conventional treatment (e.g., coagulation-flocculation, filtration, and chlorination) and the advanced treatment (e.g., membrane filtration, ozonation, and biofiltration) [3].
Some authors have also compared the advantages of using biochar for water treatment to low-cost methods [213]. They consider that biochar treatment has several merits compared to methods such as sand filtration, boiling, solar disinfection, or chlorination because, although some methods remove pathogens, biochar removes chemical, biological, and physical contaminants. Moreover, it maintains the organoleptic properties of water, while other treatments, such as chlorination, might produce carcinogenic by-products [213]. Recent work has focused on using engineering biologically enhanced biochar (BEB) for biological water treatment [210], focusing on the scope, potential benefits, and challenges of sustainable water treatment using BEB. The work examines BEB’s dynamic and complex biofilm–biochar interactions in water treatment. The authors also suggest the use of BEB instead of biological activated carbon (BAC) in the tertiary treatment of drinking water due to the immobilization of microbes on the surface facilitating contaminants removal via a combined adsorption and biodegradation process, on the basis that the biofilms can degrade and remove a wide range of organic, inorganic, and biological waterborne contaminants.
Inexpensive and available biochar and woodchips were used for anaerobic wastewater filtration, and their suitability was evaluated compared to gravel as a standard reference material [224]. Filters were fed with raw sewage from a municipal full-scale wastewater treatment plant in Germany at room temperature. The performance of the biochar filters was much better over the experiment compared to woodchip and gravel filters concerning chemical oxygen demand, total organic carbon, turbidity, and fecal indicator bacteria removal efficiency, showing the superior properties of biochar for wastewater treatment. Advanced oxidation processes are proven to be efficient in water treatment (reduction of toxic, organic pollutants) and elimination of emerging concerns like pollutants (toxins, pesticides, dyes, etc.) and include UV/O3, UV/H2O2, Fenton, photo-Fenton, nonthermal plasmas, sonolysis, photocatalysis, radiolysis, supercritical water oxidation processes, etc. [225]. In this point, it is very important to mention that advanced oxidation can be achieved using biochar because of the radical groups, mainly hydroxyl radical, introduced by chemical treatments such as acid or alkali hydrolysis. Biochar functionalized with hydroxyl groups enhances soil structure and reduces soil erosion, facilitates water and nutrient retention, etc.
4. Potential Drawbacks and Future Perspectives of the Use of Biochar-Based Materials
In recent years, biochar has gained significant attention as a promising alternative to mitigate environmental and climate change issues through efficient and inexpensive water treatment and soil amendment methods. However, despite its advantages, using this carbonaceous material can generate long-term drawbacks, especially for soil health and ecosystems [25,226]. One of the potential drawbacks of biochar is related to the synthesis conditions and the use of chemicals to improve its physicochemical properties [115]. For example, for the pyrolysis process, it is necessary to reach high temperatures (above 400 °C) under anoxic atmospheres [58,79,90,221]. To avoid these conditions, biochar-based materials can be prepared using HTC. However, it is necessary to use a large amount of water to carry out the washing cycles of the carbonaceous material. In addition, hydrothermal reactors may be used by HTC to obtain biochar, which, until now, has only been used at a laboratory scale. Due to this, the HTC of biomass has not been able to be used on a large scale. Another potential drawback related to biochar synthesis is the available feedstock. Although biochar can be produced from any biomass, the process yield is generally less than 60% [119,133]. For this reason, a more significant amount of biochar would be needed to obtain a considerable amount of biomass, which, in many cases, can be used directly as fertilizer or organic fertilizer.
On the other hand, some drawbacks are related directly to the biochar application. One of them is the generation of sludge containing biochar with contaminants after adsorption processes, which, sometimes, must be treated or incinerated, generating a large amount of gases that can harm the environment [2]. Likewise, the efficiency of biochar is sometimes lower than that of other types of adsorbents, like zeolites [227,228,229,230], clays [32,50,231,232], and hydrogels [233,234,235], which can be even cheaper than the carbonaceous materials.
When biochar is used as a soil amendment, its application can affect the soil biota because the carbonaceous material can potentially alter essential biogeochemical processes, like nutrient cycling and decomposition [34,104]. According to Han et al. [41], high biochar concentrations can inhibit the growth of specific microbial communities and favor others, generating considerable changes in microbial diversity and function in soils. In addition, biochar can influence nutrient dynamics and their interactions in soils. Its presence provides a porous surface for nutrient adsorption and immobilization, making it less accessible to plants [39,226]. Moreover, biochar may interact with pesticides and fertilizers, altering their bioavailability and potential environmental effects [34,236]. Furthermore, the presence of biochar in soils could modify their water-holding capacity, aeration, root penetration, and aggregate stability [39,104]. For all these reasons, it is necessary to pay attention to the type and amount of biochar used for soil remediation and not to considerably alter its physicochemical properties.
Even though biochar is obtained from residual biomass, it can be produced from contaminated raw materials. In this sense, when this type of biochar is used in soil remediation, contaminants from the carbonaceous materials may lead to the introduction of pollutants into the soil, generating potential environmental and human health risks [237,238]. Therefore, careful selection of raw materials and monitoring pollutant levels are significant to ensure the environmental safety of biochar application.
There is scarce information about long-term data on the biochar effects on soil health, water, and ecosystems [239,240]. Hence, conducting long-term field research studies may be crucial to evaluate the cumulative effects of biochar application and identify potential unintended consequences. Considering the drawbacks of the synthesis and applications of biochar, it is necessary to develop alternatives to improve its use, considering the principles of sustainable development. Among them, one can consider:
- Optimizing biochar synthesis methods and their physicochemical properties: Research studies are necessary to optimize the production processes for a carbonaceous material with tailored properties for specific applications, avoiding the use of complicated synthesis conditions and hazardous chemicals, as well as the generation of residues. This includes understanding the effects of the type of raw materials, temperature conditions, and post-synthesis treatments on the biochar properties and their efficiency in the corresponding application.
- Understanding the complex interactions between biochar and water bodies and soil biota is essential for predicting and mitigating potential negative impacts. This includes identifying microbial communities sensitive to biochar and developing alternatives to minimize disruptions to soil biodiversity and water bodies [63,104].
- To quantify and analyze the effects of biochar on the availability, retention, and interactions of soil nutrients with fertilizers and pesticides [60,69]. It enables the development of biochar-nutrient management strategies to optimize nutrient use efficiency and minimize environmental risks.
- To evaluate the long-term effects of using biochar on soil health, crop productivity, and ecosystem services. This fact may provide enough data for sustainable biochar management practices [239,240].
- Developing novel standardized biochar characterization and assessment protocols to compare biochar-based materials produced from different raw materials with specific synthesis methods and conditions [241,242]. This, in turn, could facilitate the development of evidence-based recommendations for using different types of biochar in specific applications.
- To address social, economic, and policy considerations necessary to ensure the sustainable application of biochar-based technologies [243]. It includes identifying potential socioeconomic implications, assessing the costs and benefits of biochar use, and developing supportive policies that promote the sustainable production and utilization of this carbonaceous material [226].
Therefore, although biochar has potential applications in improving soil and water properties, mitigating climate change, and remediating contaminated environments, its potential drawbacks must be identified and addressed. Future research efforts should optimize industrial biochar production conditions and methods, understand its interactions with water and soil biota and nutrient dynamics, evaluate its long-term effects on ecosystems, develop standardized characterization protocols, and address social, economic, and political considerations. By considering these challenges and understanding the long-term effects of using biochar, it will be possible to ensure its use as a sustainable and responsible application to mitigate environmental problems, maximizing its benefits and minimizing its potential drawbacks.
5. Conclusions and Final Remarks
Biochar emerges as a product with high environmental value, which has a low cost and is suitable for wastewater purification and soil amendment. This carbonaceous material has a significant adsorption capacity for heavy metals and other industrial pollutants in polluted water bodies. Biochar’s synthesis, activation, modification, and thermochemical treatment processes are crucial in obtaining the desired physicochemical properties. All these changes can alter the material’s structure and, thus, the pollutant removal performance obtained during the treatment. Biochar is typically used as an adsorbent. However, it can be used in other wastewater treatment technologies, like advanced oxidation processes. Combining primary and secondary treatment processes with biochar makes it possible to achieve high removal percentages of any pollutants, toxins, or impurities in industrial wastewater. On the other hand, current research has shown that adding dopants, additives, or composites to biochar can improve its physicochemical, molecular, and structural properties. Likewise, physicochemical or biological modifications of biochar can change its structural properties (e.g., specific surface area and pore structure) or modify the type and concentration of the surface oxygen functional group content, enhancing its performance in pollutant removal.
The type of raw material is essential to produce biochar-based materials with particular physicochemical properties and characteristics and, therefore, with a specific use. Among the raw materials used to obtain biochar, wood materials have been widely studied because their high content of lignin, cellulose, and hemicellulose has allowed the production of carbonaceous materials with high carbon content and high adsorption character. The widely used technique to produce biochar has been the pyrolysis process since this method favors high yields in biochar production. This process converts biomass into biochar with a high fixed carbon content and stability, where 500–800 °C is the ideal range for pyrolysis temperature in biochar production. On the other hand, HTC is also an interesting synthesis method of biochar because it has many advantages compared to others. Among them, the operating temperature is lower than pyrolysis, it is not necessary to control the inert atmosphere during the carbonization, and the generation of functional groups at the biochar surface can be controlled by varying the operating conditions.
Biochar can be used to treat agricultural and industrial wastewater. However, strategies to maximize its adsorption capability and stability must be developed. Thus, in recent years, the use of precursors, dopants, or additives during biochar synthesis has been widely studied to improve the capture of chemical molecules that are difficult to treat using conventional methods. Furthermore, modification and activation processes have been considered promising alternatives to enhance biochar’s physicochemical and structural properties and, therefore, its performance in removing pollutants in soils and water bodies. Most current scientific investigations on biochar and its applications have been performed at a laboratory scale because small-scale trials and studies should be conducted before carrying out biochar applications on an industrial scale. Biochar is a promising alternative to mitigate environmental issues since it improves the quality of water and soil, allows the use of biomass waste, and adds value to it, meeting the aim of the circular economy.
Author Contributions
Conceptualization, B.D., M.R. and C.N.-C.; methodology, B.D. and C.N.-C.; validation, P.E.O., A.S.-M. and E.B.-G.; formal analysis, P.E.O., A.S.-M., E.B.-G., M.R. and C.N.-C.; investigation, B.D., M.R. and C.N.-C.; data curation, B.D., M.R. and C.N.-C.; writing—original draft preparation, B.D. and C.N.-C.; writing—review and editing, P.E.O., A.S.-M., E.B.-G., M.R. and C.N.-C.; visualization, M.R. and C.N.-C.; supervision, M.R. and C.N.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The Ecuadorian Corporation for the Development of Research and the Academy (CEDIA) funded this research, project number CEPRA XVI-2022-13 BioFe.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding authors (M.R. and C.N.-C.) upon reasonable request.
Acknowledgments
The authors acknowledge the support of the School of Chemical Sciences and Engineering at Yachay Tech University.
Conflicts of Interest
The authors declare that this study received funding from the Ecuadorian Corporation for the Development of Research and the Academy (CEDIA). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Jenkins, J.R.; Viger, M.; Arnold, E.C.; Harris, Z.M.; Ventura, M.; Miglietta, F.; Girardin, C.; Edwards, R.J.; Rumpel, C.; Fornasier, F.; et al. Biochar alters the soil microbiome and soil function: Results of next-generation amplicon sequencing across Europe. GCB Bioenergy 2017, 9, 591–612. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of pollutants from wastewater by biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Yang, Y.; Tsang, Y.F.; Sarkar, B.; Hou, D.; Cao, X.; Meers, E.; Rinklebe, J.; Kim, K.-H.; Ok, Y.S. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 549–611. [Google Scholar] [CrossRef]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y. Combustion, pyrolysis and char CO2-gasification characteristics of hydrothermal carbonization solid fuel from municipal solid wastes. Fuel 2016, 181, 905–915. [Google Scholar] [CrossRef]
- Lehmann, J. Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; ISBN 9780203762264. [Google Scholar]
- Tripathi, M.; Sahu, J.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants (dyes) from aqueous environment: A Review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Groot, H.; Fernholz, K.; Frank, M.; Howe, J.; Bowyer, J.; Bratkovich, S. Biochar 101: An Introduction to an Ancient Product Offering Modern Opportunities; Dovetail Partners, Inc.: Minneapolis, MN, USA, 2016. [Google Scholar]
- Pandey, B.; Prajapati, Y.K.; Sheth, P.N. Recent progress in thermochemical techniques to produce hydrogen gas from biomass: A state of the art review. Int. J. Hydrog. Energy 2019, 44, 25384–25415. [Google Scholar] [CrossRef]
- Ferrer, I.; Garfí, M.; Uggetti, E.; Ferrer-Martí, L.; Calderon, A.; Velo, E. Biogas production in low-cost household digesters at the Peruvian Andes. Biomass Bioenergy 2011, 35, 1668–1674. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.-J.; Hao, X.; Peng, P.; Shi, J.-Y.; Peng, F.; Sun, R.-C. Hydrothermal synthesis and applications of advanced carbonaceous materials from biomass: A review. Adv. Compos. Hybrid Mater. 2020, 3, 267–284. [Google Scholar] [CrossRef]
- Arellano, O.; Guerra, J.; Hidalgo, A.; Flores, M.; Rojas, D.; Strubinger, A. hydrothermal carbonization (HTC) of corncob and characterization of the obtained hydrochar. Chem. Eng. Trans. 2016, 50, 235–240. [Google Scholar] [CrossRef]
- Hoffmann, V.; Jung, D.; Zimmermann, J.; Rodriguez Correa, C.; Elleuch, A.; Halouani, K.; Kruse, A. Conductive carbon materials from the hydrothermal carbonization of vineyard residues for the application in electrochemical double-layer capacitors (EDLCs) and direct carbon fuel cells (DCFCs). Materials 2019, 12, 1703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Z.; Chen, H.; Cai, T.; Liu, Z. Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: A critical review. J. Clean. Prod. 2021, 268, 115910. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, D.A.; Díaz-Robles, L.A.; Cubillos, F.; Gómez, J.; Reyes, A.; Vallejo, F.; Pino-Cortés, E. Drying process study of hydrothermal carbonized biomass. Dry. Technol. 2022, 40, 273–283. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Navas-Cárdenas, C.; Caetano, M.; Endara, D.; Jiménez, R.; Lozada, A.B.; Manangón, L.E.; Navarrete, A.; Reinoso, C.; Sommer-Márquez, A.E.; Villasana, Y. The role of oxygenated functional groups on cadmium removal using pyrochar and hydrochar derived from Guadua angustifolia residues. Water 2023, 15, 525. [Google Scholar] [CrossRef]
- Pineda, M.; Flóres, D. Evaluación del Hydrochar Producido por Tratamiento Hidrotermal Como Medio Adsorbente de Color de un Agua Residual; Universidad de La Salle: Bogota, Colombia, 2019. [Google Scholar]
- Torres, C.; Cuartas, J. Use of amazonian anthropogenic soils: Comparison between Caboclos communities and Tikunas indigenous group. Gestión Y Ambient. 2013, 16, 5–18. [Google Scholar]
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. The “Terra Preta” phenomenon: A model for sustainable agriculture in the humid tropics. Naturwissenschaften 2001, 88, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, T.; Ringer, M.; Biró, B. Characteristics and Applications of biochar in soil–plant systems: A short review of benefits and potential drawbacks. Appl. Sci. 2022, 12, 4051. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, Y.; Wang, Y.; Wang, L.; Sun, Y.; Liu, L. Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Sci. Technol. 2022, 4, 100059. [Google Scholar] [CrossRef]
- Thengane, S.K.; Bandyopadhyay, S. Biochar mines: Panacea to climate change and energy crisis? Clean Technol. Environ. Policy 2020, 22, 5–10. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 8, 183. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Qu, J.; Meng, Q.; Peng, W.; Shi, J.; Dong, Z.; Li, Z.; Hu, Q.; Zhang, G.; Wang, L.; Ma, S.; et al. Application of functionalized biochar for adsorption of organic pollutants from environmental media: Synthesis strategies, removal mechanisms and outlook. J. Clean. Prod. 2023, 423, 138690. [Google Scholar] [CrossRef]
- Chu, Y.; Khan, M.A.; Zhu, S.; Xia, M.; Lei, W.; Wang, F.; Xu, Y. Microstructural modification of organo-montmorillonite with Gemini surfactant containing four ammonium cations: Molecular dynamics (MD) simulations and adsorption capacity for copper ions. J. Chem. Technol. Biotechnol. 2019, 94, 3585–3594. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Sherpa, K.; Bui, X.T.; Nguyen, V.T.; Vo, T.D.H.; Ho, H.T.T.; Chen, C.W.; Dong, C. Di biochar for soil remediation: A comprehensive review of current research on pollutant removal. Environ. Pollut. 2023, 337, 122571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, L.; Lu, Z.; Wang, Y.; Wang, Y.; Wan, S. Enhanced removal of heavy metals from water by hydrous ferric oxide-modified biochar. ACS Omega 2020, 5, 28702–28711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Liu, L.; Ju, M.; Zheng, K. Adsorption behavior of selective recognition functionalized biochar to Cd(II) in wastewater. Materials 2018, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Fabietti, G.; Biasioli, M.; Barberis, R.; Ajmone-Marsan, F. Soil contamination by organic and inorganic pollutants at the regional scale: The case of Piedmont, Italy. J. Soils Sediments 2009, 10, 290–300. [Google Scholar] [CrossRef]
- Cui, D.J.; Zhang, Y.L. Current situation of soil contamination by heavy metals and research advances on the remediation techniques. Chin. J. Soil Sci. 2004, 35, 366–370. [Google Scholar]
- Lu, Y.; Gu, K.; Shen, Z.; Tang, C.S.; Shi, B.; Zhou, Q. Biochar implications for the engineering properties of soils: A review. Sci. Total Environ. 2023, 888, 164185. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Han, M.; Zhang, J.; Zhang, L.; Wang, Z. Effect of biochar addition on crop yield, water and nitrogen use efficiency: A meta-analysis. J. Clean. Prod. 2023, 420, 138425. [Google Scholar] [CrossRef]
- Yargicoglu, E.N.; Sadasivam, B.Y.; Reddy, K.R.; Spokas, K. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Brownsort, P.; Rovere, M.; Tagliaferro, A.; Zhao, L.; Cao, X.; Xu, G. Potassium doping increases biochar carbon sequestration potential by 45%, facilitating decoupling of carbon sequestration from soil improvement. Sci. Rep. 2019, 9, 5514. [Google Scholar] [CrossRef] [PubMed]
- Samanta, M.; Mitra, D. Treatment of petroleum hydrocarbon pollutants in water. In Water Pollution and Remediation: Organic Pollutants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 229–275. [Google Scholar] [CrossRef]
- Lou, Y.; Joseph, S.; Li, L.; Graber, E.R.; Liu, X.; Pan, G. Water extract from straw biochar used for plant growth promotion: An initial test. BioResources 2016, 11, 249–266. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Présiga-López, D.; Rubio-Clemente, A.; Pérez, J.F. Uso del biocarbón como material alternativo para el tratamiento de aguas residuales contaminadas. Rev. UIS Ing. 2020, 20, 121–134. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Ho, S. Low-cost adsorbents for the removal of phenol/phenolics, pesticides, and dyes from wastewater systems: A review. Water 2022, 14, 3203. [Google Scholar] [CrossRef]
- Jiménez-Oyola, S.; García-Martínez, M.J.; Ortega, M.F.; Chavez, E.; Romero, P.; García-Garizabal, I.; Bolonio, D. Ecological and probabilistic human health risk assessment of heavy metal(loid)s in river sediments affected by mining activities in Ecuador. Environ. Geochem. Health 2021, 43, 4459–4474. [Google Scholar] [CrossRef]
- Rae, P.; Di Lullo, G.; bte Ahmad, A. Towards environmentally-friendly additives for well completion and stimulation operations. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition; SPE, Jakarta, Indonesia, 17 April 2001. [Google Scholar]
- Hedar, Y. Budiyono Pollution impact and alternative treatment for produced water. E3S Web Conf. 2018, 31, 03004. [Google Scholar] [CrossRef]
- Neff, J.; Lee, K.; DeBlois, E.M. Produced water: Overview of composition, fates, and effects. In Produced Water; Springer: New York, NY, USA, 2011; pp. 3–54. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of soil polluted with petroleum hydrocarbons and its reuse for agriculture: Recent progress, challenges, and perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef]
- Song, X.; Wu, X.; Song, X.; Shi, C.; Zhang, Z. Sorption and desorption of petroleum hydrocarbons on biodegradable and nondegradable microplastics. Chemosphere 2021, 273, 128553. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Saeed, T.; Jupp, B.; Faizuddin, M. Petroleum hydrocarbon pollution in sediments from the Gulf and Omani waters: Status and review. Mar. Pollut. Bull. 2021, 173, 112913. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, Y.K. Applications of modified biochar-based materials for the removal of environment pollutants: A mini review. Sustainability 2020, 12, 6112. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.J. Radionuclides in fracking wastewater: Managing a toxicblend. Environ. Health Perspect. 2014, 122, A50–A55. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Bilek, E.; Bergman, R.; Mani, S. Techno-economic analysis of producing solid biofuels and biochar from forest residues using portable systems. Appl. Energy 2019, 235, 578–590. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Z.; Wang, H.; Bolan, N.S.; Wang, Y.; Chen, W. Visualizing the emerging trends of biochar research and applications in 2019: A scientometric analysis and review. Biochar 2020, 2, 135–150. [Google Scholar] [CrossRef]
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in water and wastewater treatment—A sustainability assessment. Chem. Eng. J. 2021, 420, 129946. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pal, D.B.; Prasad, N. Agricultural waste biomass utilization in waste water treatment. In Utilization of Waste Biomass in Energy, Environment and Catalysis; CRC Press: Boca Raton, FL, USA, 2022; pp. 19–41. [Google Scholar] [CrossRef]
- McGlashan, N.; Shah, N.; Caldecott, B.; Workman, M. High-level techno-economic assessment of negative emissions technologies. Process Saf. Environ. Prot. 2012, 90, 501–510. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123964885. [Google Scholar]
- Adilla Rashidi, N.; Yusup, S. A Mini Review of Biochar Synthesis, Characterization, and Related Standardization and Legislation. In Applications of Biochar for Environmental Safety; IntechOpen: London, UK, 2020; p. 16. [Google Scholar]
- Leithaeuser, A.; Gerber, M.; Span, R.; Schwede, S. Bioresource Technology Comparison of pyrochar, hydrochar and lignite as additive in anaerobic digestion and NH4+ adsorbent. Bioresour. Technol. 2022, 361, 127674. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Hawkins, B.; Li, X.; Day, D.M. Biochar Fertilizer for Soil Amendment and Carbon Sequestration. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; Volume 9781461433, pp. 57–68. ISBN 9781461433484. [Google Scholar]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E.W. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.; Bucheli, T. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef]
- Anderson, N.; Jones, J.G.; Page-Dumroese, D.; McCollum, D.; Baker, S.; Loeffler, D.; Chung, W. A comparison of producer gas, biochar, and activated carbon from two distributed scale thermochemical conversion systems used to process forest biomass. Energies 2013, 6, 164–183. [Google Scholar] [CrossRef]
- Bourke, J.; Manley-Harris, M.; Fushimi, C.; Dowaki, K.; Nunoura, T.; Antal, M.J. Do all carbonized charcoals have the same chemical structure? 2. A model of the chemical structure of carbonized charcoal. Ind. Eng. Chem. Res. 2007, 46, 5954–5967. [Google Scholar] [CrossRef]
- Rangarajan, G.; Jayaseelan, A.; Farnood, R. Photocatalytic reactive oxygen species generation and their mechanisms of action in pollutant removal with biochar supported photocatalysts: A review. J. Clean. Prod. 2022, 346, 131155. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Bouafif, H.; Hamza, N.; Neculita, C.M.; Koubaa, A. Production, characterization, and potential of activated biochar as adsorbent for phenolic compounds from leachates in a lumber industry site. Environ. Sci. Pollut. Res. 2018, 25, 26562–26575. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzaman, M. Biochar based nanocomposites for photocatalytic degradation of emerging organic pollutants from water and wastewater. Mater. Res. Bull. 2021, 140, 111262. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.V.N.; Gnana Prakash, D.; Adithya Joseph, A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Hunt, J.; DuPonte, M.; Sato, D.; Kawabata, A. The basics of biochar: A natural soil amendment. Soil. Crop. Manag. 2010, 30, 1–6. [Google Scholar]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2010, 44, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Odega, C.A.; Ayodele, O.O.; Ogutuga, S.O.; Anguruwa, G.T.; Adekunle, A.E.; Fakorede, C.O. Potential application and regeneration of bamboo biochar for wastewater treatment: A review. Adv. Bamboo Sci. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Dattatraya Saratale, G.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Sirohi, R.; Kant Bhatia, S.; Atabani, A.E.; Mulone, V.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R.L. Influence of pyrolysis temperature on physico-chemical properties of corn stover (zea mays l.) biochar and feasibility for carbon capture and energy balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef]
- Ercan, B.; Alper, K.; Ucar, S.; Karagoz, S. Comparative studies of hydrochars and biochars produced from lignocellulosic biomass via hydrothermal carbonization, torrefaction and pyrolysis. J. Energy Inst. 2023, 109, 101298. [Google Scholar] [CrossRef]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.U.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-fuels from thermochemical conversion of renewable resources: A review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Inayat, M.; Shahbaz, M.; Naqvi, S.R.; Sulaiman, S.A. Advance strategies for tar elimination from biomass gasification techniques. In Bioenergy Resources and Technologies; Academic Press: Cambridge, MA, USA, 2021; pp. 61–88. [Google Scholar] [CrossRef]
- Moreno-Riascos, S.; Ghneim-Herrera, T.; Moreno-Riascos, S.; Ghneim-Herrera, T. Impact of biochar use on agricultural production and climate change. A review. Agron. Colomb. 2020, 38, 367–381. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A review of biochar-based sorbents for separation of heavy metals from water. Int. J. Phytoremediation 2020, 22, 111–126. [Google Scholar] [CrossRef]
- Gargiulo, V.; Gomis-Berenguer, A.; Giudicianni, P.; Ania, C.O.; Ragucci, R.; Alfè, M. Assessing the Potential of Biochars Prepared by Steam-Assisted Slow Pyrolysis for CO2 Adsorption and Separation. Energy Fuels 2018, 32, 10218–10227. [Google Scholar] [CrossRef]
- Jiang, Y.; Zong, P.; Ming, X.; Wei, H.; Zhang, X.; Bao, Y.; Tian, B.; Tian, Y.; Qiao, Y. High-temperature fast pyrolysis of coal: An applied basic research using thermal gravimetric analyzer and the downer reactor. Energy 2021, 223, 119977. [Google Scholar] [CrossRef]
- Wijitkosum, S.; Jiwnok, P. Elemental Composition of Biochar Obtained from Agricultural Waste for Soil Amendment and Carbon Sequestration. Appl. Sci. 2019, 9, 3980. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Mohd Taib, R.; Mohamad Aziz, N.S.; Omar, M.R.; Md Disa, N. Banana pseudo-stem biochar derived from slow and fast pyrolysis process. Heliyon 2023, 9, e12940. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Sajjadi, B.; Chen, W.Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic-Based Amination of Biochar for Efficient CO2 Adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.T.; Fan, M.; Daugaard, D.; Brown, R.C. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Fiori, L.; Basso, D.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of biomass: Design of a batch reactor and preliminary experimental results. Chem. Eng. Trans. 2014, 37, 55–60. [Google Scholar] [CrossRef]
- Gamgoum, R.; Dutta, A.; Santos, R.M.; Chiang, Y.W. Hydrothermal Conversion of Neutral Sulfite Semi-Chemical Red Liquor into Hydrochar. Energies 2016, 9, 435. [Google Scholar] [CrossRef]
- Weiner, B.; Baskyr, I.; Poerschmann, J.; Kopinke, F.D. Potential of the hydrothermal carbonization process for the degradation of organic pollutants. Chemosphere 2013, 92, 674–680. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.W.; Kim, H.J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Ahmad, F.; Silva, E.L.; Varesche, M.B.A. Hydrothermal processing of biomass for anaerobic digestion—A review. Renew. Sustain. Energy Rev. 2018, 98, 108–124. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Yihunu, E.W.; Minale, M.; Abebe, S.; Limin, M. Preparation, characterization and cost analysis of activated biochar and hydrochar derived from agricultural waste: A comparative study. SN Appl. Sci. 2019, 1, 873. [Google Scholar] [CrossRef]
- Shackley, S.; Sohi, S. An Assessment of the Benefits and Issues Associated with the Application of Biochar to Soil; Department for Environment, Food and Rural Affairs, UK Government: London, UK, 2010. [Google Scholar]
- Ralebitso-Senior, K.; Orr, C. Biochar Application: Essential Soil Microbial Ecology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128034330. [Google Scholar]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefinery 2022, 12, 925–947. [Google Scholar] [CrossRef]
- Anto, S.; Sudhakar, M.P.; Shan Ahamed, T.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.; Belver, C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. C—J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Baghel, P.; Anand, A.; Vijay, V.K.; Kaushal, P. A comparative study of physical and chemical activation of rice straw derived biochar to enhance Zn+2 adsorption. Bioresour. Technol. Rep. 2021, 15, 100774. [Google Scholar] [CrossRef]
- Micháleková-Richveisová, B.; Frišták, V.; Pipíška, M.; Ďuriška, L.; Moreno-Jimenez, E.; Soja, G. Iron-impregnated biochars as effective phosphate sorption materials. Environ. Sci. Pollut. Res. 2016, 24, 463–475. [Google Scholar] [CrossRef]
- Frankel, M.L.; Bhuiyan, T.I.; Veksha, A.; Demeter, M.A.; Layzell, D.B.; Helleur, R.J.; Hill, J.M.; Turner, R.J. Removal and biodegradation of naphthenic acids by biochar and attached environmental biofilms in the presence of co-contaminating metals. Bioresour. Technol. 2016, 216, 352–361. [Google Scholar] [CrossRef]
- Erdem, A.; Dogru, M. Process intensification: Activated carbon production from biochar produced by gasification highly porous carbon substances with low production costs. Johns. Matthey Technol. Rev. 2021, 65, 352–365. [Google Scholar] [CrossRef]
- Trakal, L.; Veselská, V.; Šafařík, I.; Vítková, M.; Číhalová, S.; Komárek, M. Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour. Technol. 2016, 203, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, J.; Jin, Q.; Li, Z.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, H. Sludge-based biochar activation to enhance Pb(II) adsorption. Fuel 2019, 252, 101–108. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crops Prod. 2019, 128, 405–423. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Bushra, B.; Remya, N. Biochar from pyrolysis of rice husk biomass—Characteristics, modification and environmental application. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Timur, S.; Kantarli, I.C.; Ikizoglu, E.; Yanik, J. Preparation of Activated Carbons from Oreganum Stalks by Chemical Activation. Energy Fuels 2006, 20, 2636–2641. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2019, 35, 777–815. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Pedicini, R.; Maisano, S.; Chiodo, V.; Conte, G.; Policicchio, A.; Agostino, R.G. Posidonia oceanica and wood chips activated carbon as interesting materials for hydrogen storage. Int. J. Hydrog. Energy 2020, 45, 14038–14047. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; García-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Ellis, N. Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Thermal behaviour of lignocellulosic material in the presence of phosphoric acid. Influence of the acid content in the initial solution. Carbon N. Y. 2006, 44, 2347–2350. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. An insight into the KOH activation mechanism through the production of microporous activated carbon for the removal of Pb2+ cations. Appl. Surf. Sci. 2009, 255, 3723–3730. [Google Scholar] [CrossRef]
- Fierro, V.; Muñiz, G.; Basta, A.H.; El-Saied, H.; Celzard, A. Rice straw as precursor of activated carbons: Activation with ortho-phosphoric acid. J. Hazard. Mater. 2010, 181, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, R.; Jamalluddin, N.A.; Abu Bakar, M.Z. Effect of impregnation ratio and activation temperature on the yield and adsorption performance of mangrove based activated carbon for methylene blue removal. Results Mater. 2021, 10, 100183. [Google Scholar] [CrossRef]
- Yakout, S.M.; Sharaf El-Deen, G. Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Yorgun, S.; Yildiz, D. Preparation and characterization of activated carbons from Paulownia wood by chemical activation with H3PO4. J. Taiwan Inst. Chem. Eng. 2015, 53, 122–131. [Google Scholar] [CrossRef]
- Marzbali, M.H.; Esmaieli, M.; Abolghasemi, H.; Marzbali, M.H. Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: A batch study. Process Saf. Environ. Prot. 2016, 102, 700–709. [Google Scholar] [CrossRef]
- Srinivasakannan, C.; Abu Bakar, Z.M. Production of activated carbon from rubber wood sawdust. Biomass Bioenergy 2004, 27, 89–96. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Yang, R.; Li, G.; Hu, C. The role of H3PO4 in the preparation of activated carbon from NaOH-treated rice husk residue. RSC Adv. 2015, 5, 32626–32636. [Google Scholar] [CrossRef]
- Zięzio, M.; Charmas, B.; Jedynak, K.; Hawryluk, M.; Kucio, K. Preparation and characterization of activated carbons obtained from the waste materials impregnated with phosphoric acid(V). Appl. Nanosci. 2020, 10, 4703–4716. [Google Scholar] [CrossRef]
- Yu, J.; Chang, J.S.; Guo, H.; Han, S.; Lee, D.J. Sodium ions removal by sulfuric acid-modified biochars. Environ. Res. 2023, 235, 116592. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Liu, R.; Xu, S.; Mohamed, B.A.; Yang, Z.; Hu, Y.; Chen, J.; Zhao, S.; Wu, Z.; Peng, H.; et al. An overview of sulfur-functional groups in biochar from pyrolysis of biomass. J. Environ. Chem. Eng. 2022, 10, 107185. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, R.; Song, G.; Wu, L.; Ye, H.; Xu, L.; Parikh, S.J.; Nguyen, T.; Khan, E.; Vithanage, M.; et al. Enhanced removal of ammonium from water using sulfonated reed waste biochar-A lab-scale investigation. Environ. Pollut. 2022, 292, 118412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, T.; Cai, Y.; Zhao, Y.; Song, S.; Quintana, M. Engineering sulfuric acid-pretreated biochar supporting MnO2 for efficient toxic organic pollutants removal from aqueous solution in a wide pH range. J. Clean. Prod. 2023, 416, 137968. [Google Scholar] [CrossRef]
- Quah, R.V.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Abdullah, E.C.; Abdullah, M.O. Magnetic biochar derived from waste palm kernel shell for biodiesel production via sulfonation. Waste Manag. 2020, 118, 626–636. [Google Scholar] [CrossRef]
- Cao, M.; Lu, M.; Yin, H.; Zhu, Q.; Xing, K.; Ji, J. Effect of hemicellulose extraction pretreatment on sulfonated corncob biochar for catalytic biodiesel production. J. Environ. Chem. Eng. 2023, 11, 109058. [Google Scholar] [CrossRef]
- Kumari, N.; Chhabra, T.; Kumar, S.; Krishnan, V. Nanoarchitectonics of sulfonated biochar from pine needles as catalyst for conversion of biomass derived chemicals to value added products. Catal. Commun. 2022, 168, 106467. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Feedstock type drives surface property, demineralization and element leaching of nitric acid-activated biochars more than pyrolysis temperature. Bioresour. Technol. 2022, 344, 126316. [Google Scholar] [CrossRef] [PubMed]
- Güzel, F.; Sayğılı, H.; Akkaya Sayğılı, G.; Koyuncu, F.; Yılmaz, C. Optimal oxidation with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J. Clean. Prod. 2017, 144, 260–265. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Prodromou, M.; Pashalidis, I. Activated biochar derived from cactus fibres—Preparation, characterization and application on Cu(II) removal from aqueous solutions. Bioresour. Technol. 2014, 159, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, R.; Heidarpour, M.; Mousavi, S.F.; Afyuni, M. Characterization and sodium sorption capacity of biochar and activated carbon prepared from rice husk. J. Agric. Sci. Technol. 2015, 17, 1057–1069. [Google Scholar]
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH activation mechanism during biomass pyrolysis: Chemical reactions between O-containing groups and KOH. Appl. Energy 2020, 278, 115730. [Google Scholar] [CrossRef]
- Pizzanelli, S.; Maisano, S.; Pinzino, C.; Manariti, A.; Chiodo, V.; Pitzalis, E.; Forte, C. The effect of activation on the structure of biochars prepared from wood and from posidonia oceanica: A spectroscopic study. Physchem 2022, 2, 286–304. [Google Scholar] [CrossRef]
- Deng, H.; Li, G.; Yang, H.; Tang, J.; Tang, J. Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J. 2010, 163, 373–381. [Google Scholar] [CrossRef]
- Trakal, L.; Šigut, R.; Šillerová, H.; Faturíková, D.; Komárek, M. Copper removal from aqueous solution using biochar: Effect of chemical activation. Arab. J. Chem. 2014, 7, 43–52. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef]
- Fahmi, A.H.; Samsuri, A.W.; Jol, H.; Singh, D. Physical modification of biochar to expose the inner pores and their functional groups to enhance lead adsorption. RSC Adv. 2018, 8, 38270–38280. [Google Scholar] [CrossRef]
- Dieguez Alonso, A. Fixed-Bed Biomass Pyrolysis: Mechanisms and Biochar Production; Technische Universitat: Berlin, Germany, 2015. [Google Scholar]
- Bruni, G.O.; Klasson, K.T. Aconitic acid recovery from renewable feedstock and review of chemical and biological applications. Foods 2022, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Patel, M.; Pittman, C.U.; Mohan, D. Batch and continuous fixed-bed lead removal using himalayan pine needle biochar: Isotherm and kinetic studies. ACS Omega 2020, 5, 16366–16378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, B.; Liu, Q.; Wu, C.; Li, Z. Preparation of porous biochar from heavy bio-oil for adsorption of methylene blue in wastewater. Fuel Process. Technol. 2022, 238, 107485. [Google Scholar] [CrossRef]
- Li, H.; Kong, J.; Zhang, H.; Gao, J.; Fang, Y.; Shi, J.; Ge, T.; Fang, T.; Shi, Y.; Zhang, R.; et al. Mechanisms and adsorption capacities of ball milled biomass fly ash/biochar composites for the adsorption of methylene blue dye from aqueous solution. J. Water Process Eng. 2023, 53, 103713. [Google Scholar] [CrossRef]
- Yang, E.; Yao, C.; Liu, Y.; Zhang, C.; Jia, L.; Li, D.; Fu, Z.; Sun, D.; Robert Kirk, S.; Yin, D. Bamboo-derived porous biochar for efficient adsorption removal of dibenzothiophene from model fuel. Fuel 2018, 211, 121–129. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Min, L.; Ren, C. Biochars change the sorption and degradation of thiacloprid in soil: Insights into chemical and biological mechanisms. Environ. Pollut. 2018, 236, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah, I.; Khan, M.T.; Zubair, M.; Bilal, M.; Sajid, M. Removal of pharmaceuticals from water using sewage sludge-derived biochar: A review. Chemosphere 2022, 289, 133196. [Google Scholar] [CrossRef]
- Ma, H.; Ai, J.; Lu, C.; Christian Bruun Hansen, H. Element doping of biochars enhances catalysis of trichloroethylene dechlorination. Chem. Eng. J. 2022, 428, 132496. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Jha, E. Thiol Functionalized and Manganese Dioxide Doped Biochar for the Removal of Toxic Organic and Inorganic Contaminants from Water; Stockholm Junior Water Prize; Lynbrook High School: San Jose, CA, USA, 2021. [Google Scholar]
- Di Stasi, C.; Renda, S.; Greco, G.; González, B.; Palma, V.; Manyà, J.J. Wheat-straw-derived activated biochar as a renewable support of Ni-CeO2 catalysts for CO2 methanation. Sustainability 2021, 13, 8939. [Google Scholar] [CrossRef]
- Sajjadi, B.; Shrestha, R.M.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Double-layer magnetized. J. Water Process Eng. 2021, 39, 2214–7144. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, N.; Feng, C.; Li, M.; Gao, Y. Chromium removal using a magnetic corncob biochar/polypyrrole composite by adsorption combined with reduction: Reaction pathway and contribution degree. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 201–209. [Google Scholar] [CrossRef]
- Wang, P.; Tang, L.; Wei, X.; Zeng, G.; Zhou, Y.; Deng, Y.; Wang, J.; Xie, Z.; Fang, W. Synthesis and application of iron and zinc doped biochar for removal of p-nitrophenol in wastewater and assessment of the influence of co-existed Pb(II). Appl. Surf. Sci. 2017, 392, 391–401. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, Z.; Sun, Y.; Cao, X.; Hou, D.; Alessi, D.S.; Ok, Y.S.; Tsang, D.C.W. Unraveling iron speciation on Fe-biochar with distinct arsenic removal mechanisms and depth distributions of As and Fe. Chem. Eng. J. 2021, 425, 131489. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Pham, T.H.; Nguyen Thi, H.T.; Nguyen, T.N.; Nguyen, M.V.; Tran Dinh, T.; Nguyen, M.P.; Do, T.Q.; Phuong, T.; Hoang, T.T.; et al. Synthesis of iron-modified biochar derived from rice straw and its application to arsenic removal. J. Chem. 2019, 2019, 5295610. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, P.; Wei, Z.; Xiao, F.; Liu, S.; Guo, H.; Qu, C.; Xiong, J.; Sun, H.; Tan, W. Porous Fe-doped graphitized biochar: An innovative approach for co-removing per-/polyfluoroalkyl substances with different chain lengths from natural waters and wastewater. Chem. Eng. J. 2023, 476, 146888. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Bhattacharya, J. Potassium-iron rice straw biochar composite for sorption of nitrate, phosphate, and ammonium ions in soil for timely and controlled release. Sci. Total Environ. 2020, 712, 136337. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Wang, J.; Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests. Environ. Pollut. 2020, 261, 114223. [Google Scholar] [CrossRef]
- Liu, D.X.; Mu, J.; Yao, Q.; Bai, Y.; Qian, F.; Liang, F.; Shi, F.N.; Gao, J. Design of iron-ion-doped pomelo peel biochar composites towards removal of organic pollutants. Springer Nat. Appl. Sci. 2019, 1, 184–191. [Google Scholar] [CrossRef]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation. J. Hazard. Mater. 2020, 397, 122764. [Google Scholar] [CrossRef]
- Cai, W.; Wei, J.; Li, Z.; Liu, Y.; Zhou, J.; Han, B. Preparation of amino-functionalized magnetic biochar with excellent adsorption performance for Cr(VI) by a mild one-step hydrothermal method from peanut hull. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 102–111. [Google Scholar] [CrossRef]
- Singh, P.; Sarswat, A.; Pittman, C.U.; Mlsna, T.; Mohan, D. Sustainable low-concentration arsenite [As(III)] removal in single and multicomponent systems using hybrid iron oxide-biochar nanocomposite adsorbents—A mechanistic study. ACS Omega 2020, 5, 2575–2593. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis biochars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Jia, L.; Yu, Y.; Li, Z.-P.; Qin, S.-N.; Guo, J.-R.; Zhang, Y.-Q.; Wang, J.-C.; Zhang, J.-C.; Fan, B.-G.; Jin, Y. Study on the Hg0 removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals. Bioresour. Technol. 2021, 332, 125086. [Google Scholar] [CrossRef] [PubMed]
- Eniola, J.O.; Sizirici, B.; Khaleel, A.; Yildiz, I. Fabrication of engineered biochar-iron oxide from date palm frond for the effective removal of cationic dye from wastewater. J. Water Process Eng. 2023, 54, 104046. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Wang, X.; Wang, K.; Zhu, L.; Wang, S. Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption. Energy Fuels 2019, 33, 12459–12468. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, C.; Yin, C.; Kang, S.; Liu, Y.; Ge, Z.; Xia, Q.; Wang, Y.; Li, X. Facile large-scale synthesis of macroscopic 3D porous graphene-like carbon nanosheets architecture for efficient CO2 adsorption. Carbon N. Y. 2019, 145, 751–756. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.W.; Khan, E.; Yip, A.C.K.; Ng, Y.H.; Rinklebe, J.; Ok, Y.S. Customised fabrication of nitrogen-doped biochar for environmental and energy applications. Chem. Eng. J. 2020, 401, 126136. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, G.; Zhu, L.; Wang, S. Front Cover: Green Conversion of Microalgae into High-Performance Sponge-Like Nitrogen-Enriched Carbon (ChemElectroChem 3/2019). ChemElectroChem 2019, 6, 598. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, H.; Deng, L.; Wang, Y.; Kang, D.; Li, C.; Chen, H. Enhanced adsorption of Pb(II) by nitrogen and phosphorus co-doped biochar derived from Camellia oleifera shells. Environ. Res. 2020, 191, 110030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Q.; Islam, S.M.; Liu, Y.; Ma, S.; Kanatzidis, M.G. Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS42− on. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar composites: Emerging trends, field successes and sustainability implications. Soil Use Manag. 2022, 38, 14–38. [Google Scholar] [CrossRef]
- Yuan, S.; Hong, M.; Li, H.; Ye, Z.; Gong, H.; Zhang, J.; Huang, Q.; Tan, Z. Contributions and mechanisms of components in modified biochar to adsorb cadmium in aqueous solution. Sci. Total Environ. 2020, 733, 139320. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, S.; Yuan, R.; Wang, P. Biomass pyrolysis with alkaline-earth-metal additive for co-production of bio-oil and biochar-based soil amendment. Sci. Total Environ. 2020, 743, 140760. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cao, X.; Zhao, L.; Wang, J.; Ding, Z. Effects of Mineral Additives on Biochar Formation: Carbon Retention, Stability, and Properties. Environ. Sci. Technol. 2014, 48, 11211–11217. [Google Scholar] [CrossRef]
- Campbell, L.S.; Davies, B.E. Experimental investigation of plant uptake of caesium from soils amended with clinoptilolite and calcium carbonate. Plant Soil 1997, 189, 65–74. [Google Scholar] [CrossRef]
- Chan, K.Y.; Zwieten, L.V.; Meszaros, I.; Downie, A.; Joseph, S.; Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C.E. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Peng, X.; Ye, L.L.; Wang, C.H.; Zhou, H.; Sun, B. Temperature- and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 2011, 112, 159–166. [Google Scholar] [CrossRef]
- Antonangelo, J.; Zhang, H. the use of biochar as a soil amendment to reduce potentially toxic metals (PTMs) phytoavailability. In Applications of Biochar for Environmental Safety; Abdelhafez, A., Mohammed, A., Eds.; IntechOpen: London, UK, 2020; p. 276. [Google Scholar]
- Manasa, R.L.; Mehta, A. Wastewater: Sources of pollutants and its remediation. In Environmental Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 197–219. [Google Scholar]
- Veloz, C.; Pazmiño-Arias, E.; Gallardo, A.M.; Montenegro, J.; Sommer-Márquez, A.; Ricaurte, M. Predictive modeling of the primary settling tanks based on artificial neural networks for estimating TSS and COD as typical effluent parameters. Water Sci. Technol. 2022, 85, 3451–3464. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manage. 2017, 197, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lu, Q.; Zhou, H.; Shen, F.; Liu, Z.; Zhu, W.; Huang, H. Tuning oxygenated functional groups on biochar for water pollution control: A critical review. J. Hazard. Mater. 2021, 420, 126547. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.C.C.d.S.; Nzediegwu, C.; Benally, C.; Messele, S.A.; Kwak, J.H.; Naeth, M.A.; Ok, Y.S.; Chang, S.X.; Gamal El-Din, M. Pristine and engineered biochar for the removal of contaminants co-existing in several types of industrial wastewaters: A critical review. Sci. Total Environ. 2022, 809, 151120. [Google Scholar] [CrossRef]
- Pustahija, L.; Kern, W. Surface Functionalization of (Pyrolytic) Carbon—An Overview. C 2023, 9, 38. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.H.; Ahn, Y.T.; Chung, J.W. Comparison of Heavy Metal Adsorption by Peat Moss and Peat Moss-Derived Biochar Produced Under Different Carbonization Conditions. Water Air Soil Pollut. 2015, 226, 9. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Smith, M.A.; Heinemeyer, A.; Lord, R.; Ennis, C.J.; Hodgson, E.M.; Farrar, K. Production Factors Controlling the Physical Characteristics of Biochar Derived from Phytoremediation Willow for Agricultural Applications. BioEnergy Res. 2013, 7, 371–380. [Google Scholar] [CrossRef]
- Rajapaksha, P.P.; Power, A.; Chandra, S.; Chapman, J. Graphene, electrospun membranes and granular activated carbon for eliminating heavy metals, pesticides and bacteria in water and wastewater treatment processes. Analyst 2018, 143, 5629–5645. [Google Scholar] [CrossRef] [PubMed]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment—Conversion technologies and applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Yang, H.; Doran, J.; Rooney, D.W. Industrial Biochar Systems for Atmospheric Carbon Removal: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- Khiari, Z.; Alka, K.; Kelloway, S.; Mason, B.; Savidov, N. Integration of Biochar Filtration into Aquaponics: Effects on Particle Size Distribution and Turbidity Removal. Agric. Water Manag. 2020, 229, 105874. [Google Scholar] [CrossRef]
- Kaetzl, K.; Lübken, M.; Gehring, T.; Wichern, M. Efficient Low-Cost Anaerobic Treatment of Wastewater Using Biochar and Woodchip Filters. Water 2018, 10, 818. [Google Scholar] [CrossRef]
- Ameta, S.C. Introduction. In Advanced Oxidation Processes for Waste Water Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–12. [Google Scholar]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties—New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the usefulness of sorbents in the remediation of soil exposed to the pressure of cadmium and cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef]
- Hira, N.E.; Lock, S.S.M.; Shoparwe, N.F.; Lock, I.S.M.; Lim, L.G.; Yiin, C.L.; Chan, Y.H.; Hassam, M. Review of adsorption studies for contaminant removal from wastewater using molecular simulation. Sustainability 2023, 15, 1510. [Google Scholar] [CrossRef]
- Vogelsang, C.; Umar, M. Municipal solid waste fly ash-derived zeolites as adsorbents for the recovery of nutrients and heavy metals—A Review. Water 2023, 15, 3817. [Google Scholar] [CrossRef]
- Sodha, V.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Bandyopadhyay, R.; Sridewi, N. Comprehensive review on zeolite-based nanocomposites for treatment of effluents from wastewater. Nanomaterials 2022, 12, 3199. [Google Scholar] [CrossRef]
- Undabeytia, T.; Shuali, U.; Nir, S.; Rubin, B. Applications of chemically modified clay minerals and clays to water purification and slow release formulations of herbicides. Minerals 2021, 11, 9. [Google Scholar] [CrossRef]
- Chen, M.; Yang, T.; Han, J.; Zhang, Y.; Zhao, L.; Zhao, J.; Li, R.; Huang, Y.; Gu, Z.; Wu, J. The Application of Mineral Kaolinite for Environment Decontamination: A Review. Catalysts 2023, 13, 123. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Immanuel Edison, T.N.J.; Babu, R.S.; Karpagavinayagam, P.; Vedhi, C. A short review on recent advances of hydrogel-based adsorbents for heavy metal ions. Metals 2021, 11, 864. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Ur Rashid, T.U.; Kabir, S.M.F. Cellulose-based hydrogels for wastewater treatment: A concise review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Shin, W.S.; Al Masud, M.A.; Nandi, R.; Islam, T. A critical review of sustainable pesticide remediation in contaminated sites: Research challenges and mechanistic insights. Environ. Pollut. 2023, 341, 122940. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, C.; Wang, H.; Zhou, L.; Yang, J.; Zhang, Y.; Li, H.; Zhou, D. Distribution, toxicity, and impacts of nano-biochar in mice following dietary exposure: Insights into environmental risks and mammalian effects. Environ. Pollut. 2023, 338, 122652. [Google Scholar] [CrossRef]
- Xue, P.; Hou, R.; Fu, Q.; Li, T.; Wang, J.; Zhou, W.; Shen, W.; Su, Z.; Wang, Y. Potentially migrating and residual components of biochar: Effects on phosphorus adsorption performance and storage capacity of black soil. Chemosphere 2023, 336, 139250. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Zhou, Y.; Zhang, B.; Peng, Y.; Zhuo, Y.; Ai, W.; Gao, C.; Wu, B.; Liu, D.; et al. Straw and straw biochar differently affect fractions of soil organic carbon and microorganisms in farmland soil under different water regimes. Environ. Technol. Innov. 2023, 32, 103412. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Yang, M.; Zhang, F. Exploring biochar addition impacts on soil erosion under natural rainfall: A study based on four years of field observations on the Loess Plateau. Soil Tillage Res. 2024, 236, 105935. [Google Scholar] [CrossRef]
- Haider Jaffari, Z.; Jeong, H.; Shin, J.; Kwak, J.; Son, C.; Lee, Y.G.; Kim, S.; Chon, K.; Hwa Cho, K. Machine-learning-based prediction and optimization of emerging contaminants’ adsorption capacity on biochar materials. Chem. Eng. J. 2023, 466, 143073. [Google Scholar] [CrossRef]
- Lakshmi, D.; Akhil, D.; Kartik, A.; Gopinath, K.P.; Arun, J.; Bhatnagar, A.; Rinklebe, J.; Kim, W.; Muthusamy, G. Artificial intelligence (AI) applications in adsorption of heavy metals using modified biochar. Sci. Total Environ. 2021, 801, 251. [Google Scholar] [CrossRef] [PubMed]
- Nematian, M.; Keske, C.; Ng’ombe, J.N. A techno-economic analysis of biochar production and the bioeconomy for orchard biomass. Waste Manag. 2021, 135, 467–477. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).