Transforming Biomass Waste into Hydrochars and Porous Activated Carbon: A Characterization Study
Abstract
:1. Introduction
2. Materials and Methods
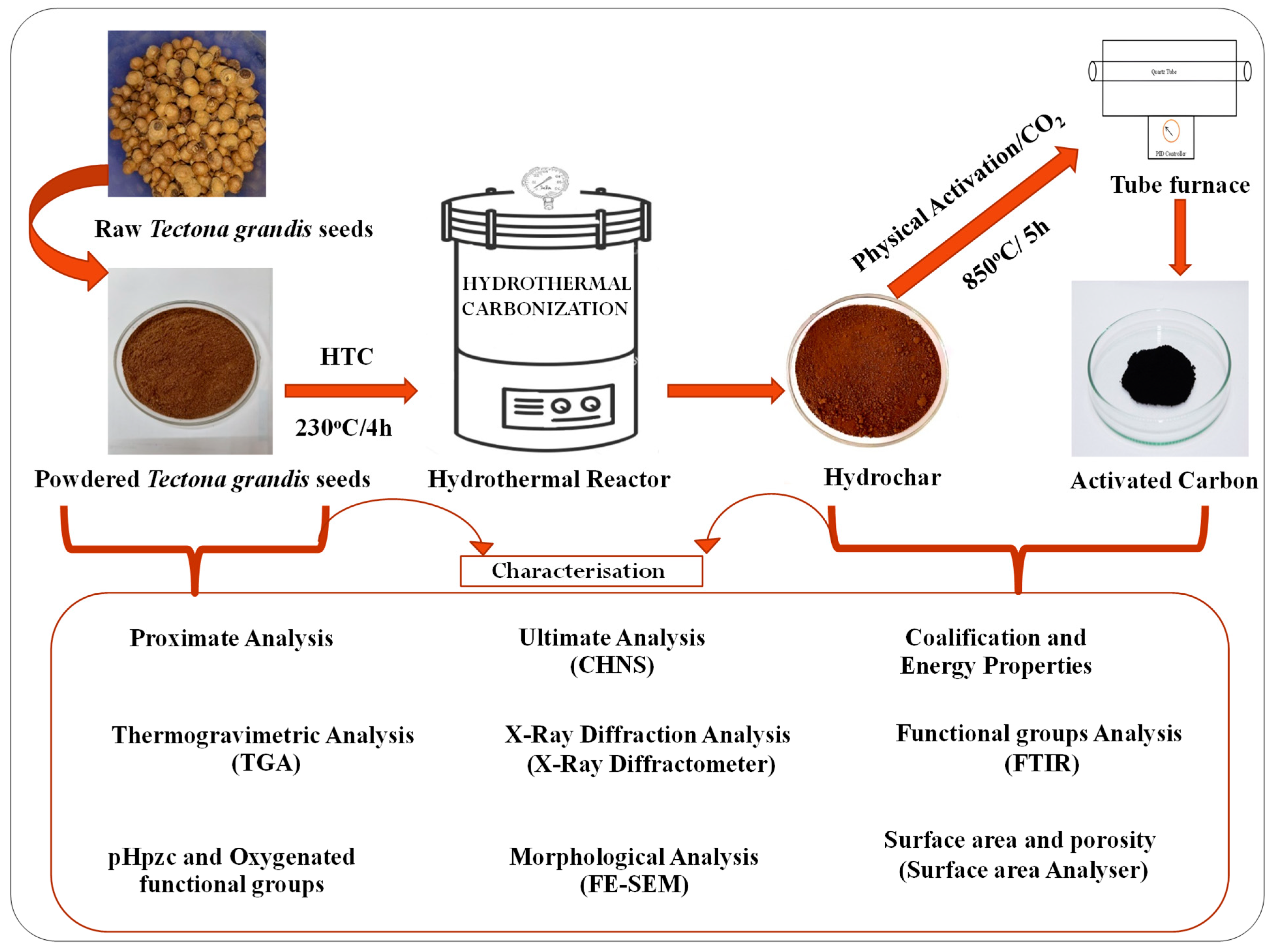
2.1. Pretreatment of Precursor
2.2. Hydrochar Production
2.3. Activated Carbon Production
2.4. Characterization of Tectona grandis Seeds, Hydrochars, and Activated Carbons
2.4.1. Proximate, Ultimate, and Yield Analysis
2.4.2. Higher Heating Value (HHV), Fuel Ratio, Energy Yield, and Ratio of Energy Densification
2.4.3. XRD Analysis
2.4.4. Thermogravimetric Analysis
2.4.5. Point of Zero Charge (pHpzc) and Oxygen Containing Functional Groups
2.4.6. FTIR Analysis
2.4.7. Morphological Analysis
2.4.8. Textural Analysis
3. Results
3.1. Proximate, Ultimate, and Yield Analysis
3.2. Energy Properties of Hydrochars
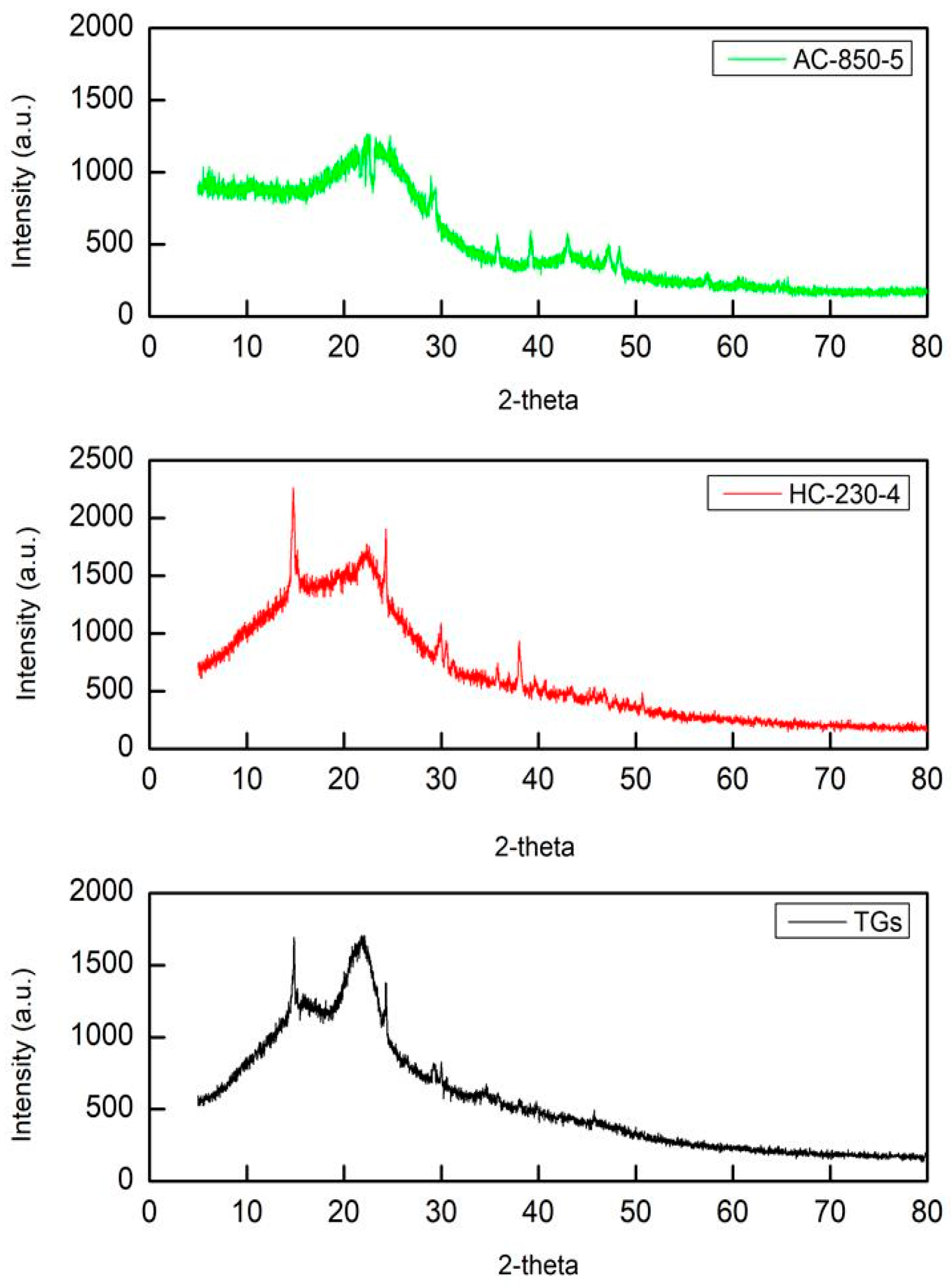
3.3. XRD Analysis
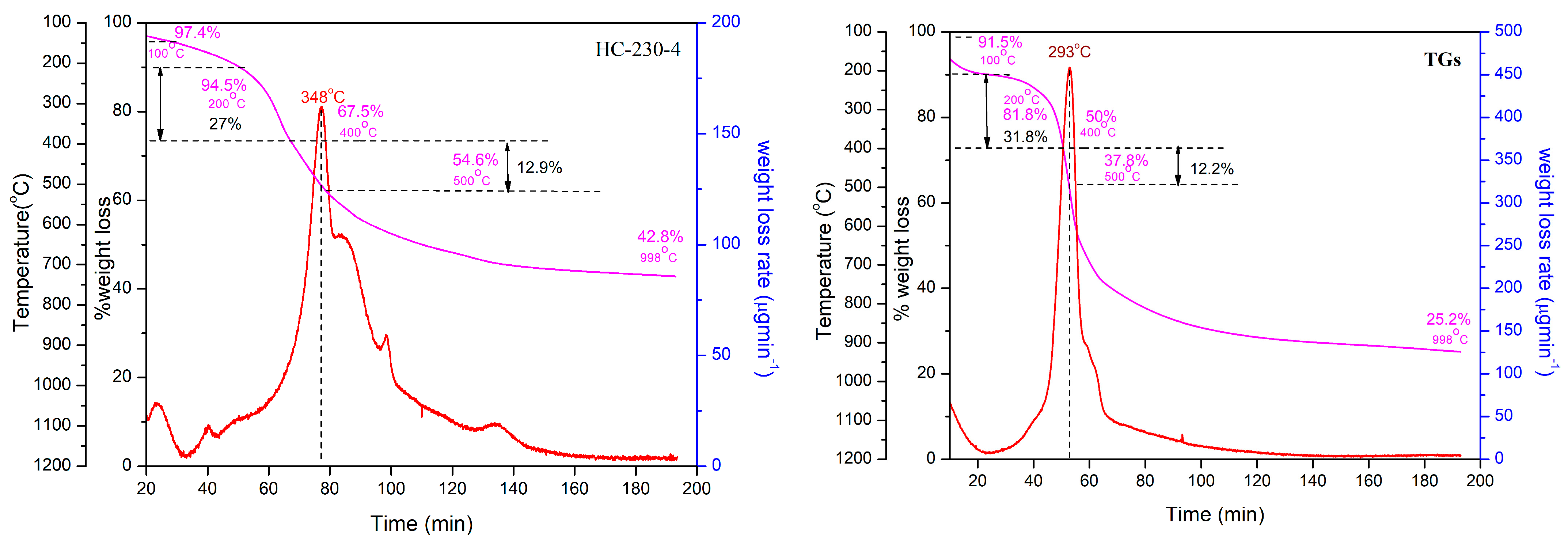
3.4. Thermogravimetric Analysis
3.5. Point of Zero Charge and Oxygen Containing Functional Groups Determination
3.6. Fourier Transform Infrared Spectroscopic (FTIR) Analysis
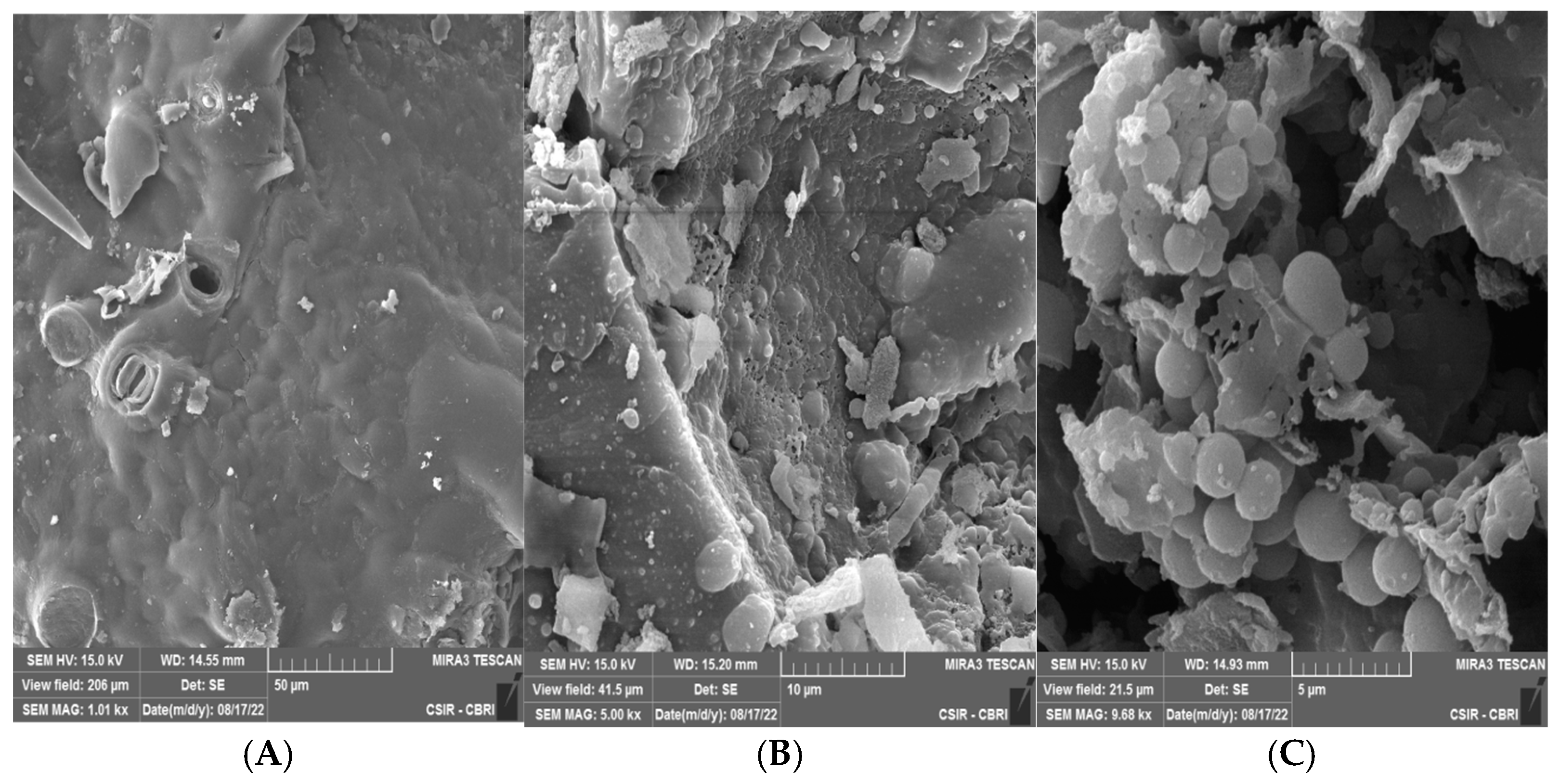
3.7. Morphological Analysis
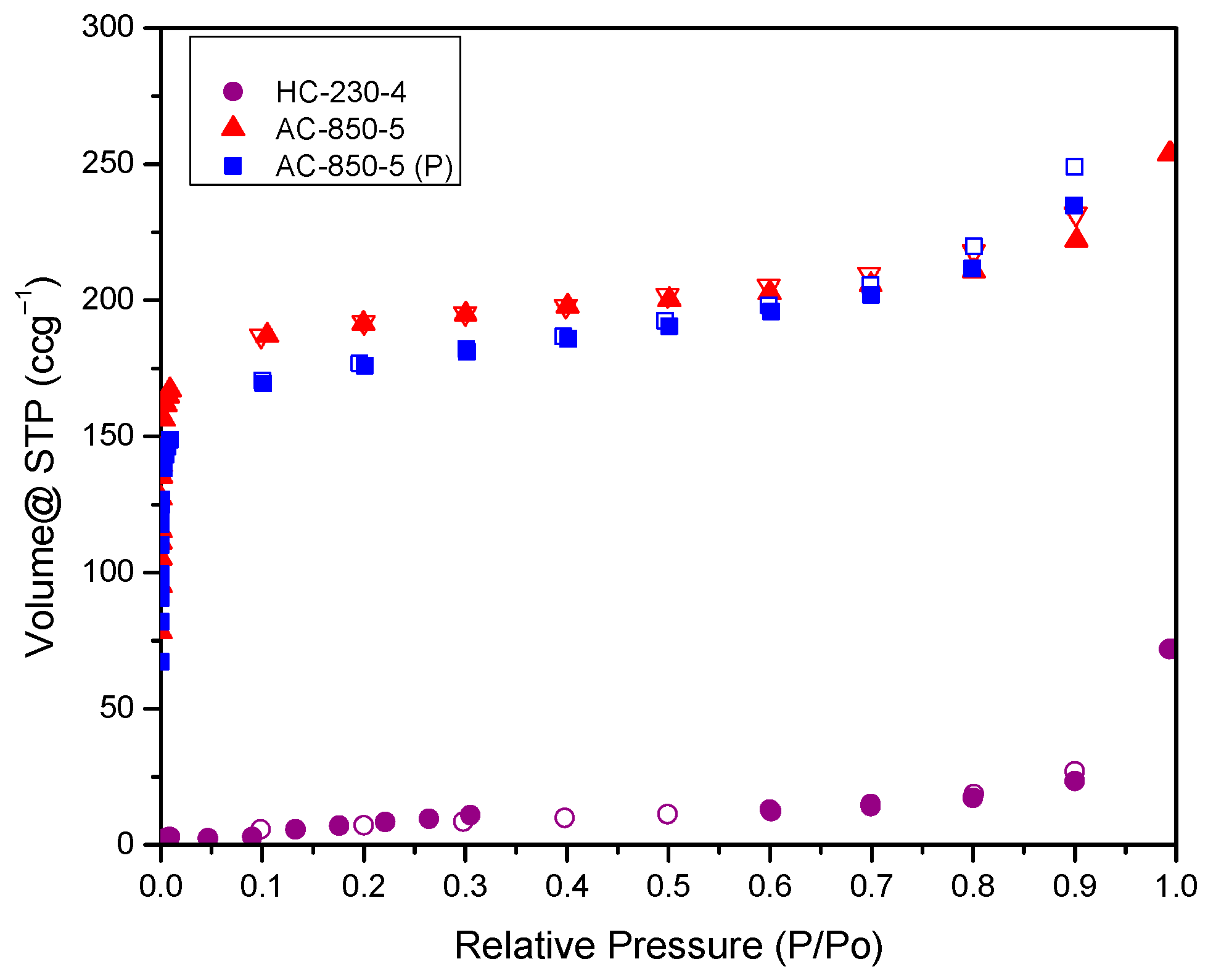
3.8. Textural Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Singh, L.P.; Chaudhary, M.; Kushwaha, S. A novel approach to develop activated carbon by an ingenious hydrothermal treatment methodology using Phyllanthus emblica fruit stone. J. Clean. Prod. 2021, 288, 125643. [Google Scholar] [CrossRef]
- Diaz, E.; Manzano, F.J.; Villamil, J.; Rodriguez, J.J.; Mohedano, F.A. Low-Cost Activated Grape Seed-Derived Hydrochar through Hydrothermal Carbonization and Chemical Activation for Sulfamethoxazole Adsorption. Appl. Sci. 2019, 9, 5127. [Google Scholar] [CrossRef]
- Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M. Low-cost activated carbon preparation from Corn stigmata fibers chemically activated using H3PO4, ZnCl2 and KOH: Study of methylene blue adsorption, stochastic isotherm and fractal kinetic. Ind. Crops Prod. 2022, 178, 114546. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Geraldo, P.F.; Mourão, P.A.M.; Castanheiro, J.E.; Carreiro, E.P.; Suhas. Utilization of Biomass Waste at Water Treatment. Resources 2024, 13, 37–52. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Belo, C.R.; Mourão, P.A.M. Valorisation of Tectona Grandis tree sawdust through the production of high activated carbon for environment applications. Bioresour. Technol. 2018, 249, 328–333. [Google Scholar] [CrossRef]
- Gunaga, R.P. Status of fruit yield in seed production areas of teak (Tectona grandis Linn.) in India: Implications for management. Teaknet Bull 2011, 4, 1–9. [Google Scholar]
- Hanna Fathima, A.C.; Adithya, D.; George, N. Exploring Tectona grandis: Phytochemicals, medicinal value, and green innovations: A review article. J. Pharmacogn. Phytochem. 2024, 13, 102–111. [Google Scholar] [CrossRef]
- Palanisamy, K.; Hegde, M.; Yi, J.-S. Teak (Tectona grandis Linn. f.): A renowned commercial timber species. J. For. Environ. Sci. 2009, 25, 1–24. [Google Scholar]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Isemin, R.; Melezhyk, A.; Kuzmin, S.; Nebyvayev, A.; Muratova, N.; Mikhalev, A.; Milovanov, O.; Teplitskii, Y.; Buchilko, E.; Pitsukha, E. Production of Activated Carbon from Biochar Obtained by Wet Torrefaction of Chicken Manure as Sole Feedstock, and in Mixture with Sawdust in a Fluidized Bed Powered with Superheated Steam. Chem. Eng. Trans. 2021, 86, 1519–1524. [Google Scholar]
- Maneerung, T.; Liew, J.; Dai, Y.; Kawi, S.; Chong, C.; Wang, C.-H. Activated carbon derived from carbon residue from biomass gasification and its application for dye adsorption: Kinetics, isotherms and thermodynamic studies. Bioresour. Technol. 2016, 200, 350–359. [Google Scholar] [CrossRef]
- Smith, A.M.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Masoumi, S.; Dalai, A.K. Optimized production and characterization of highly porous activated carbon from algal-derived hydrochar. J. Clean. Prod. 2020, 263, 121427. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Herklotz, B.; Busch, D.; Pielert, J.; Lynam, J.; Mumme, J. Review Article: Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Dubey, B.K. Hydrothermal carbonization of yard waste for solid bio-fuel production: Study on combustion kinetic, energy properties, grindability and flowability of hydrochar. Waste Manag. 2019, 91, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Carrott, P.J.M.; Carrott, M.M.L.R.; Vale, T.S.C.; Marques, L.; Nabais, J.M.V.; Mourão, P.A.M.; Suhas, P.A.M. Characterisation of Surface Ionisation and Adsorption of Phenol and 4-Nitrophenol on Non-Porous Carbon Blacks. Adsorpt. Sci. Technol. 2008, 26, 827–841. [Google Scholar] [CrossRef]
- Boehm, H.-P. Surface Chemical Characterization of Carbons from Adsorption Studies. In Adsorption by Carbons; Eduardo, J., Bottani, J.M.D.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 301–327. [Google Scholar]
- Boehm, H.P. Chemical Identification of Surface Groups. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1966; Volume 16, pp. 179–274. [Google Scholar]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z. A Mechanism Study on Hydrothermal Carbonization of Waste Textile. Energy Fuels 2016, 30, 7746–7754. [Google Scholar] [CrossRef]
- Saetea, P.; Tippayawong, N. Recovery of Value-Added Products from Hydrothermal Carbonization of Sewage Sludge. ISRN Chem. Eng. 2013, 2013, 268947. [Google Scholar] [CrossRef]
- Park, S.-W.; Jang, C.-H. Characteristics of carbonized sludge for co-combustion in pulverized coal power plants. Waste Manag. 2011, 31, 523–529. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Characterization of Energy-Rich Hydrochars from Microwave-Assisted Hydrothermal Carbonization of Coconut Shell. Waste Biomass Valorization 2019, 10, 1979–1987. [Google Scholar] [CrossRef]
- Xu, Q.; Qian, Q.; Quek, A.; Ai, N.; Zeng, G.; Wang, J. Hydrothermal Carbonization of Macroalgae and the Effects of Experimental Parameters on the Properties of Hydrochars. ACS Sustain. Chem. Eng. 2013, 1, 1092–1101. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, B.; Yang, G.; Lucia, L.A.; Chen, J. Hydrothermal Carbonization of Corncob Residues for Hydrochar Production. Energy Fuels 2015, 29, 872–876. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Wu, T.; Shi, F.; Zhu, C. Characteristic evolution of hydrochar from hydrothermal carbonization of corn stalk. J. Anal. Appl. Pyrolysis 2015, 116, 1–9. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, G.; Li, J.; Yang, Q.; Huang, S.; Cai, J. Hydrothermal conversion of bamboo shoot shell to biochar: Preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal. J. Anal. Appl. Pyrolysis 2019, 143, 104694. [Google Scholar] [CrossRef]
- Huang, G.-g.; Liu, Y.-f.; Wu, X.-x.; Cai, J.-j. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Jayakumar, N.S.; Sahu, J.N.; Ganesan, P.; Bhutto, A.W.; Mubarak, N.M. Hydrothermal carbonization of oil palm shell. Korean J. Chem. Eng. 2015, 32, 1789–1797. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, W.; Wang, B.; Fang, J.; Zou, W.; He, F.; Li, Y.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Adsorption of acetone and cyclohexane onto CO2 activated hydrochars. Chemosphere 2020, 245, 125664. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Román, S.; Ledesma, B.; Sabio, E. Study of variables in energy densification of olive stone by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 307–314. [Google Scholar] [CrossRef]
- Saha, N.; Saba, A.; Reza, M.T. Effect of hydrothermal carbonization temperature on pH, dissociation constants, and acidic functional groups on hydrochar from cellulose and wood. J. Anal. Appl. Pyrolysis 2019, 137, 138–145. [Google Scholar] [CrossRef]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Borel, L.D.M.S.; Lira, T.S.; Ribeiro, J.A.; Ataíde, C.H.; Barrozo, M.A.S. Pyrolysis of brewer’s spent grain: Kinetic study and products identification. Ind. Crops Prod. 2018, 121, 388–395. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, R.C.; Menezes, R.S.G.; Fiuza-Jr, R.A.; Andrade, H.M.C. Activated carbon microspheres derived from hydrothermally treated mango seed shells for acetone vapor removal. Carbon Lett. 2021, 31, 779–793. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Ross, A.B.; Mariner, T.; Hammerton, J.; Fitzsimmons, M. Hydrochars produced by hydrothermal carbonisation of seaweed, coconut shell and oak: Effect of processing temperature on physicochemical adsorbent characteristics. SN Appl. Sci. 2022, 4, 203. [Google Scholar] [CrossRef]
- Shen, F.; Wang, Y.; Li, L.; Zhang, K.; Smith, R.L.; Qi, X. Porous carbonaceous materials from hydrothermal carbonization and KOH activation of corn stover for highly efficient CO2 capture. Chem. Eng. Commun. 2018, 205, 423–431. [Google Scholar] [CrossRef]
- Diaz, E.; Sanchis, I.; Coronella, C.J.; Mohedano, A.F. Activated Carbons from Hydrothermal Carbonization and Chemical Activation of Olive Stones: Application in Sulfamethoxazole Adsorption. Resources 2022, 11, 43. [Google Scholar] [CrossRef]
- Román, S.; Valente Nabais, J.M.; Ledesma, B.; González, J.F.; Laginhas, C.; Titirici, M.M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater. 2013, 165, 127–133. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Yu, S.-H.; Müller, J.-O.; Antonietti, M. A Direct Synthesis of Mesoporous Carbons with Bicontinuous Pore Morphology from Crude Plant Material by Hydrothermal Carbonization. Chem. Mater. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. Production and applications of activated carbons. In Handbook of Porous Solids; Wiley: Hoboken, NJ, USA, 2002; pp. 1766–1827. [Google Scholar]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Ab Rahim, M.H.; Park, J.-W.; Kim, H.-J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Hydrothermal conversion of urban food waste to chars for removal of textile dyes from contaminated waters. Bioresour. Technol. 2014, 161, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.A.; Phihusut, D.; Tuntiwiwattanapun, N. Preparation of rice husk hydrochar as an atrazine adsorbent: Optimization, characterization, and adsorption mechanisms. J. Environ. Chem. Eng. 2022, 10, 107575. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Carbonization temperature and feedstock type interactively affect chemical, fuel, and surface properties of hydrochars. Bioresour. Technol. 2021, 330, 124976. [Google Scholar] [CrossRef]
- Ishibashi, N.; Yamamoto, K.; Wakisaka, H.; Kawahara, Y. Influence of the hydrothermal pre-treatments on the adsorption characteristics of activated carbons from woods. J. Polym. Environ. 2014, 22, 267–271. [Google Scholar] [CrossRef]
- Genli, N.; Kutluay, S.; Baytar, O.; Şahin, Ö. Preparation and characterization of activated carbon from hydrochar by hydrothermal carbonization of chickpea stem: An application in methylene blue removal by RSM optimization. Int. J. Phytoremediat. 2022, 24, 88–100. [Google Scholar] [CrossRef] [PubMed]



| TGs | Hydrochars | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC- 180-1 | HC- 190-1 | HC- 200-1 | HC- 210-1 | HC- 220-1 | HC- 230-1 | HC- 240-1 | HC- 250-1 | HC- 230-2 | HC- 230-3 | HC- 230-4 | HC- 230-5 | |||
| Proximate Analysis | VM | 71.83 | 68.63 | 64.73 | 63.98 | 63.78 | 63.46 | 63.14 | 62.98 | 62.83 | 62.81 | 62.20 | 60.19 | 59.61 |
| FC * | 16.20 | 20.25 | 24.20 | 25.00 | 25.31 | 25.64 | 26.38 | 26.34 | 26.36 | 26.61 | 27.44 | 29.40 | 30.10 | |
| M | 8.7 | 8.2 | 8.14 | 8.11 | 8.03 | 8.07 | 7.71 | 7.69 | 7.65 | 7.69 | 7.64 | 7.61 | 7.59 | |
| A | 3.268 | 2.912 | 2.924 | 2.901 | 2.872 | 2.821 | 2.765 | 2.761 | 2.759 | 2.732 | 2.719 | 2.711 | 2.703 | |
| Ultimate Analysis | C | 48.47 | 51.16 | 53.83 | 58.98 | 59.53 | 59.24 | 60.11 | 59.12 | 59.32 | 61.73 | 63.22 | 66.19 | 66.14 |
| H | 6.402 | 6.637 | 5.934 | 5.138 | 3.57 | 5.107 | 5.004 | 5.228 | 5.057 | 4.937 | 5.039 | 5.219 | 5.176 | |
| N | 1.196 | 1.62 | 1.8 | 0.942 | 1.404 | 0.456 | 0.704 | 1.021 | 1.048 | 0.432 | 0.207 | 0.05 | 0.08 | |
| S | 1.078 | 0.055 | 0.065 | 0 | 0.065 | 0 | 0 | 0.039 | 0 | 0 | 0 | 0.021 | 0 | |
| O ** | 39.58 | 37.61 | 35.4 | 32.03 | 32.55 | 32.37 | 31.41 | 31.83 | 31.81 | 30.16 | 28.81 | 25.80 | 25.83 | |
| H/C | 1.584 | 1.556 | 1.322 | 1.045 | 0.719 | 1.034 | 0.998 | 1.061 | 1.022 | 0.959 | 0.956 | 0.946 | 0.938 | |
| O/C | 0.612 | 0.551 | 0.493 | 0.407 | 0.410 | 0.409 | 0.391 | 0.403 | 0.402 | 0.366 | 0.341 | 0.292 | 0.292 | |
| Yield (%) | - | 81.92 | 76.39 | 69.88 | 69.11 | 66.82 | 63.41 | 60.58 | 59.49 | 61.73 | 57.31 | 53.99 | 53.78 | |
| Fuel Ratio | 0.22 | 0.29 | 0.37 | 0.39 | 0.39 | 0.40 | 0.41 | 0.41 | 0.41 | 0.42 | 0.44 | 0.48 | 0.50 | |
| HHV (MJ kg−1) | 20.39 | 21.71 | 22.03 | 23.25 | 21.54 | 23.28 | 23.56 | 23.43 | 23.30 | 24.18 | 24.96 | 26.53 | 26.48 | |
| Energy Densification Ratio | - | 1.06 | 1.08 | 1.14 | 1.05 | 1.14 | 1.15 | 1.14 | 1.14 | 1.14 | 1.22 | 1.30 | 1.29 | |
| Energy Yield | - | 87.21 | 82.54 | 79.69 | 73.02 | 76.29 | 73.26 | 69.62 | 67.98 | 73.21 | 70.17 | 70.24 | 69.85 | |
| Surface Area | 3.60 | 4.41 | 6.82 | 8.18 | 8.18 | 9.10 | 9.64 | 9.92 | 10.09 | 10.1 | 12.51 | 14.41 | 12.63 | |
| Sample | AC- 750-1 | AC- 850-1 | AC- 950-1 | AC- 850-3 | AC- 850-5 | AC- 850-7 | AC- 850-5(P) | ||
|---|---|---|---|---|---|---|---|---|---|
| Preparation Conditions | Temperature (°C) | 750 | 850 | 950 | 850 | 850 | 850 | 850 | |
| Activation time (h) | 1 | 1 | 1 | 3 | 5 | 7 | 5 | ||
| Ultimate Analysis | C | 79.11 | 79.41 | 79.35 | 81.27 | 83.57 | 83.93 | 82.11 | |
| H | 1.297 | 1.369 | 1.421 | 1.542 | 1.597 | 1.586 | 1.321 | ||
| N | 0.062 | 0.057 | 0 | 0 | 0 | 0 | 0.071 | ||
| S | 0.011 | 0.019 | 0.017 | 0.023 | 0.054 | 0.058 | 0 | ||
| Yield (%) | 53.26 | 35.98 | 33.54 | 18.86 | 13.76 | 11.82 | 10.54 | ||
| Surface Area (m2g−1) | 337.7 | 486.8 | 367.4 | 519.1 | 729.7 | 700.9 | 664.6 |
| Sample | pHpzc | Carboxylic Groups | Lactonic Groups | Phenolic Groups | Total Acidic Groups | Total Basic Groups |
|---|---|---|---|---|---|---|
| (mmol g−1) | (mmol g−1) | (mmol g−1) | (mmol g−1) | (mmol g−1) | ||
| TGs | 4.2 | 0.116 | 0.104 | 0.100 | 0.320 | 0.23 |
| HC-230-4 | 4.3 | 0.660 | 0.540 | 0.120 | 1.32 | 0.19 |
| AC-850-5 | 6.2 | 0.317 | BDL | 0.115 | 0.432 | 0.21 |
| Sample | Vibration Band (cm−1) | Assignment | Inference |
|---|---|---|---|
| TGs | 3405 | O-H | Stretching vibrations due to hydroxyl groups |
| 2927 | C-H | Stretching vibrations due to aliphatic structures | |
| 1631, 1511 | C=O | Stretching vibrations of carboxylic groups in aromatic rings | |
| 1403 | C=C | Bending vibrations due to methyl groups | |
| 1255 | C-O | Ester and phenolic groups | |
| 1048 | C-O-C | Stretching vibrations of polysaccharides bands | |
| 606 | C-H | Bending vibrations due to aromatic structure | |
| HC-230-4 | 3379 | O-H | Stretching vibrations due to hydroxyl groups |
| 2905 | C-H | Stretching vibrations due to aliphatic structures | |
| 1605, 1513 | C=O | Stretching vibrations due to carboxylic groups and aromatic rings. | |
| 1458, 1428, 1318, 1271, 1210, 1159, 1111, 1058, 1031 | C-O | Stretching vibrations in hydroxyl, ester or ether, polysaccharides, and O-H bending vibrations | |
| 558 | C-H | Deformation vibration due to aromatic structure | |
| AC-850-5 | 3500–3400 | O-H | Stretching vibrations due to hydroxyl groups |
| 2970 | C-H | Stretching vibrations due to aliphatic structures | |
| 1622 | C=O | Stretching vibrations due to carboxylic groups | |
| 1384 | O-H | Bending vibrations due to aromatic rings | |
| 1115 | C-O-C | Due to aromatic structures |
| Sample | Feedstock | Preparation Temperature | Surface Area (BET) | Total Pore Volume | Mesopore Volume | Micropore Volume | Pore Diameter |
|---|---|---|---|---|---|---|---|
| (°C) | (m2g−1) | (cm3g−1) | (cm3g−1) | (cm3g−1) | (nm) | ||
| HC-230-4 | TGs | 230 | 14.41 | 0.111 | 0.105 | 0.006 | -- |
| AC-850-5 | HC-230-4 | 850 | 729.70 | 0.392 | 0.106 | 0.286 | 2.15 |
| AC-850-5(P) | TGs | 850 | 664.52 | 0.505 | 0.256 | 0.249 | 3.60 |
| Material | Biomass/Water Ratio (gm:mL) | Carbonization Temperature (°C) | Residence Time (h) | Surface Area (m2g−1) | Reference |
|---|---|---|---|---|---|
| Saw dust | 1:12 | 200 | 20 | 4.4139 | [38] |
| Wheat straw | 1:12 | 200 | 20 | 9.1428 | [38] |
| Urban food waste | 1:4 | 250 | 20 | 6.07 | [52] |
| Rice husk | 1:5 | 175 | 40 | 13.09 | [53] |
| Wheat straw | 1:8 | 240 | 4 | 4.54 | [54] |
| Canola straw | 1:8 | 240 | 4 | 3.42 | [54] |
| Tectona grandis seeds | 1:10 | 230 | 4 | 14.41 | This study |
| Material | Type of Activation | Activation Temperature (°C) | Residence Time (h) | Surface Area (m2g−1) | Reference |
|---|---|---|---|---|---|
| Orange peel-derived hydrochar | Physical/CO2 | 750 | 20 | 301 | [43] |
| Orange peel-derived hydrochar | Physical/Air | 300 | 20 | 499 | [43] |
| Hickory wood chip-derived hydrochar | Physical/CO2 | 900 | 6 | 928 | [35] |
| Oak wood-derived hydrochar | Physical/CO2 | 900 | 1 | 903 | [55] |
| Chickpea stem-derived hydrochar | Chemical/KOH | 500 | 5 | 455 | [56] |
| Tectonagrandis seed-derived hydrochar | Physical/CO2 | 850 | 4 | 729.7 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhas; Chaudhary, M.; Chaudhary, S.; Chaubey, S.; da Paixão Cansado, I.P.; Dehghani, M.H.; Tyagi, I.; Gaur, R. Transforming Biomass Waste into Hydrochars and Porous Activated Carbon: A Characterization Study. Resources 2025, 14, 34. https://doi.org/10.3390/resources14030034
Suhas, Chaudhary M, Chaudhary S, Chaubey S, da Paixão Cansado IP, Dehghani MH, Tyagi I, Gaur R. Transforming Biomass Waste into Hydrochars and Porous Activated Carbon: A Characterization Study. Resources. 2025; 14(3):34. https://doi.org/10.3390/resources14030034
Chicago/Turabian StyleSuhas, Monika Chaudhary, Shubham Chaudhary, Shivangi Chaubey, Isabel Pestana da Paixão Cansado, Mohammad Hadi Dehghani, Inderjeet Tyagi, and Rama Gaur. 2025. "Transforming Biomass Waste into Hydrochars and Porous Activated Carbon: A Characterization Study" Resources 14, no. 3: 34. https://doi.org/10.3390/resources14030034
APA StyleSuhas, Chaudhary, M., Chaudhary, S., Chaubey, S., da Paixão Cansado, I. P., Dehghani, M. H., Tyagi, I., & Gaur, R. (2025). Transforming Biomass Waste into Hydrochars and Porous Activated Carbon: A Characterization Study. Resources, 14(3), 34. https://doi.org/10.3390/resources14030034









