Yield, Bioactive Compounds, and Antioxidant Potential of Twenty-Three Diverse Microgreen Species Grown Under Controlled Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Materials and Microgreens Production Under Controlled Conditions
2.2. Sample Extraction and Determination of Antioxidants and Their Activities
2.3. Determination of Phenolic Acids and Flavonoids
2.4. Tyrosinase Inhibitory Assay
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Morphology, Yield, and Percentage of Dry Matter
3.2. Bioactive Compounds, Antioxidant, and Tyrosinase Inhibitory Activities
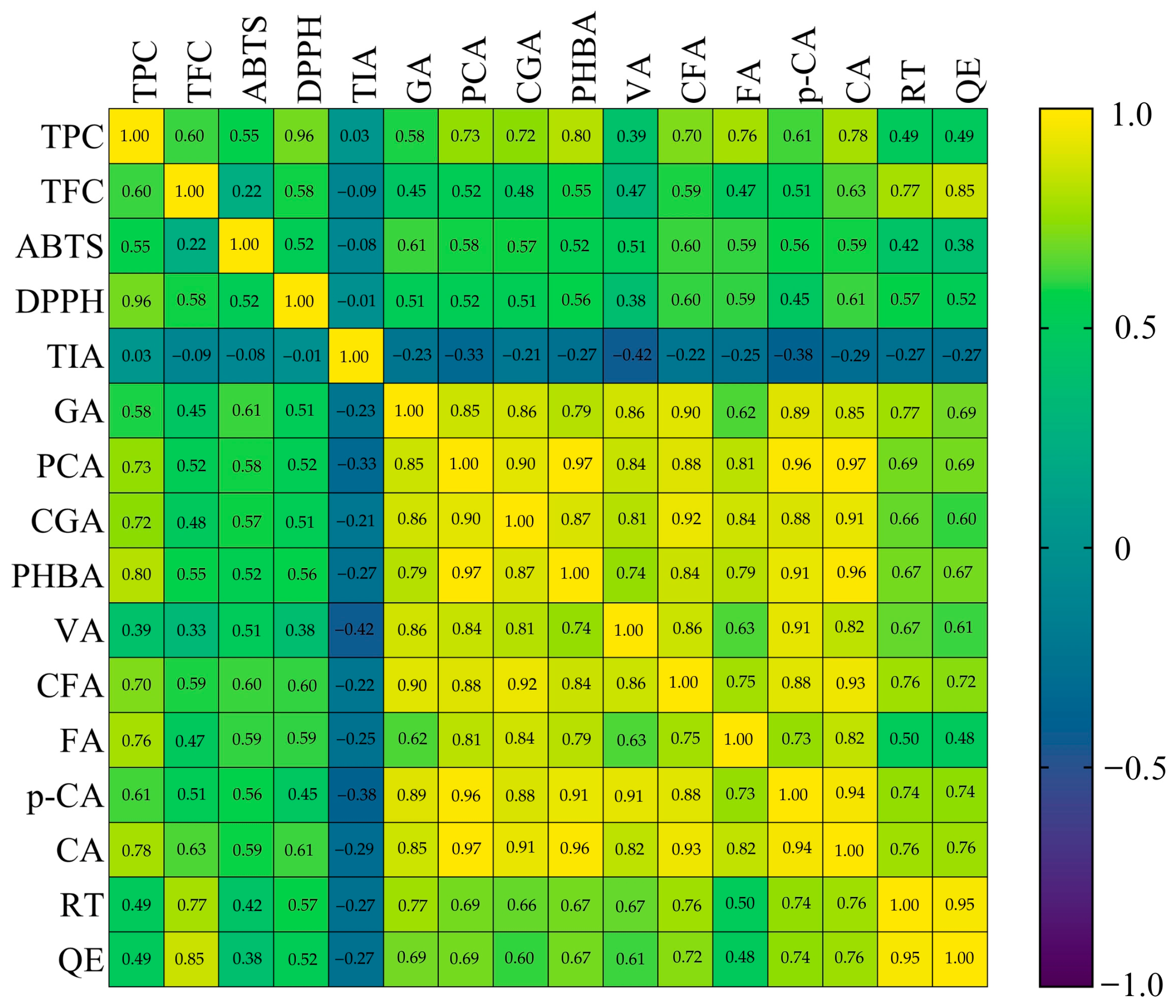
3.3. Principal Component Analysis (PCA) and Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino bis-3-ethylbenzthiazoline-6-sulphonic acid |
| DAP | Days After Planting |
| DPPH | 1-diphenyl-2-picrylhydrazyl |
| LED | Light-Emitting Diode |
| PCA | Principal Component Analysis |
| PDM | Percentage of Dry Matter |
| PPFD | Photosynthetic Photon Flux Density |
| TIA | Tyrosinase Inhibitory Activity |
| TFC | Total Flavonoid Content |
| TPC | Total Phenolic Content |
References
- Lile, R.; Ocnean, M.; Balan, I.M. Challenges for zero hunger (SDG 2): Links with other SDGs. In Transitioning to Zero Hunger; Kiba, D.I., Ed.; MDPI: Basel, Switzerland, 2023; pp. 9–66. [Google Scholar]
- Cohen, M.J. Advances in Food Security and Sustainability; Elsevier Inc.: Philadelphia, PA, USA, 2023. [Google Scholar]
- United Nations Statistics Division, Development Data and Outreach Branch. Zero Hunger. 2023. Available online: https://unstats.un.org/sdgs/report/2023/goal-02/ (accessed on 14 June 2024).
- Candib, L.M. Obesity and diabetes in vulnerable populations: Reflection on proximal and distal causes. Ann. Fam. Med. 2007, 6, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, J.; Kaur, S.; Guanjal, M.; Kaur, J.; Nanda, V.; Ullah, R.; Ercisli, S.; Rasane, P. Emergence of microgreens as a valuable food, current understanding of their market and consumer perception: A review. Food Chem. 2024, 23, 2024. [Google Scholar] [CrossRef] [PubMed]
- Hemler, E.C.; Hu, F.B. Plant-based diets for personal, population, and planetary health. Adv. Nutr. 2019, 10, S275–S283. [Google Scholar] [CrossRef]
- Yeargin, T.A.; Lin, Z.; Prado, I.D.; Sirsat, S.A.; Gibson, K.E. Consumer practices and perceptions regarding the purchasing and handling of microgreens in the United States. Food Control 2023, 145, 109470. [Google Scholar] [CrossRef]
- Mlinarić, S.; Piškor, A.; Melnjak, A.; Mikuška, A.; Gajdošik, M.Š.; Begović, L. Antioxidant capacity and shelf life of radish microgreens affected by growth light and cultivars. Horticulturae 2023, 9, 76. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.; Aguiar, A.A.; Ferreira, I.M.P.L.V.O. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food. Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Dimita, R.; Min, A.S.; Luvisi, A.; Greco, D.; Bellis, L.D.; Accogli, R.; Minini, C.; Negro, C. Volatile compounds and total phenolic content of Perilla frutescens at microgreens and mature stages. Horticulturae 2022, 8, 71. [Google Scholar] [CrossRef]
- Koppert Cress. Koppert Cress. 2016. Available online: https://www.koppertcress.com/en (accessed on 18 June 2024).
- Kowitcharoen, L.; Phornvillay, S.; Lekkham, P.; Pongprasert, N.; Srilaong, V. Bioactive composition and nutritional profile of microgreens cultivated in Thailand. Appl. Sci. 2021, 11, 7981. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A comprehensive review of bioactive molecules and health benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef]
- Dereje, B.; Jacquier, J.C.; Elliott-Kingston, C.; Harty, M.; Harbourne, N. Brassicaceae microgreens: Phytochemical compositions, influences of growing practices, postharvest technology, health, and food applications. ACS Food Sci. Technol. 2023, 3, 981–998. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities, and horizons for novel research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Yang, Z.; Guo, Q.; Gu, Z. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. J. Funct. Foods 2014, 9, 70–77. [Google Scholar] [CrossRef]
- Le, T.N.; Chiu, C.H.; Hsieh, P.C. Bioactive compounds and bioactivities of Brassica oleracea L. var. italica sprouts and microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Fuente, B.D.L.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Antiproliferative effect of bioaccessible fractions of four Brassicaceae microgreens on human colon cancer cells linked to their phytochemical composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef]
- Lone, J.K.; Pandey, R.; Gayacharan. Microgreens on the rise: Expanding our horizons from farm to fork. Heliyon 2024, 10, e25870. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Pinnaco, P.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pinnaco, P.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Palladino, M.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Phenolic constitution, phytochemical and macronutrient content in three species of microgreens as modulated by natural fiber and synthetic substrates. Antioxidants 2020, 9, 252. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Zarrelli, A.; Soteriou, G.A.; Kyratzis, A.; Antoniou, C.; Pizzolongo, F.; Romano, R.; et al. Ontogenetic variation in the mineral, phytochemical and yield attributes of Brassicaceous microgreens. Foods 2021, 10, 1032. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Soteriou, G.A.; Graziani, G.; Kyratzis, A.; Antoniou, C.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Preharvest nutrient deprivation reconfigures nitrate, mineral, and phytochemical content of microgreens. Foods 2021, 10, 1333. [Google Scholar] [CrossRef]
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae microgreens: A novel and promissory source of sustainable bioactive compounds. Curr. Res. Food Sci. 2023, 6, 100480. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Rouphael, Y.; Gioia, F.D.; Kyratzis, A.; Serio, F.; Renna, M.; de Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Butkutė, B.; Taujenis, L.; Norkevičienė, E. Small-seeded legumes as a novel food source. Variation of nutritional, mineral and phytochemical profiles in the chain: Raw seeds-sprouted seeds-microgreens. Molecules 2019, 24, 133. [Google Scholar] [CrossRef] [PubMed]
- National Science and Technology Development Agency. NSTDA and Partners to Boost Medicinal Plant Production in Thung Kula Ronghai. 2022. Available online: https://www.bcg.in.th/eng/nstda-and-partners-to-boost-medicinal-plant-production-in-thung-kula-ronghai/ (accessed on 18 June 2024).
- Steven, S.; Islam, M.S.; Ghimire, A.; Methela, N.J.; Kwon, E.H.; Yun, B.W.; Lee, I.J.; Kim, S.H.; Kim, Y. Chitosan-GSNO nanoparticles and silicon priming enhance the germination and seedling growth of soybean (Glycine max L.). Plants 2024, 13, 1290. [Google Scholar] [CrossRef]
- Arya, K.S.; Kutty, M.S.; Pradeepkumar, T. Microgreens of tropical edible-seed species, an economical source of phytonutrients- insights into nutrient content, growth environment and shelf life. Future Foods 2023, 8, 100262. [Google Scholar] [CrossRef]
- Dubey, S.; Harbourne, N.; Harty, M.; Hurley, D.; Elliott-Kingston, C. Microgreens production: Exploiting environmental and cultural factors for enhanced agronomical benefits. Plants 2024, 13, 2631. [Google Scholar] [CrossRef]
- Harakotr, B.; Srijunteuk, S.; Rithichai, P.; Tabunhan, S. Effects of light-emitting diode light irradiance levels on yield, antioxidants and antioxidant capacities of indigenous vegetable microgreens. Sci. Technol. Asia 2019, 4, 59–66. [Google Scholar] [CrossRef]
- Li, T.; Lalk, G.T.; Bi, G. Fertilization and pre-sowing seed soaking affect yield and mineral nutrients of ten microgreen species. Horticulturae 2021, 7, 14. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Ruangnoo, S.; Sangmukdee, K.; Chamchusri, K.; Rithichai, P. Enhancement of plumbagin production through elicitation in in vitro-regenerated shoots of Plumbago indica L. Plants 2024, 13, 1450. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S.; Meeso, N. Phytochemicals, vitamin C and sugar content of Thai wild fruits. Food Chem. 2011, 126, 972–981. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Panala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Leb. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mizzi, L.; Chatzitzika, C.; Gatt, R.; Valdramidis, V. HPLC analysis of phenolic compounds and flavonoids with overlapping peaks. Food Technol. Biotechnol. 2020, 58, 12–19. [Google Scholar] [CrossRef]
- Nurrochmad, A.; Wirasti, W.; Dirman, A.; Lukitaningsih, E.; Rahmawati, A.; Fakhrudin, N. Effects of antioxidant, anti-collagenase, anti-elastase, anti-tyrosinase of the extract and fraction from Turbinaria decurrens Bory. Indones. J. Pharm. 2018, 29, 188–199. [Google Scholar] [CrossRef]
- Motulsky, H.J. GraphPad Statistics Guide. 2016. Available online: http://www.graphpad.com/guides/prism/10/statistics/index.htm (accessed on 15 June 2024).
- Budavári, N.; Pék, Z.; Helyes, L.; Takács, S.; Nemeskéri, E. An overview on the use of artificial lighting for sustainable lettuce and microgreens production in an indoor vertical farming system. Horticulturae 2024, 10, 938. [Google Scholar] [CrossRef]
- Parkes, M.G.; Azevedo, D.L.; Cavallo, A.C.; Domingos, T.; Teixeira, R.F.M. Life cycle assessment of microgreen production: Effects of indoor vertical farm management on yield and environmental performance. Sci. Rep. 2023, 13, 11324. [Google Scholar] [CrossRef]
- Samuoliene, G.; Brazaityte, A.; Jankauskiene, J.; Virsile, A.; Sirtautaus, R.; Novickovas, A.; Sakalauskaite, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; de Pascale, S.; Rouphael, Y. Sensory attributes and consumer acceptability of 12 microgreens species. Agronomy 2020, 7, 1043. [Google Scholar] [CrossRef]
- Tan, L.; Nuffer, H.; Feng, J.; Kwan, S.H.; Chen, H.; Tong, X.; Kong, L. Antioxidant properties and sensory evaluation of microgreens from commercial and local farms. Food Sci. Human Wellness 2020, 9, 45–51. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and microgreens-novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.; Shah, M.; Mir, M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Dhaka, A.S.; Dikshit, H.K.; Mishra, G.P.; Tontang, M.T.; Meena, N.L.; Kumar, R.R.; Ramesh, S.V.; Narwal, S.; Aski, M.; Thimmegowda, V.; et al. Evaluation of growth conditions, antioxidant potential, and sensory attributes of six diverse microgreens species. Agriculture 2023, 13, 676. [Google Scholar] [CrossRef]
- Renna, M.; di Gioia, F.; Leoni, B.; Minini, C.; Santamaria, P. Culinary assessment of self-produced microgreens as basic ingredients in sweet and savory dishes. J. Culin. Sci. Technol. 2016, 15, 126–142. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Jones-Baumgardt, C.; Zheng, Y. Responses of yield and appearance quality of four Brassicaceae microgreens to varied blue light proportion in red and blue light-emitting diodes lighting. Sci. Hortic. 2020, 259, 108857. [Google Scholar] [CrossRef]
- Flores, M.; Hernández-Adasme, C.; Guevara, M.J.; Escalona, V.H. Effect of different light intensities on agronomic characteristics and antioxidant compounds of Brassicaceae microgreens in a vertical farm system. Front. Sustain. Food Syst. 2024, 8, 1349423. [Google Scholar] [CrossRef]
- Jones-Baumgardt, C.; Llewellyn, D.; Ying, Q.; Zheng, Y. Intensity of sole-source light-emitting diodes affects growth, yield, and quality of Brassicaceae microgreens. HortScience 2019, 54, 1168–1174. [Google Scholar] [CrossRef]
- Johnson, R.; Kong, Y.; Zheng, Y. Elongation growth mediated by blue light varies with light intensities and plant species: A comparison with red light in arugula and mustard seedlings. Environ. Exp. Bot. 2020, 169, 103898. [Google Scholar] [CrossRef]
- Hernández-Adasme, C.; Palma-Dias, R.; Escalona, V.H. The effect of light intensity and photoperiod on the yield and antioxidant activity of beet microgreens produced in an indoor system. Horticulturae 2023, 9, 493. [Google Scholar] [CrossRef]
- Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Biochemical compounds, antioxidant capacity, leaf color profile and yield of basil (Ocimum sp.) microgreens in floating system. Plants 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, J.L. Stommel, microgreens of Brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.D. Impact of light emitting-diodes (LEDs) and its potential on plant growth and development in controlled-environment plant production system. Curr. Biotechnol. 2016, 5, 28–43. [Google Scholar] [CrossRef]
- Lester, G.E.; Makus, D.J.; Hodges, D.K.; Jifon, J.L. Summer (Subarctic) versus winter (Subtropic) production affects spinach (Spinacia oleracea L.) leaf bionutrients: Vitamins (C, E, Folate, K1, provitamin A), lutein, phenolics, and antioxidants. J. Agric. Food Chem. 2013, 61, 7019–7027. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compost. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in Acyanic and Cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Tomas, M.; Zhang, L.; Zengin, G.; Rocchetti, G.; Capanoglu, E.; Lucini, L. Metabolomic insight into the profile, in vitro bioaccessibility and bioactive properties of polyphenols and glucosinolates from four Brassicaceae microgreens. Food Res. Int. 2021, 140, 110039. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, Z.; Lin, L.Z.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS(n.). J. Agric. Food Chem. 2013, 61, 10960–109670. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of phenolic compounds and antioxidant activity of 12 Cruciferous vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef]
- Yang, J.; Lee, H.; Sung, J.; Kim, Y.; Joeng, H.S.; Lee, J. Conversion of rutin to quercetin by acid treatment in relation to biological activities. Prev. Nutr. Food Sci. 2019, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, L.; Xue, B.; Liu, Q.; Ou, S.; Wang, Y.; Peng, X. Different flavonoids can shape unique gut microbiota profile in vitro. J. Food Sci. 2016, 81, H2273–H2279. [Google Scholar] [CrossRef] [PubMed]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; de Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Haldipur, A.C.; Srividya, N. Comparative evaluation of phytochemical content, antioxidant capacities and overall antioxidant potential of select culinary microgreens. J. Agric. Food Res. 2020, 2, 100046. [Google Scholar] [CrossRef]
- Pohntadavit, K.; Duangmano, S.; Osiriphan, M.; Leksawasdi, N.; Techapun, C.; Sumonsiri, N.; Sommano, S.R.; Rachtanapun, P.; Nunta, R.; Khemacheewakul, J. Tyrosinase inhibitory activity of crude procyanidin extract from green soybean seed and the stability of bioactive compounds in an anti-aging skin care formulation. Cosmetics 2024, 11, 178. [Google Scholar] [CrossRef]


| No. | Common Name | Scientific Name | Family | Seeding Rate/Tray (g) 1/ | Days After Planting (DAP) | Biomass-to-Seed Ratio |
|---|---|---|---|---|---|---|
| 1 | Red amaranth | Amaranthus viridis L. | Amaranthaceae | 9 | 6 | 6.77 |
| 2 | Leaf celery | Apium graveolens var. secalinum. | Apiaceae | 9 | 16 | 4.51 |
| 3 | Coriander | Coriandrum sativum L. | Apiaceae | 25 | 13 | 1.47 |
| 4 | Dill | Anethum graveolens L. | Apiaceae | 8 | 13 | 2.69 |
| 5 | Garland chrysanthemum | Chrysanthemum coronarium | Asteraceae | 9 | 13 | 3.15 |
| 6 | Sunflower | Helianthus annuus L. | Asteraceae | 30 | 6 | 3.62 |
| 7 | Indian mustard | Brassica juncea L. Czern. | Brassicaceae | 8 | 6 | 13.29 |
| 8 | Chinese mustard | B. juncea L var. rugosa | Brassicaceae | 8 | 6 | 8.86 |
| 9 | Chinese cabbage | B. rapa L. subsp. pekinensis | Brassicaceae | 8 | 6 | 13.63 |
| 10 | Daikon radish | Raphanus sativus L. var. longipinnatus | Brassicaceae | 25 | 6 | 10.83 |
| 11 | Red radish | R. sativus L. var. radicula | Brassicaceae | 25 | 6 | 10.66 |
| 12 | Rat-tailed radish | R. caudatus L. var. caudatus Alef | Brassicaceae | 25 | 6 | 9.11 |
| 13 | Cabbage | B. oleracea L. var. capitata | Brassicaceae | 8 | 6 | 11.26 |
| 14 | Cauliflower | B. oleracea var. botrytis | Brassicaceae | 8 | 6 | 11.58 |
| 15 | Broccoli | B. oleracea L. var. italica Plenck. | Brassicaceae | 8 | 6 | 11.27 |
| 16 | Chinese kale | B. oleracea L. var. alboglabra | Brassicaceae | 15 | 8 | 9.73 |
| 17 | Water convolvulus | Ipomoea aquatica Forssk. | Convolvulaceae | 50 | 9 | 4.00 |
| 18 | Thai water convolvulus | I. aquatica Forssk. | Convolvulaceae | 60 | 8 | 2.53 |
| 19 | Sugar pea | Pisum sativum L. | Fabaceae | 100 | 9 | 1.08 |
| 20 | Red holy basil | Ocimum tenuiflorum L. | Lamiaceae | 8 | 20 | 2.68 |
| 21 | Lemon basil | O. × africanum L. | Lamiaceae | 10 | 16 | 4.15 |
| 22 | Okra | Abelmoschus esculentus Moench. | Malvaceae | 40 | 9 | 5.81 |
| 23 | Roselle | Hibiscus sabdariffa L. | Malvaceae | 40 | 6 | 2.06 |
| No. 1/ | FW 2/ | PDM | TPC | TFC | Antioxidant Activities (mg TE/g DW) | TIA | |
|---|---|---|---|---|---|---|---|
| (g/tray) | (%) | (mg GAE/g DW) | (mg QE/g DW) | ABTS | DPPH | (%) | |
| 1 | 60.93 ± 1.52 3/ | 1.70 ± 0.00 i | 21.57 ± 0.25 j | 30.29 ± 1.61 i | 17.37 ± 0.88 kl | 26.24 ± 0.89 g | 10.00 ± 1.47 fg |
| 2 | 40.58 ± 1.29 m | 6.21 ± 0.64 c–g | 48.89 ± 0.5 c | 55.04 ± 0.08 e | 21.82 ± 0.71 ij | 41.28 ± 0.39 de | 21.25 ± 1.68 d |
| 3 | 36.7 ± 1.53 n | 6.51 ± 0.49 c–f | 33.19 ± 3.38 g | 64.56 ± 0.56 d | 10.97 ± 0.28 m | 10.19 ± 2.05 jk | 20.33 ± 1.18 d |
| 4 | 21.52 ± 1.57 p | 5.74 ± 0.26 d–h | 13.94 ± 0.92 k | 63.27 ± 0.08 d | 8.25 ± 1.38 m | 8.61 ± 0.89 k | 10.16 ± 0.98 fg |
| 5 | 28.33 ± 1.92 o | 6.41 ± 0.74 c–g | 42.56 ± 1.12 e | 55.07 ± 0.18 e | 28.44 ± 1.48 h | 52.29 ± 0.40 c | 47.56 ± 1.19 a |
| 6 | 108.73 ± 1.64 h | 12.30 ± 0.47 a | 16.59 ± 1.25 k | 20.93 ± 1.40 l | 18.76 ± 1.94 jk | 16.53 ± 0.95 i | 6.09 ± 1.95 h |
| 7 | 106.33 ± 1.95 h | 4.56 ± 0.15 h | 41.77 ± 0.35 ef | 49.24 ± 2.01f | 36.59 ± 0.66 d–f | 32.80 ± 0.19 f | 15.4 ± 0.10 e |
| 8 | 70.87 ± 1.39 k | 6.41 ± 0.12 c-g | 28.43 ± 1.81 h | 22.62 ± 1.04 kl | 40.38 ± 4.87 bc | 15.03 ± 4.68 i | 36.08 ± 1.47 c |
| 9 | 109.06 ± 2.66 h | 4.95 ± 0.35 gh | 33.24 ± 2.01 g | 43.83 ± 2.25 g | 22.31 ± 1.09 i | 15.30 ± 0.06 i | 41.24 ± 1.48 b |
| 10 | 270.66 ± 1.66 a | 6.34 ± 0.54 c–g | 25.95 ± 0.30 hi | 26.50 ± 2.82 j | 37.46 ± 5.06 c–e | 20.74 ± 3.88 h | 15.71 ± 0.42 e |
| 11 | 266.59 ± 1.50 b | 6.64 ± 0.40 c–e | 43.64 ± 0.40 de | 64.16 ± 1.69 d | 44.33 ± 1.09 a | 41.88 ± 0.32 de | 1.87 ± 0.47 j |
| 12 | 227.75 ± 2.25 d | 7.26 ± 0.38 c | 49.74 ± 0.58 c | 92.70 ± 0.16 a | 32.97 ± 0.49 g | 32.91 ± 0.93 f | 3.74 ± 0.08 i |
| 13 | 90.1 ± 1.79 i | 5.32 ± 0.70 e–h | 69.05 ± 7.22 a | 42.38 ± 0.48 g | 38.60 ± 2.53 cd | 44.38 ± 4.27 d | 9.40 ± 0.63 g |
| 14 | 92.63 ± 2.53i | 5.36 ± 0.62 e–h | 38.69 ± 0.10 f | 65.29 ± 2.41 d | 35.11 ± 1.58 e–g | 40.03 ± 1.51 e | 9.17 ± 0.09 g |
| 15 | 90.13 ± 1.71 i | 10.50 ± 0.70 b | 53.69 ± 2.17 b | 85.93 ± 1.77 b | 40.29 ± 0.14 bc | 38.39 ± 2.23 e | 20.83 ± 0.87 d |
| 16 | 145.95 ± 4.02 g | 6.67 ± 0.76 c–e | 46.27 ± 1.21 cd | 62.79 ± 2.50 d | 32.22 ± 1.30 g | 32.73 ± 0.39 f | 8.06 ± 1.56 g |
| 17 | 200.17 ± 1.57 e | 5.05 ± 0.79 f–h | 33.98 ± 0.68 g | 52.22 ± 1.12 ef | 14.94 ± 0.07 l | 25.76 ± 1.51 g | 16.05 ± 1.74 e |
| 18 | 151.53 ± 2.29 f | 6.26 ± 0.68 c–g | 43.68 ± 1.98 de | 75.77 ± 2.58 c | 24.16 ± 1.40 i | 68.52 ± 2.10 b | 20.36 ± 1.50 d |
| 19 | 108.01 ± 2.46 h | 9.80 ± 1.15 b | 21.32 ± 0.46 j | 25.53 ± 2.50 jk | 17.1 ± 2.01 kl | 13.83 ± 0.80 ij | 0.29 ± 0.17 j |
| 20 | 21.47 ± 2.1 p | 7.03 ± 0.54 cd | 48.83 ± 1.01 c | 78.75 ± 1.69 c | 43.27 ± 0.95 ab | 75.23 ± 0.39 a | 11.78 ± 0.62 f |
| 21 | 41.54 ± 3.32 m | 6.53 ± 0.85 c–e | 49.35 ± 1.46 c | 83.75 ± 2.50 b | 33.84 ± 1.02 fg | 53.59 ± 3.35c | 3.46 ± 1.08 i |
| 22 | 232.45 ± 3.09 c | 5.68 ± 0.83 d–h | 27.78 ± 0.90 h | 34.4 ± 3.95 h | 9.23 ± 0.25 m | 13.85 ± 0.19 ij | 20.05 ± 1.88 d |
| 23 | 82.53 ± 2.39 j | 6.76 ± 0.71 c–e | 22.81 ± 1.23 ij | 26.47 ± 1.03 j | 35.45 ± 1.10 d–g | 22.80 ± 4.84 gh | 3.32 ± 0.51 i |
| C.V. (%) | 1.94 | 13.66 | 5.38 | 3.54 | 6.86 | 6.73 | 7.67 |
| No. 1/ | Hydroxybenzoic Acids | Hydroxycinnamic Acids | Flavonoids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GA 2/ | PCA | CGA | PHBA | VA | CFA | FA | p-CA | CA | RT | QE | |
| 1 | n.d. | n.d. | 0.11 ± 0.00 k | n.d. | n.d. | n.d. | 0.11 ± 0.00 i | n.d. | n.d. | 0.02 ± 0.00 l | n.d. |
| 2 | 0.53 ± 0.00 kl 3/ | 0.36 ± 0.01 g | 2.47 ± 0.23 fg | 0.09 ± 0.00 f | 0.05 ± 0.00 g–i | 1.28 ± 0.08 d | 2.77 ± 0.2 ef | 0.09 ± 0.00 g | 0.13 ± 0.00 e | 0.82 ± 0.16 g | 0.30 ± 0.01 i–k |
| 3 | 0.24 ± 0.00 q | n.d. | 0.73 ± 0.04 ij | n.d. | n.d. | 0.35 ± 0.04 hi | 0.35 ± 0.02 hi | n.d. | 0.02 ± 0.00 jk | 0.13 ± 0.02 kl | 0.58 ± 0.05 h |
| 4 | 0.24 ± 0.00 q | 0.18 ± 0.01 lm | 0.41 ± 0.02 jk | n.d. | 0.06 ± 0.01 gh | 0.12 ± 0.01 hi | 0.54 ± 0.06 g–i | 0.07 ± 0.01 gh | 0.03 ± 0.00 j | 0.39 ± 0.05 h–j | 1.03 ± 0.08 g |
| 5 | 0.51 ± 0.03 lm | 0.26 ± 0.01 ij | 2.51 ± 0.07 fg | 0.05 ± 0.00 g | 0.06 ± 0.00 gh | 1.55 ± 0.05 d | 2.36 ± 0.94 f | 0.08 ± 0.00 g | 0.09 ± 0.00 f | 0.24 ± 0.01 h–l | 0.51 ± 0.06 h |
| 6 | 0.36 ± 0.00 o | 0.27 ± 0.00 hi | 0.69 ± 0.06 i–k | 0.04 ± 0.01 g–i | 0.08 ± 0.01 e | 0.27 ± 0.04 hi | 0.89 ± 0.10 g–i | 0.11 ± 0.00 f | 0.06 ± 0.00 hi | 0.12 ± 0.00 kl | 0.12 ± 0.00 j–m |
| 7 | 0.54 ± 0.00 k | 0.22 ± 0.00 i–k | 2.81 ± 0.08 f | n.d. | 0.06 ± 0.00 fg | 1.52 ± 0.02 d | 2.82 ± 0.25 ef | 0.08 ± 0.00 gh | 0.08 ± 0.00 fg | 0.33 ± 0.05 h–k | 0.32 ± 0.04 ij |
| 8 | 0.62 ± 0.01 i | 0.23 ± 0.00 ij | 1.1 ± 0.16 hi | n.d. | 0.07 ± 0.00 f | 0.92 ± 0.14 ef | 0.99 ± 0.03 g–i | 0.08 ± 0.00 gh | 0.05 ± 0.00 i | 0.13 ± 0.00 kl | 0.12 ± 0.00 k–m |
| 9 | 0.13 ± 0.00 r | n.d. | 0.38 ± 0.06 jk | n.d. | n.d. | 0.26 ± 0.01 hi | 0.21 ± 0.00 hi | n.d. | 0.01 ± 0.00 jk | 0.05 ± 0.00 l | 0.06 ± 0.00 lm |
| 10 | 0.70 ± 0.00 h | 0.27 ± 0.00 hi | 1.41 ± 0.09 h | n.d. | 0.08 ± 0.00 e | 0.75 ± 0.02 fg | 1.06 ± 0.06 gh | 0.09 ± 0.00 g | 0.04 ± 0.00 i | 0.23 ± 0.03 h–l | 0.21 ± 0.02 j–l |
| 11 | 2.09 ± 0.01 a | 0.95 ± 0.07 a | 10.08 ± 0.45 a | 0.22 ± 0.03 b | 0.22 ± 0.00 a | 5.66 ± 0.19 a | 5.88 ± 0.39 d | 0.31 ± 0.00 a | 0.41 ± 0.00 a | 3.03 ± 0.36 b | 2.86 ± 0.11 c |
| 12 | 1.09 ± 0.01 d | 0.77 ± 0.02 c | 6.12 ± 0.67 c | 0.19 ± 0.01 c | 0.11 ± 0.00 bc | 3.03 ± 0.27 b | 6.13 ± 0.26 cd | 0.23 ± 0.01 bc | 0.31 ± 0.00 b | 3.44 ± 0.24 a | 4.1 ± 0.30 a |
| 13 | 1.13 ± 0.03 c | 0.95 ± 0.04 a | 8.05 ± 0.46 b | 0.27 ± 0.04 a | 0.10 ± 0.00 d | 2.78 ± 0.63 b | 8.2 ± 0.56 a | 0.21 ± 0.01 c | 0.33 ± 0.02 b | 0.21 ± 0.02 i–l | 0.22 ± 0.01 j–l |
| 14 | 0.50 ± 0.01 m | 0.19 ± 0.00 k–m | 2.11 ± 0.08 g | n.d. | 0.05 ± 0.00 gh | 1.22 ± 0.19 de | 2.32 ± 0.55 f | 0.06 ± 0.00 hi | 0.07 ± 0.00 gh | 1.43 ± 0.19 f | 1.36 ± 0.13 f |
| 15 | 1.18 ± 0.04 b | 0.82 ± 0.04 b | 5.97 ± 0.77 c | 0.22 ± 0.00 b | 0.11 ± 0.00 cd | 2.93 ± 0.11 b | 3.57 ± 1.11 e | 0.23 ± 0.01 b | 0.32 ± 0.00 b | 2.49 ± 0.07 d | 3.28 ± 0.13 b |
| 16 | 0.34 ± 0.00 o | 0.63 ± 0.03 d | 5.15 ± 0.57 d | 0.14 ± 0.00 d | 0.11 ± 0.00 bc | 2.90 ± 0.10 b | 6.81 ± 1.08 bc | 0.16 ± 0.01 d | 0.26 ± 0.00 c | 0.42 ± 0.02 hi | 1.10 ± 0.15 g |
| 17 | 0.58 ± 0.01 j | 0.22 ± 0.00 j–l | 2.16 ± 0.10 g | n.d. | 0.06 ± 0.00 gh | 1.50 ± 0.13 d | 0.96 ± 0.05 gh i | 0.07 ± 0.00 gh | 0.06 ± 0.00 hi | 0.45 ± 0.05 h | 0.46 ± 0.03 hi |
| 18 | 1.03 ± 0.01 e | 0.57 ± 0.01 e | 4.89 ± 0.57 d | 0.15 ± 0.00 d | 0.12 ± 0.00 b | 2.86 ± 0.33 b | 2.79 ± 0.96 ef | 0.15 ± 0.00 d | 0.25 ± 0.01 c | 2.72 ± 0.20 c | 2.8 ± 0.15 c |
| 19 | 0.42 ± 0.01 n | 0.30 ± 0.04 h | 0.85 ± 0.05 h–j | n.d. | 0.10 ± 0.01 d | 0.40 ± 0.03 hi | 0.83 ± 0.05 gh i | 0.11 ± 0.01 f | 0.05 ± 0.01 i | 0.18 ± 0.00 j–l | 0.16 ± 0.00 j–m |
| 20 | 0.87 ± 0.00 f | 0.50 ± 0.00 f | 4.29 ± 0.53 e | 0.11 ± 0.01 e | 0.08 ± 0.00 e | 2.23 ± 0.15 c | 7.26 ± 0.23 b | 0.13 ± 0.01 e | 0.21 ± 0.00 d | 1.75 ± 0.01 e | 1.94 ± 0.12 e |
| 21 | 0.82 ± 0.01 g | 0.56 ± 0.01 e | 0.76 ± 0.06 ij | 0.13 ± 0.00 d | 0.08 ± 0.00 e | 2.14 ± 0.12 c | 1.34 ± 0.13 g | 0.15 ± 0.01 d | 0.22 ± 0.00 d | 1.66 ± 0.08 e | 2.51 ± 0.04 d |
| 22 | 0.40 ± 0.00 n | 0.15 ± 0.00 m | 0.76 ± 0.07 ij | n.d. | 0.04 ± 0.00 i | 0.45 ± 0.05 gh | 0.61 ± 0.03 g–i | 0.05 ± 0.00 j | 0.02 ± 0.00 j | 0.17 ± 0.01 kl | 0.18 ± 0.00 j–m |
| 23 | 0.28 ± 0.00 p | 0.17 ± 0.00 m | 0.71 ± 0.06 i–k | n.d. | 0.05 ± 0.00 hi | 0.41 ± 0.03 hi | 0.61 ± 0.02 g–i | 0.05 ± 0.00 ij | 0.02 ± 0.00 j | 0.11 ± 0.00 kl | 0.11 ± 0.00 lm |
| C.V. (%) | 0.49 | 8.25 | 11.91 | 3.87 | 12.56 | 12.05 | 8.53 | 8.65 | 7.25 | 13.18 | 9.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harakotr, B.; Charoensup, L.; Rithichai, P.; Jirakiattikul, Y.; Suthamwong, P. Yield, Bioactive Compounds, and Antioxidant Potential of Twenty-Three Diverse Microgreen Species Grown Under Controlled Conditions. Resources 2025, 14, 71. https://doi.org/10.3390/resources14050071
Harakotr B, Charoensup L, Rithichai P, Jirakiattikul Y, Suthamwong P. Yield, Bioactive Compounds, and Antioxidant Potential of Twenty-Three Diverse Microgreen Species Grown Under Controlled Conditions. Resources. 2025; 14(5):71. https://doi.org/10.3390/resources14050071
Chicago/Turabian StyleHarakotr, Bhornchai, Lalita Charoensup, Panumart Rithichai, Yaowapha Jirakiattikul, and Patlada Suthamwong. 2025. "Yield, Bioactive Compounds, and Antioxidant Potential of Twenty-Three Diverse Microgreen Species Grown Under Controlled Conditions" Resources 14, no. 5: 71. https://doi.org/10.3390/resources14050071
APA StyleHarakotr, B., Charoensup, L., Rithichai, P., Jirakiattikul, Y., & Suthamwong, P. (2025). Yield, Bioactive Compounds, and Antioxidant Potential of Twenty-Three Diverse Microgreen Species Grown Under Controlled Conditions. Resources, 14(5), 71. https://doi.org/10.3390/resources14050071







