Thermal Conversion of Municipal Biowaste Anaerobic Digestate to Valuable Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Pyrolysis Process
2.3. Characterization Methods
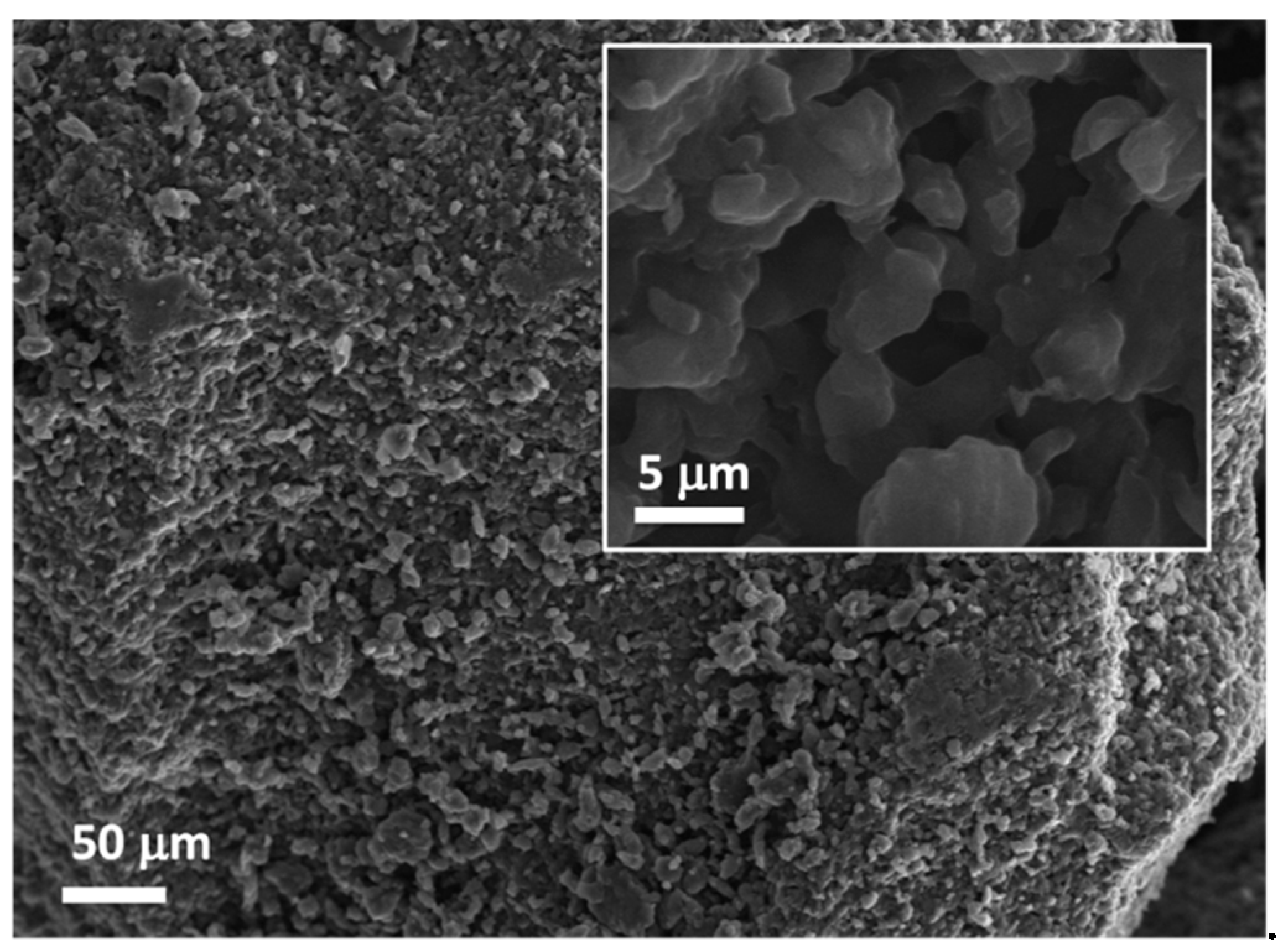
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Area, M.C.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics—A review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Franzoso, F.; Vaca-Garcia, C.; Rouilly, A.; Evon, P.; Montoneri, E.; Persico, P.; Mendichi, R.; Nisticò, R.; Francavilla, M. Extruded versus solvent cast blends of poly(vinyl alcohol-co-ethylene) and biopolymers isolated from municipal biowaste. J. Appl. Polym. Sci. 2016, 133, 43009. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Esmaeilian, B.; Wang, B.; Lewis, K.; Duarte, F.; Ratti, C.; Behdad, S. The future of waste management in smart and sustainable cities: A review and concept paper. Waste Manag. 2018, 81, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. Aquatic-derived biomaterials for a sustainable future: A European opportunity. Resources 2017, 6, 65. [Google Scholar] [CrossRef]
- Anceschi, A.; Guerretta, F.; Magnacca, G.; Zanetti, M.; Benzi, P.; Trotta, F.; Caldera, F.; Nisticò, R. Sustainable N-containing biochars obtained at low temperatures as sorbing materials for environmental application: Municipal biowaste-derived substances and nanosponges case studies. J. Anal. Appl. Pyrolysis 2018, 134, 606–613. [Google Scholar] [CrossRef]
- Guerretta, F.; Magnacca, G.; Franzoso, F.; Ivanchenko, P.; Nisticò, R. Sodium alginate conversion into char via pyrolysis at the onset temperature. Mater. Lett. 2019, 234, 339–342. [Google Scholar] [CrossRef]
- Magnacca, G.; Guerretta, F.; Vizintin, A.; Benzi, P.; Valsania, M.C.; Nisticò, R. Preparation, characterization and environmental/electrochemical energy storage testing of low-cost biochar from natural chitin obtained via pyrolysis at mild conditions. Appl. Surf. Sci. 2018, 427, 883–893. [Google Scholar] [CrossRef]
- Baxter, M.D.; Acosta, E.; Montoneri, E.; Tabasso, S. Waste biomass-extracted surfactants for heavy oil removal. Ind. Eng. Chem. Res. 2014, 53, 3612–3621. [Google Scholar] [CrossRef]
- Nisticò, R.; Evon, P.; Labonne, L.; Vaca-Medina, G.; Montoneri, E.; Francavilla, M.; Vaca-Garcia, C.; Magnacca, G.; Franzoso, F.; Negre, M. Extruded poly(ethylene-co-vinyl alcohol) composite films containing biopolymers isolated from municipal biowaste. ChemistrySelect 2016, 1, 2354–2365. [Google Scholar] [CrossRef]
- ACEA Pinerolese. Available online: https://www.aceapinerolese.it/ (accessed on 8 November 2018).
- Nisticò, R.; Celi, L.R.; Bianco Prevot, A.; Carlos, L.; Magnacca, G.; Zanzo, E.; Martin, M. Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J. Hazard. Mater. 2018, 342, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Miikki, V.; Hanninen, K.; Knuutinen, J.; Hyotylainen, J. Pyrolysis of humic acids from digested and composted sewage sludge. Chemosphere 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Palma, D.; Bianco Prevot, A.; Brigante, M.; Fabbri, D.; Magnacca, G.; Richard, C.; Mailhot, G.; Nisticò, R. New insights on the photodegradation of caffeine in the presence of bio-based substances-magnetic iron oxide hybrid nanomaterials. Materials 2018, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Franzoso, F.; Nisticò, R.; Cesano, F.; Corazzari, I.; Turci, F.; Scarano, D.; Bianco Prevot, A.; Magnacca, G.; Carlos, L.; Martire, D.O. Biowaste-derived substances as a tool for obtaining magnet-sensitive materials for environmental applications in wastewater treatments. Chem. Eng. J. 2017, 310, 307–316. [Google Scholar] [CrossRef]
- Ruppenthal, M.; Oelmann, Y.; Wilcke, W. Optimized demineralization technique for the measurement of stable isotope ratios of nonexchangeable H in soil organic matter. Environ. Sci. Technol. 2013, 47, 949–957. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and 1H NMR techniques. Sci. Total Environ. 2016, 541, 623–637. [Google Scholar] [CrossRef]
- Beebe, R.A.; Beckwith, J.B.; Honig, J.M. The determination of small surface areas by krypton adsorption at low temperatures. J. Am. Chem. Soc. 1945, 67, 1554–1558. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef]
- Agblevor, F.A.; Jahromi, H. Aqueous phase synthesis of hydrocarbons from reactions of guaiacol and low molecular weight oxygenates. ChemCatChem 2018, 10, 5201–5214. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, X.; Li, S.; Mikulcic, H.; Basenic, T.; Deng, S.; Vujanovic, M.; Tan, H.; Kumfer, B.M. Synergistic effects during co-pyrolysis of biomass and plastic: Gas, tar, soot, char products and thermogravimetric study. J. Energy Inst. 2019, 92, 108–117. [Google Scholar] [CrossRef]
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of aqueous-phase catalytic pyrolysis oil to liquid hydrocarbons using multifunctional nickel catalyst. Ind. Eng. Chem. Res. 2018, 57, 13257–13268. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 642, 190–197. [Google Scholar] [CrossRef]
- Ratajczak, P.; Suss, M.E.; Kaasik, F.; Beguin, F. Carbon electrodes for capacitive technologies. Energy Storage Mater. 2019, 16, 126–145. [Google Scholar] [CrossRef]
- Antonelli, M.; Baccioli, A.; Francesconi, M.; Psaroudakis, P.; Martorano, L. Technologies for energy recovery from waste biomasses: A study about Tuscan potentialities. Energy Procedia 2015, 81, 450–460. [Google Scholar] [CrossRef]
- Tong, X.; Chen, Z.; Zhuo, H.; Jing, S.; Liu, J.; Zhong, L. Tailoring the physicochemical properties of chitosan-derived N-doped carbon by controlling hydrothermal carbonization time for high-performance supercapacitor application. Carbohydr. Polym. 2019, 207, 764–774. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.; Liu, S.; Wang, Y.; Qiu, J. Sustainable synthesis and assembly of biomass-derived B/N co-doped carbon nanosheets with ultrahigh aspect ratio for high-performance supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. [Google Scholar] [CrossRef]
- Sridevi, G.; Narmatha, M.; Sathish, M. N-containing carbon/graphene nanocomposites for electrochemical supercapacitor applications. J. Nanosci. Nanotechnol. 2017, 17, 1267–1274. [Google Scholar] [CrossRef]


| Samples | %C ± SD | %H ± SD | %N ± SD | C/N | SSA (m2 g−1) | ζ Potential (mV) ± SD |
|---|---|---|---|---|---|---|
| MWDF | 54.5 ± 0.1 | 6.2 ± 0.1 | 7.5 ± 0.4 | 7.3 | n.d. | n.d. |
| MWDF char | 72.7 ± 4.7 | 2.2 ± 0.1 | 7.2 ± 0.3 | 10.1 | 0.51 | −45.6 ± 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisticò, R.; Guerretta, F.; Benzi, P.; Magnacca, G.; Mainero, D.; Montoneri, E. Thermal Conversion of Municipal Biowaste Anaerobic Digestate to Valuable Char. Resources 2019, 8, 24. https://doi.org/10.3390/resources8010024
Nisticò R, Guerretta F, Benzi P, Magnacca G, Mainero D, Montoneri E. Thermal Conversion of Municipal Biowaste Anaerobic Digestate to Valuable Char. Resources. 2019; 8(1):24. https://doi.org/10.3390/resources8010024
Chicago/Turabian StyleNisticò, Roberto, Federico Guerretta, Paola Benzi, Giuliana Magnacca, Davide Mainero, and Enzo Montoneri. 2019. "Thermal Conversion of Municipal Biowaste Anaerobic Digestate to Valuable Char" Resources 8, no. 1: 24. https://doi.org/10.3390/resources8010024
APA StyleNisticò, R., Guerretta, F., Benzi, P., Magnacca, G., Mainero, D., & Montoneri, E. (2019). Thermal Conversion of Municipal Biowaste Anaerobic Digestate to Valuable Char. Resources, 8(1), 24. https://doi.org/10.3390/resources8010024