Variation of the Chemical Composition of Waste Cooking Oils upon Bentonite Filtration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Clarification Procedure
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.3.1. Retention Indices
2.3.2. Headspace Solid-Phase Microextraction (HS-SPME)
2.4. NMR Analysis
2.4.1. Determination of Hunter Lab Coordinates
2.4.2. Sensory Evaluation
2.5. Statistical Analysis
2.6. Thermogravimetric Analysis
2.7. XRD
3. Results and Discussion
3.1. Characterization of the Volatile Organic Compounds (VOCs)
3.2. Principal Component Analysis
3.3. NMR
3.4. Color
3.5. Sensory Evaluation
3.6. Characterization of Bentonite
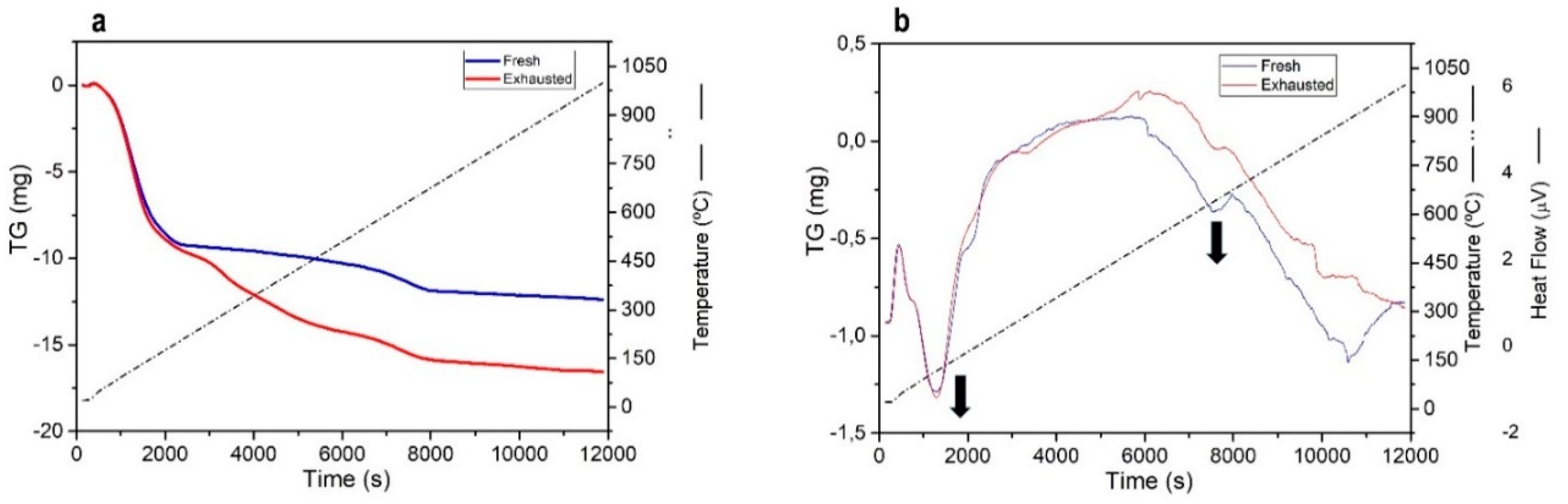
3.7. Thermogravimetric Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, J. Flavor chemistry of deep fat frying in oil. In Flavor Chemistry of Lipid Foods; Min, D.B., Smouse, T.H., Zhang, S.S., Eds.; American Oil Chemists Society: Champaign, IL, USA, 1989; p. 113. [Google Scholar]
- Ziaiifar, A.M.; Achir, N.; Courtois, F.; Trezzani, I.; Trystram, G. Review of mechanisms, conditions, and factors involved in the oil uptake phenomenon during the deep-fat frying process. Int. J. Food Sci. Technol. 2008, 43, 1410–1423. [Google Scholar] [CrossRef]
- Saguy, I.; Dana, D. Integrated approach to deep fat frying: Engineering, nutrition, health and consumer aspects. J. Food Eng. 2003, 56, 143–152. [Google Scholar] [CrossRef]
- Ramos, T.R.P.; Gomes, M.I.; Barbosa-Póvoa, A.P. Planning waste cooking oil collection systems. Waste Manag. 2013, 33, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Borrello, M.; Caracciolo, F.; Lombardi, A.; Pascucci, S.; Cembalo, L. Consumers’ Perspective on Circular Economy Strategy for Reducing Food Waste. Sustainability 2017, 9, 141. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Li, J.; Chen, Y.; Gong, Y.; Li, Y.; Zhang, J. Current Situation and Development of Kitchen Waste Treatment in China. Procedia Environ. Sci. 2016, 31, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Eubia.org. Available online: http://www.eubia.org/cms/wiki-biomass/biomass-resources/challenges-related-to-biomass/used-cooking-oil-recycling/ (accessed on 1 April 2019).
- Petran, J.; Pedisic, L.; Orlovic, M.; Podolski, S.; Bradac, V. Biolubricants from natural waste oil and fats. Goriva Maz. 2008, 47, 463–478. [Google Scholar]
- Shashidhara, Y.M.; Jayaram, S.R. Vegetable oils as a potential cutting fluid—An evolution. Tribol. Int. 2010, 43, 1073–1081. [Google Scholar] [CrossRef]
- Salemdeeb, R.; Zu Ermgassen, E.K.; Kim, M.H.; Balmford, A.; Al-Tabbaa, A. Environmental and health impacts of using food waste as animal feed: A comparative analysis of food waste management options. J. Clean. Prod. 2017, 140, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Tres, A.; Bou, R.; Guardiola, F.; Nuchi, C.D.; Magrinya, N.; Codony, R. Use of recovered frying oils in chicken and rabbit feeds: Effect on the fatty acid and tocol composition and on the oxidation levels of meat, liver and plasma. Animal 2013, 7, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Panadare, D.C.; Rathod, V.K. Applications of Waste Cooking Oil Other Than Biodiesel: A Review. Iran. J. Chem. Eng. 2015, 12, 55–76. [Google Scholar]
- Singhabhandhu, A.; Tezuka, T. The waste-to-energy framework for integrated multi-waste utilization: Waste cooking oil, waste lubricating oil, and waste plastics. Energy 2010, 35, 2544–2551. [Google Scholar] [CrossRef]
- Singhabhandhu, A.; Tezuka, T. Prospective framework for collection and exploitation of waste cooking oil as feedstock for energy conversion. Energy 2010, 35, 1839–1847. [Google Scholar] [CrossRef]
- Namoco, C.S., Jr.; Comaling, V.C.; Buna, C.C., Jr. Utilization of used cooking oil as an alternative cooking fuel resource. ARPN J. Eng. Appl. Sci. 2017, 12, 435–442. [Google Scholar]
- Capuano, D.; Costa, M.; Di Fraia, S.; Massarotti, N.; Vanoli, L. Direct use of waste vegetable oil in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 759–770. [Google Scholar] [CrossRef]
- No, S.-Y. Inedible vegetable oils and their derivatives for alternative diesel fuels in CI engines: A review. Renew. Sustain. Energy Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar]
- Sun, D.; Lu, T.; Xiao, F.; Zhu, X.; Sun, G. Formulation and aging resistance of modified bio-asphalt containing high percentage of waste cooking oil residues. J. Clean. Prod. 2017, 161, 1203–1214. [Google Scholar] [CrossRef]
- Asli, H.; Ahmadinia, E.; Zargar, M.; Karim, M.R. Investigation on physical properties of waste cooking oil – Rejuvenated bitumen binder. Constr. Build. Mater. 2012, 37, 398–405. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying Waste to Mercury: Inexpensive Sorbents Made from Sulfur and Recycled Cooking Oils. Chem. A Eur. J. 2017, 23, 16219–16230. [Google Scholar] [CrossRef]
- Waste Framework Directive. Available online: http://ec.europa.eu/environment/waste/framework/list.htm (accessed on 1 April 2019).
- Vlahopoulou, G.; Petretto, G.L.; Garroni, S.; Piga, C.; Mannu, A. Variation of density and flash point in acid degummed waste cooking oil. J. Food Process. Preserv. 2018, 42, e13533. [Google Scholar] [CrossRef]
- Mannu, A.; Vlahopoulou, G.; Sireus, V.; Petretto, G.L.; Mulas, G.; Garroni, S. Bentonite as a Refining Agent in Waste Cooking Oils Recycling: Flash Point, Density and Color Evaluation. Nat. Prod. Commun. 2018, 13, 613–616. [Google Scholar] [CrossRef]
- Van Del Dool, H.; Kartz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Petretto, G.L.; Tuberoso, C.I.G.; Vlahopoulou, G.; Atzei, A.; Mannu, A.; Zrira, S.; Pintore, G. Volatiles, color characteristics and other physico–chemical parameters of commercial Moroccan honeys. Nat. Prod. Res. 2016, 30, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Del Caro, A.; Balestra, F.; Piga, A.; Fadda, C. Bee pollen as a functional ingredient in gluten-free bread: A physical-chemical, technological and sensory approach. LWT Food Sci. Technol. 2018, 90, 1–7. [Google Scholar] [CrossRef]
- Gruppo di Chemiometria. Available online: http://gruppochemiometria.it/index.php/software (accessed on 1 March 2019).
- Zhang, Q.; Liu, C.; Sun, Z.; Hu, X.; Shen, Q.; Wu, J. Authentication of edible vegetable oils adulterated with used frying oil by Fourier Transform Infrared Spectroscopy. Food Chem. 2012, 132, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Alencar, J.W.; Alves, P.B.; Craveiro, A.A. Pyrolysis of tropical vegetable oils. J. Agric. Food Chem. 1983, 31, 1268–1270. [Google Scholar] [CrossRef]
- Chung, T.Y.; Eiserich, J.P.; Shibamoto, T. Volatile compounds identified in headspace samples of peanut oil heated under temperatures ranging from 50 to 200 °C. J. Agric. Food Chem. 1993, 41, 1467–1470. [Google Scholar] [CrossRef]
- Wu, C.-M.; Chen, S.-Y. Volatile compounds in oils after deep frying or stir frying and subsequent storage. J. Am. Oil Chem. Soc. 1992, 69, 858–865. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Full, G.H.; Dao, L.T. Effect of Heating on the Characteristics and Chemical Composition of Selected Frying Oils and Fats. J. Agric. Food Chem. 1997, 45, 3244–3249. [Google Scholar] [CrossRef]
- Urgeghe, P.; Piga, C.; Addis, M.; Di Salvo, R.; Piredda, G.; Scintu, M.F.; Wolf, I.V.; Sanna, G. SPME/GC-MS Characterization of the Volatile Fraction of an Italian PDO Sheep Cheese to Prevalent Lypolitic Ripening: The Case of Fiore Sardo. Food Anal. Methods 2012, 5, 723–730. [Google Scholar] [CrossRef]
- Dais, P.; Spyros, A.; Christophoridou, S.; Hatzakis, E.; Fragaki, G.; Agiomyrgianaki, A.; Salivaras, E.; Siragakis, G.; Daskalaki, D.; Tasioula-Margari, M.; et al. Comparison of Analytical Methodologies Based on1H and31P NMR Spectroscopy with Conventional Methods of Analysis for the Determination of Some Olive Oil Constituents. J. Agric. Food Chem. 2007, 55, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, C.; Belsito, E.; De Marco, R.; Di Gioia, M.L.; Leggio, A.; Liguori, A. Quantitative determination of fatty acid chain composition in pork meat products by high resolution 1H NMR spectroscopy. Food Chem. 2013, 136, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, R.; Wu, S.; Wang, Q.; Zhang, Z. Correlations between 1H NMR and conventional methods for evaluating soybean oil deterioration during deep frying. J. Food Meas. Charact. 2018, 12, 1420–1426. [Google Scholar] [CrossRef]
- Castejón, D.; Mateos-Aparicio, I.; Molero, M.D.; Cambero, M.I.; Herrera, A. Evaluation and Optimization of the Analysis of Fatty Acid Types in Edible Oils by 1H-NMR. Food Anal. Methods 2014, 6, 1285–1297. [Google Scholar] [CrossRef]
- Corsaro, C.; Mallamace, D.; Vasi, S.; Ferrantelli, V.; Dugo, G.; Cicero, N. 1H HR-MAS NMR Spectroscopy and the Metabolite Determination of Typical Foods in Mediterranean Diet. J. Anal. Methods Chem. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatzakis, E.; Agiomyrgianaki, A.; Kostidis, S.; Dais, P. High-Resolution NMR Spectroscopy: An Alternative Fast Tool for Qualitative and Quantitative Analysis of Diacylglycerol (DAG) Oil. J. Am. Oil Chem. Soc. 2011, 88, 1695–1708. [Google Scholar] [CrossRef]
- Vigli, G.; Philippidis, A.; Spyros, A.; Dais, P. Classification of Edible Oils by Employing31P and1H NMR Spectroscopy in Combination with Multivariate Statistical Analysis. A Proposal for the Detection of Seed Oil Adulteration in Virgin Olive Oils. J. Agric. Food Chem. 2003, 51, 5715–5722. [Google Scholar] [CrossRef]
- Camin, F.; Pavone, A.; Bontempo, L.; Wehrens, R.; Paolini, M.; Faberi, A.; Marianella, R.M.; Capitani, D.; Vista, S.; Mannina, L. The use of IRMS, 1 H NMR and chemical analysis to characterise Italian and imported Tunisian olive oils. Food Chem. 2016, 196, 98–105. [Google Scholar] [CrossRef]
- Popescu, R.; Costinel, D.; Dinca, O.R.; Marinescu, A.; Stefanescu, I.; Ionete, R.E. Discrimination of vegetable oils using NMR spectroscopy and chemometrics. Food Control 2015, 48, 84–90. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Guillen, M.D. A study by 1H nuclear magnetic resonance of the influence on the frying medium composition of some soybean oil-food combinations in deep-frying. Food Res. Int. 2014, 55, 347–355. [Google Scholar] [CrossRef]
- Collar, C.; Jiménez, T.; Conte, P.; Fadda, C. Impact of ancient cereals, pseudocereals and legumes on starch hydrolysis and antiradical activity of technologically viable blended breads. Carbohydr. Polym. 2014, 113, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheok, S.C.; Lim, E.E. Mechanism of palm oil bleaching by montmorillonite clay activated at various acid concentrations. J. Am. Oil Chem. Soc. 1982, 59, 129–131. [Google Scholar] [CrossRef]
- Taylor, D.R.; Jenkins, D.B.; Ungermann, C.B. Bleaching with alternative layered minerals: A comparison with acid-activated montmorillonite for bleaching soybean oil. J. Am. Oil Chem. Soc. 1989, 66, 334–341. [Google Scholar] [CrossRef]
- Makhoukhi, B.; Didi, M.A.; Villemin, D.; Azzouz, A. Acid activation of Bentonite for use as a vegetable oil bleaching agent. Grasas y Aceites 2009, 60, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Mannu, A.; Vlachopoulou, G.; Sireus, V.; Mulas, G.; Petretto, G.L. Characterization of Sardinian Bentonite. J. Sci. Res. 2019, 11, 145–150. [Google Scholar] [CrossRef]
- Suchithra, P.; Vazhayal, L.; Mohamed, A.P.; Ananthakumar, S. Mesoporous organic–inorganic hybrid aerogels through ultrasonic assisted sol–gel intercalation of silica–PEG in bentonite for effective removal of dyes, volatile organic pollutants and petroleum products from aqueous solution. Chem. Eng. J. 2012, 200, 589–600. [Google Scholar] [CrossRef]
- De Oliveira, C.I.R.; Rocha, M.C.G.; Da Silva, A.L.N.; Bertolino, L.C. Characterization of bentonite clays from Cubati, Paraíba (Northeast of Brazil). Cerâmica 2016, 62, 272–277. [Google Scholar] [CrossRef]
- Caglar, B.; Afsin, B.; Tabák, Á.; Eren, E. Characterization of the cation-exchanged bentonites by XRPD, ATR, DTA/TG analyses and BET measurement. Chem. Eng. J. 2009, 149, 242–248. [Google Scholar] [CrossRef]




| PCA | RT | WCO | SD | WCO1 | SD | WCO2 | SD | RI | RI Literature | Δ | Compound |
|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 5.444 | 0.42 | 0.08 | 0.72 * | 0.14 | 0.96 | 0.08 | 702 | 700 | 2 | heptane |
| V2 | 5.573 | 0.89 | 0.03 | 0.28 ** | 0.13 | 0.11 | 0.01 | 711 | 702 | 9 | acetaldehyde |
| V3 | 6.13 | 0.53 | 0.07 | 0.10 ** | 0.07 | 0.13 | 0.02 | 748 | n.a. | Methylamine, N,N-dimethyl- | |
| V4 | 6.881 | 1.12 | 0.26 | 2.22 * | 0.72 | 3.46 | 0.53 | 799 | 800 | 1 | octane |
| V5 | 9.65 | 0.20 | 0.04 | 0.10 * | 0.01 | 0.05 | 0.00 | 910 | 907 | 3 | 2-Butanone |
| V6 | 10.132 | 0.72 | 0.39 | 0.48 | 0.28 | 0.43 | 0.10 | 925 | 918 | 7 | Butanal, 3-methyl- |
| V7 | 10.579 | 0.14 | 0.00 | n.d. | n.d. | 939 | 971 | 32 | Butyl-cyclopentane | ||
| V8 | 12.109 | 1.00 | 0.19 | 1.18 | 0.28 | 1.54 | 0.33 | 988 | 979 | 9 | pentanal |
| V9 | 15.527 | 4.43 | 0.87 | 6.79 | 1.33 | 7.42 | 0.84 | 1091 | 1083 | 8 | hexanal |
| V10 | 18.842 | 0.26 | 0.03 | 0.47 | 0.22 | 0.53 | 0.01 | 1193 | 1182 | 11 | 2-Heptanone |
| V11 | 18.94 | 0.31 | 0.24 | 0.86 | 0.08 | 1.19 | 0.50 | 1196 | 1184 | 12 | Heptanal |
| V12 | 19.133 | 0.28 | 0.12 | 0.07 * | 0.01 | n.d. | 1202 | 1185 | 17 | Pyridine | |
| V13 | 19.404 | 0.23 | 0.09 | 0.42 * | 0.02 | 0.56 | 0.04 | 1211 | 1200 | 11 | Limonene |
| V14 | 19.997 | 0.42 | 0.20 | 0.06 * | 0.03 | n.d. | 1230 | 1212 | 18 | Pyrazine | |
| V15 | 20.184 | 0.50 | 0.14 | 0.24 * | 0.09 | n.d. | 1237 | 1216 | 21 | 2-Hexenal, (E)- | |
| V16 | 20.297 | 0.44 | 0.15 | 0.66 | 0.18 | 1.00 | 0.06 | 1240 | 1231 | 9 | Furan, 2-pentyl- |
| V17 | 20.675 | 0.56 | 0.21 | 0.66 | 0.06 | 0.53 | 0.09 | 1253 | 1250 | 3 | 1-Pentanol |
| V18 | 20.884 | 0.48 | 0.17 | 0.56 | 0.02 | 0.70 | 0.09 | 1260 | 1246 | 14 | γ-terpinene |
| V19 | 21.500 | 0.20 | 0.15 | n.d. | n.d. | 1280 | 1272 | 8 | Hexyl acetate | ||
| V20 | 21.728 | 0.81 | 0.15 | 0.06 ** | 0.02 | 0.03 | 0.01 | 1287 | 1266 | 21 | Pyrazine, methyl- |
| V21 | 22.061 | 0.12 | 0.02 | 0.12 | 0.02 | 0.05 | 0.01 | 1298 | 1287 | 11 | 2-Octanone |
| V22 | 22.199 | 1.11 | 0.28 | 1.46 | 0.51 | 0.98 | 0.80 | 1303 | 1289 | 14 | Octanal |
| V23 | 22.841 | 1.16 | 0.25 | 0.20 ** | 0.06 | n.d. | 1325 | 1303 | 22 | 2-Propanone, 1-hydroxy- | |
| V24 | 23.448 | 2.09 | 0.44 | 2.28 | 0.24 | 1.87 | 0.53 | 1346 | 1322 | 24 | 2-Heptenal |
| V25 | 23.632 | 0.27 | 0.09 | 0.43 | 0.10 | 0.26 | 0.05 | 1353 | 1338 | 15 | 5-Hepten-2-one, 6-methyl- |
| V26 | 23.821 | 0.81 | 0.08 | 0.10 ** | 0.01 | n.d. | 1360 | 1326 | 34 | Formamide, N,N-dimethyl- | |
| V27 | 25.27 | 5.07 | 1.10 | 6.42 | 0.40 | 6.59 | 0.25 | 1410 | 1391 | 19 | Nonanal |
| V28 | 26.032 | 1.25 | 0.25 | 0.23 ** | 0.08 | 0.38 | 0.00 | 1437 | 1414 | 23 | N,N-Dimethylacetamide |
| V29 | 26.305 | 0.22 | 0.08 | 0.19 | 0.04 | 0.12 | 0.02 | 1447 | 1441 | 6 | Ethyl octanoate |
| V30 | 26.463 | 0.79 | 0.08 | 0.69 | 0.04 | 0.56 | 0.20 | 1453 | 1429 | 24 | 2-Octenal, (E)- |
| V31 | 26.616 | 0.24 | 0.06 | 0.30 | 0.05 | 0.46 | 0.10 | 1458 | 1453 | 5 | Heptanol |
| V32 | 26.756 | 11.05 | 0.74 | n.d. | n.d. | 1463 | 1449 | 14 | Acetic acid | ||
| V33 | 27.498 | 12.72 | 1.03 | 3.64 ** | 1.69 | 0.17 * | 0.19 | 1489 | 1461 | 28 | Furfural |
| V34 | 28.121 | 0.97 | 0.20 | n.d. | 0.10 | 0.10 | 1511 | n.a. | Pyridine, 4-ethenyl- | ||
| V35 | 28.554 | 0.64 | 0.06 | 0.32 ** | 0.06 | 0.12 | 0.03 | 1525 | 1495 | 30 | 2,4-Heptadienal, |
| V36 | 29.538 | 0.52 | 0.03 | n.d. | n.d. | 1557 | 1535 | 22 | Propanoic acid | ||
| V37 | 29.787 | 1.90 | 0.38 | 1.66 | 0.13 | 2.00 | 0.40 | 1565 | 1534 | 31 | 2-Nonenal, |
| V38 | 31.011 | 1.32 | 0.04 | 0.38 ** | 0.23 | 0.08 | 0.01 | 1607 | 1570 | 37 | 2-Furancarboxaldehyde, 5-methyl- |
| V39 | 31.951 | 0.36 | 0.10 | n.d. | n.d. | 1646 | 1625 | 21 | Butanoic acid | ||
| V40 | 32.627 | 2.00 | 0.14 | 1.75 | 0.24 | 1.07 * | 0.17 | 1674 | 1644 | 30 | 2-Decenal, (E)- |
| V41 | 32.695 | 1.21 | 0.15 | 0.33 ** | 0.19 | n.d. | 1677 | 1660 | 17 | Furanmethanol | |
| V42 | 34.497 | 2.27 | 0.11 | 2.09 | 0.13 | 1.66 * | 0.13 | 1764 | 1746 | 18 | α-Farnesene |
| V43 | 34.857 | 1.06 | 0.07 | 0.72 ** | 0.02 | 0.35 | 0.07 | 1782 | 1752 | 30 | 2-Undecenal |
| V44 | 35.117 | 1.01 | 0.05 | 0.77 ** | 0.05 | 0.29 | 0.09 | 1795 | 1797 | 2 | 2,4-Decadienal, isomer 1 |
| V45 | 35.997 | 4.48 | 0.02 | 2.76 ** | 0.45 | 0.39 | 0.20 | 1847 | 1811 | 36 | 2,4-Decadienal, isomer 2 |
| V46 | 36.215 | 1.91 | 0.17 | n.d. | n.d. | 1860 | 1846 | 14 | Hexanoic acid |
| Analyte | Prior to Filtration (WCO) | After Filtration (WCO2) |
|---|---|---|
| Linolenic acid | <3% | <3% |
| Linoleic acid | 13% | 10% |
| Oleic acid | 70% | 70% |
| SFA a | 16% | 16% |
| IV b | 83.2 | 83.8 |
| Entry | Sample | Color (CIE) a (S) b | |||
|---|---|---|---|---|---|
| L c | a d | b e | ΔE (ΔS) | ||
| 1 | WCO | 23.11 (0.02) | 3.25 (0.05) | 9.33 (0.04) | |
| 2 | WCO1 | 25.22 (0.02) | 2.19 (0.10) | 12.62 (0.05) | 4.04 (0.06) |
| 3 | WCO2 | 27.17 (0.01) | 0.21 (0.05) | 15.25 (0.05) | 7.79 (0.05) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannu, A.; Vlahopoulou, G.; Urgeghe, P.; Ferro, M.; Del Caro, A.; Taras, A.; Garroni, S.; Rourke, J.P.; Cabizza, R.; Petretto, G.L. Variation of the Chemical Composition of Waste Cooking Oils upon Bentonite Filtration. Resources 2019, 8, 108. https://doi.org/10.3390/resources8020108
Mannu A, Vlahopoulou G, Urgeghe P, Ferro M, Del Caro A, Taras A, Garroni S, Rourke JP, Cabizza R, Petretto GL. Variation of the Chemical Composition of Waste Cooking Oils upon Bentonite Filtration. Resources. 2019; 8(2):108. https://doi.org/10.3390/resources8020108
Chicago/Turabian StyleMannu, Alberto, Gina Vlahopoulou, Paolo Urgeghe, Monica Ferro, Alessandra Del Caro, Alessandro Taras, Sebastiano Garroni, Jonathan P. Rourke, Roberto Cabizza, and Giacomo L. Petretto. 2019. "Variation of the Chemical Composition of Waste Cooking Oils upon Bentonite Filtration" Resources 8, no. 2: 108. https://doi.org/10.3390/resources8020108
APA StyleMannu, A., Vlahopoulou, G., Urgeghe, P., Ferro, M., Del Caro, A., Taras, A., Garroni, S., Rourke, J. P., Cabizza, R., & Petretto, G. L. (2019). Variation of the Chemical Composition of Waste Cooking Oils upon Bentonite Filtration. Resources, 8(2), 108. https://doi.org/10.3390/resources8020108