Abstract
Ultramafic areas are critical for nickel (Ni) phytomining due to the high concentration of this element in their soils and the number of hyperaccumulators they harbor. The aim of the present study was to evaluate the potential of the Morais massif, an ultramafic area in Portugal, for phytomining using the hyperaccumulator species Alyssum serpyllifolium subsp. lusitanicum. Soil samples and A. serpyllifolium specimens were collected in four locations of the Morais massif. After determination of Ni concentrations in the samples, the results show that soil pseudo-total Ni concentrations in sites number 1 and 2 are significantly higher than in the soil samples collected in the other two locations, with 1918 and 2092 mg kg−1, respectively. Nickel accumulation is significantly greater in the aerial parts of plants collected at sites 1, 2, and 4, presenting Ni harvestable amount means of 88.36, 93.80, and 95.56 mg per plant, respectively. These results suggest that the sites with highest potential for phytomining are sites 1, 2, and 4. A nickel agromining system in these locations could represent an additional source of income to local farmers, since ultramafic soils have low productivity for agriculture and crop production.
1. Introduction
Serpentine (ultramafic) soils cover approximately 1% of the Earth’s terrestrial surface. Featuring an uneven distribution, they can be found especially in tropical (e.g., New Caledonia, Cuba, and Indonesia) and temperate regions (e.g., Italy, Spain, Turkey, and California) [1]. Serpentine soils result from the weathering of ultramafic rocks that have a low silica (SiO2) content (<45%) and are rich in ferromagnesian (ultramafic) minerals (at least 70% of minerals within olivine and pyroxene groups) [2]. Thus, serpentine soils present high concentrations of magnesium (Mg) and iron (Fe), low calcium-magnesium (Ca:Mg) ratio, and high concentrations of trace elements such as cobalt (Co), chromium (Cr), and nickel (Ni) [1]. These characteristics, particularly the elevated values of Mg and Ni and low Ca soil concentrations, are considered the main cause of serpentine soil toxicity [3]. Whereas Ni concentrations in normal soils may vary from 7 to 500 mg kg−1, serpentine soils present values that are typically in the range of 700 to 8000 mg kg−1 [4,5]. Serpentine soils are also poor in important macronutrients [e.g., nitrogen (N), potassium (K), and phosphorus (P)] and micronutrients [e.g., boron (B) and molybdenum (Mo)] and have a low organic matter content and low water holding capacity [6]. These physicochemical properties make these soils adverse environments for normal plant growth [7,8]. However, serpentine soils endemic flora have developed specific resistance mechanisms under these environmental conditions over thousands of years of natural selection [9].
The Morais massif is a serpentinized region from Portugal and therefore has a specialized flora that thrives on metal rich substrates [10]. In Portugal, serpentine outcrops are restricted to Morais and Bragança massifs localized in the Trás-os-Montes region and harbor the nickel hyperaccumulator species Alyssum serpyllifolium subsp. lusitanicum [11]. Hyperaccumulators are remarkable plants that accumulate extreme amounts of metals (such as cadmium, cobalt, magnesium, and zinc) in their tissues while remaining sufficiently healthy to maintain a self-sustaining population [12]. It has been hypothesized that high metal accumulation in these plants may serve various ecological functions such as metal tolerance, resistance to environmental stress, competitive strategy (allelopathy), and defense against pathogens and herbivores [13,14,15,16]. In the case of A. serpyllifolium, there is evidence that the high concentrations of Ni protect the species against herbivores [13]. The first discovery of a Ni hyperaccumulator was made in 1948 by Minguzzi and Vergnano with the species Alyssum bertolonii [17]. In 1977, Brooks et al. defined a threshold for nickel hyperaccumulation of 1000 mg kg−1 in the dry matter of plant shoots [18]. Currently, there are approximately 500 plant species identified that hyperaccumulate nickel, making up 70% of all known hyperaccumulators [19]. Nickel hyperaccumulators are widely dispersed, reflecting the distribution of serpentine soils from which they absorb this element [20]. The plant A. serpyllifolium subsp. lusitanicum T. R. Dudley & P. Silva (or Alyssum pintodasilvae) was described by Dudley in 1967 [21,22]. It belongs to the Brassicaceae family, and it is classified as an herbaceous and perennial plant that presents a ramified stem that can reach 10 to 30 cm and yellow flowers. It is endemic to Morais and Bragança massifs and can accumulate up to 8000 mg kg−1 (dry weight) of nickel [11].
Phytomining is a recent phytotechnology based on the use of hyperaccumulators to recover economically relevant amounts of valuable metals from mineralized or contaminated soils [23,24]. The growing Ni demand and its market price together with the existence of extensive ultramafic areas with high Ni concentrations and the number of hyperaccumulator species they shelter have strengthened the importance of Ni phytomining [23]. In 2015, the concept of agromining (variant of phytomining) was proposed as an agricultural strategy that would allow local communities to farm for metals, providing them an economic profit [25]. Therefore, the two main approaches to consider with this phytotechnology are (i) phytomining functioning jointly with phytoremediation as part of a rehabilitation strategy (e.g., in degraded mine soils and quarries); (ii) agromining in soils unsuitable for conventional agriculture and pasture (e.g., serpentine soils) [23,25]. The Ni phytomining process consists of (i) the selection of an ultramafic area with high bioavailable nickel concentrations; (ii) the cultivation of a hyperaccumulator species in the area (preferably native) that will be harvested when maturity is reached (maximum biomass); (iii) the incineration of the harvested biomass and the chemical processing that results in nickel recovery [1]. In this context, the survey of potential areas for phytomining and hyperaccumulator species is crucial for future implementation of these phytomining/agromining systems.
This study consisted primarily of the assessment of nickel concentrations in soils and A. serpyllifolium plant species from four different sites in the Morais massif. To draw further conclusions, other soil physicochemical properties and plant metal uptake parameters were also analyzed. The main objective of this work is to provide support towards the implementation of nickel phytomining in the Morais massif.
Morais Massif Location and Geology
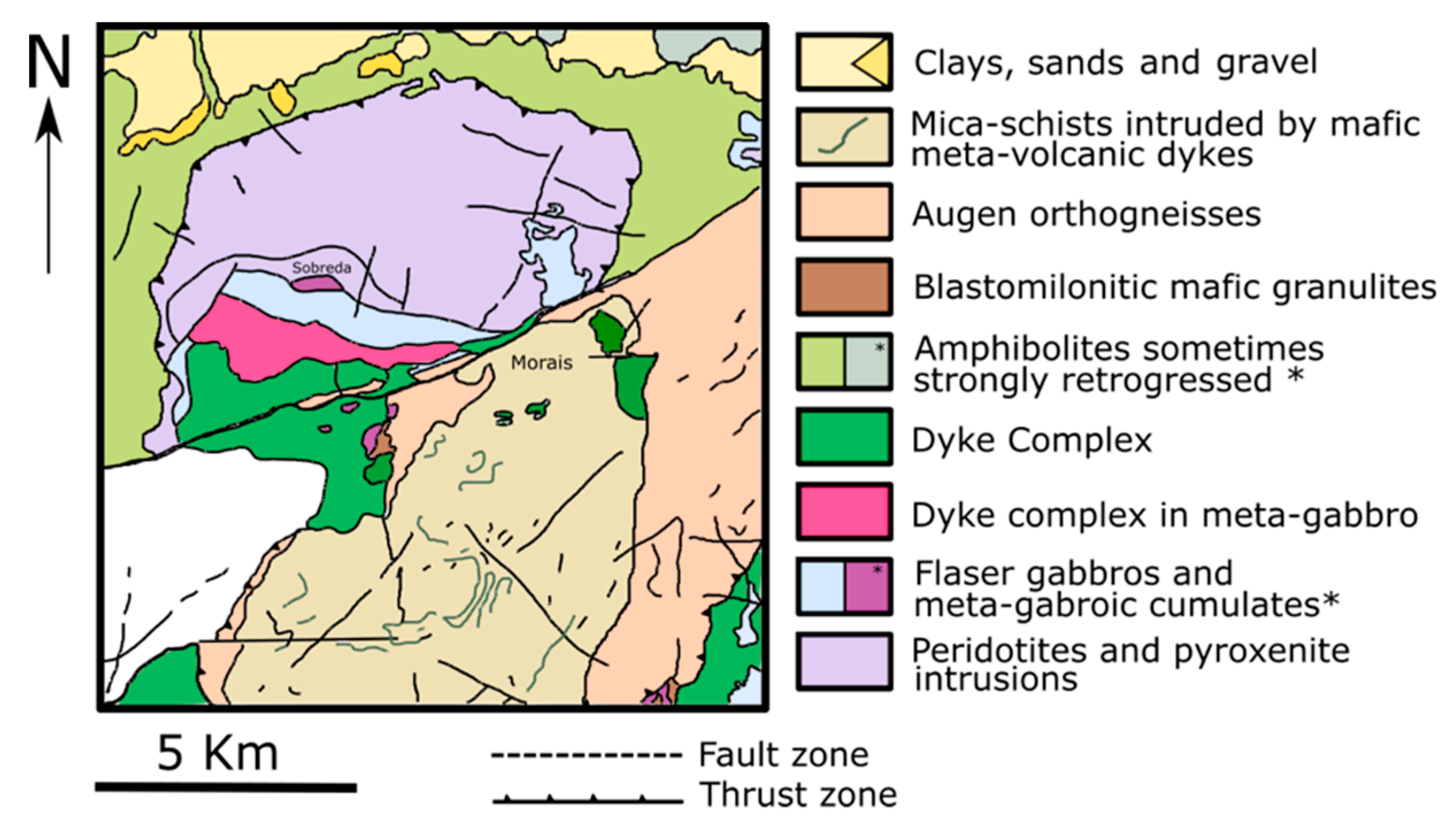
The Morais massif is located in the eastern part of the Trás-os-Montes region in the north of Portugal. It belongs to the Bragança district and is found mostly in the municipality of Macedo de Cavaleiros. Geologically, the study area integrates the Galicia-Trás-os-Montes Zone (GTMZ) of the Iberian Massif. The GTMZ is composed of four main sequences [26,27]: (i) parautochthonous complex (schistose domain) with diversified metasedimentary and metavolcanic rocks; (ii) lower allochthonous complex with gneiss, metasedimentary, and metavolcanic rocks; (iii) ophiolite complex (intermediate allochthonous complex) composed by thrust sheets made of ophiolitic rocks such as amphibolites, serpentinized peridotites, sheeted dike/gabbro complexes, flaser gabbros, and mafic cumulates; (iv) upper allochthonous complex made of high-grade metamorphic rocks such as paragneisses, eclogites, mafic granulites, pyroxenites, and peridotites. Figure 1 presents the main geological composition of the Morais massif.
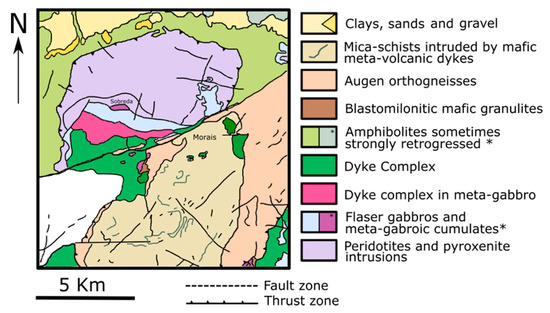
Figure 1.
Simplified geological map of the Morais massif.
2. Materials and Methods
To evaluate the phytomining potential of the Morais massif, samples of soil and A. serpyllifolium subsp. lusitanicum specimens were collected from 4 sites in the study area with the following coordinates: (1) 41°31′10.54′′ N, 6°49′7.03′′ W; (2) 41°31′14.56′′ N, 6°48′50.58′′ W; (3) 41°29′38.47′′ N, 6°47′26.68′′ W; (4) 41°30′33.60′′ N, 6°45′23.74′′ W. Sites were randomly selected, with sites number 1 and 2 being located to the west and site number 4 further east within the ultramafic complex. Site number 3 is located at a southern point, close to the urban area of Morais town. From each selected site, 3 soil samples and 3 A. serpyllifolium specimens were collected, making a total of 12 soil samples and 12 plant samples that were analyzed in this study. Sampling was performed in May 2018, and it is worth noting that plants still had not reached full maturity (which would be normally expected around 2–3 months later). At each site, soil samples were collected with a hand auger from surface horizons covering a depth of approximately 15 cm, since it corresponded to the A. serpyllifolium rhizosphere zone. Plant sampling followed a uniform criterion in terms of plant maturity and size. The specimens were collected in an area of 1 m2, and the most mature plants were selected for analysis. Shoot sizes varied between 12 and 15 cm and dry weights between 18 and 22 g.
Soil samples were air-dried and sieved through a 2 mm sieve. The plant samples were washed once with tap water and twice with deionized water in order to remove any dust deposits and oven-dried at 50 °C for 48 h. The dry weight of the different plant parts (roots, stems, leaves, and flowers) was measured, and then the plants were ground into a homogenous powder using an electric grinder (Selecline 851680/SHG269). Soil and plant samples were subjected to acid digestion with HNO3 and HCl (3:1, v/v), and elemental analysis was assessed through inductively coupled plasma mass spectrometry (ICP-MS) [28]. Using this method, the pseudo total concentrations of Ca, Mg, P, Cr, and Ni in the soil were determined, as well as Cr and Ni concentrations in the different plant tissues. The former was analyzed since, along with Ni, it is the most abundant trace element in ultramafic soils, and competition for the uptake of both elements could occur [29]. The bioavailable fractions of Cr and Ni in the soil samples were determined through extraction with ammonium acetate (1 M NH4Ac, pH 7) [30]. The levels of Cr and Ni in the filtrate were assessed by ICP-MS. The pH and the electrical conductivity in the soil were determined using a suspension of 10 g of soil and 50 mL of deionized water (1:5) [31]. The suspension was homogeneously mixed in an orbital shaker for 2 h at 150 rpm, and the values were measured using a pH/conductivity meter (Peak Instruments S-620).
All results were based on the statistical analyses from three replicates. The Levene, the Brown–Forsythe, and the Welch tests were used to evaluate the homogeneity of variances. One-way ANOVA was conducted to assess the statistical significance of differences among values. Tukey and Dunnett’s T3 tests were used in cases of equal and unequal variances, respectively. Pearson correlation coefficients were used to examine the relationship between different parameters. Statistical analyses were carried out using SPSS statistical program (ver. 25.0, IBM Corp., Armonk, NY, USA).
3. Results and Discussion
3.1. Soil
The physicochemical properties of the soil collected in different sampling sites are presented in Table 1. All soil samples exhibit an approximately neutral to slightly acidic pH (6.8–6.4), typical of serpentine soils that are characterized by a pH that ranges between 6 to 8 [32]. The electrical conductivity values are low, meaning that there are no dissolved salts [33]. As expected for serpentinite soils, the Morais massif presents high concentrations of Mg, Ni, and Cr (Figure 2), as well as a low Ca:Mg ratio. The Ca concentration is significantly higher at sites 3 and 4 in comparison to the values from sites 1 and 2. These results suggest that plants from sites 1 and 2 might be under more stress due to the lower values of Ca, since this is considered one of the main causes of serpentine soil toxicity [3]. The concentration of P, which is one of the macronutrients essential to plants, is significantly lower at site 3 in comparison to the other sampling sites.

Table 1.
Selected physicochemical characteristics of the soils from each sampling site.
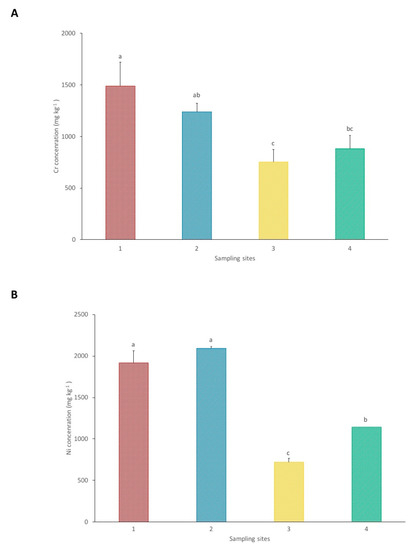
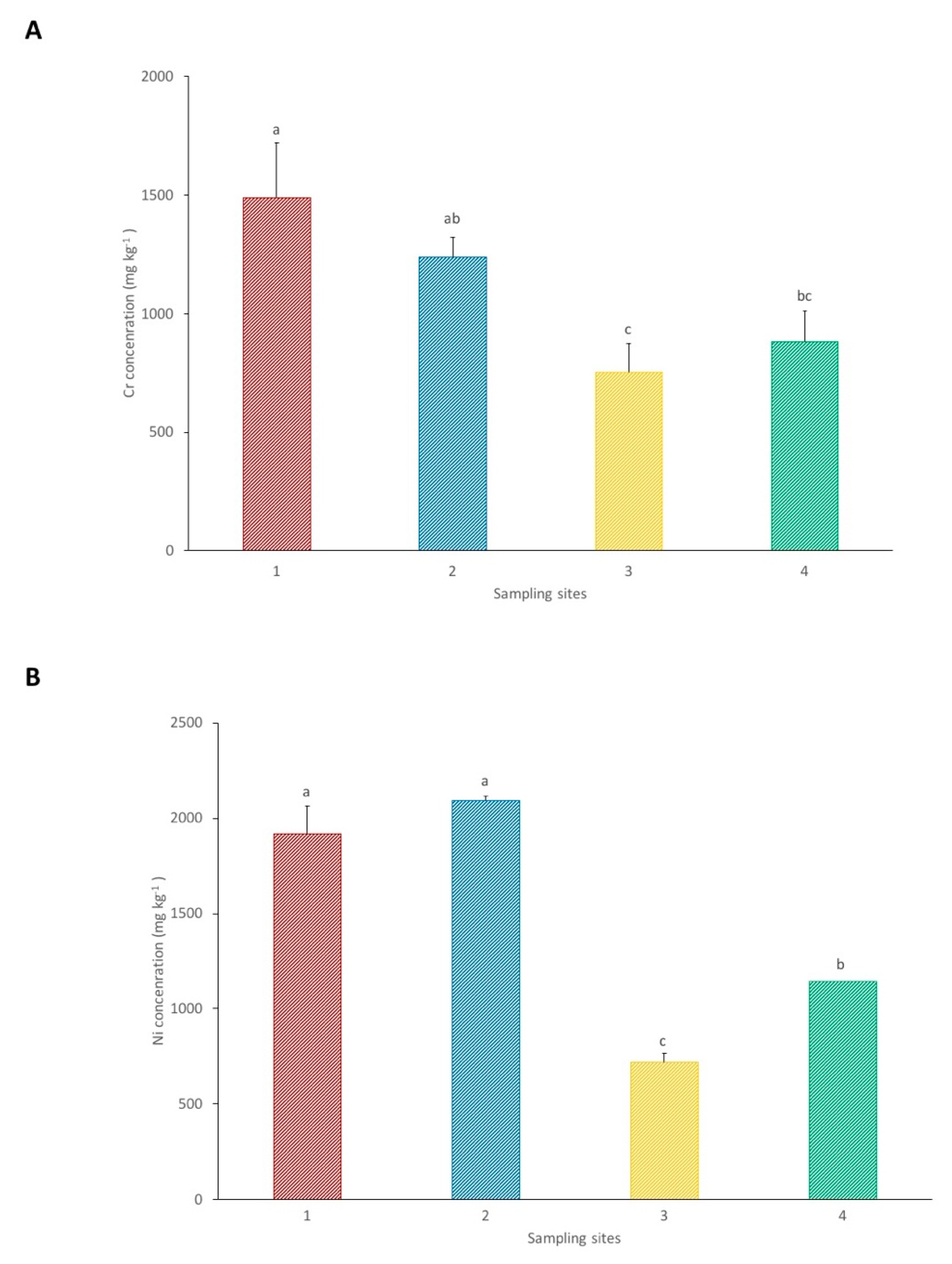
Figure 2.
Pseudo total concentrations of Cr (A) and Ni (B) in the soils from each sampling site. Each value represents the mean of three replicate measurements ± SD. Different letters indicate significant differences between sites at p < 0.05.
The results of the pseudo total concentrations of Cr and Ni in the soil from each sampling site are presented in Figure 2. Chromium concentrations are significantly lower in site 3 in comparison with the other sites, with a mean value of 753 mg kg−1. Sampling site 1 presents a significantly higher Cr concentration in relation to site 4, with 1491 and 881 mg kg−1, respectively. The results from site 2, with a mean of 1240 mg kg−1, do not show significant differences in comparison to sites 1 and 4.
Regarding Ni concentrations, sites 1 and 2, with 1918 and 2092 mg kg−1, respectively, present significantly higher values in comparison with sites 3 and 4. Site 3 shows significantly lower concentrations of Ni, with a mean of 721 mg kg−1, in relation to site 4, which presents a Ni concentration of 1145 mg kg−1.
Table 2 shows the results of some physicochemical characteristics of the ultramafic soil in the Morais complex and the values obtained from studies in other ultramafic areas located in temperate regions [1]. The values representing Morais correspond to the ones obtained at site number 2, since it is the location analyzed with the highest Ni pseudo-total concentration in the soil. The Melide complex is close to the village of Eidián in Galicia (Northwest Spain), and it corresponds to an ultramafic area located very close to the Morais ultramafic complex [1]. As aforementioned, ultramafic soils present low Ca:Mg ratios, scarcity of macronutrients, pH close to neutrality, and elevated levels of metals such as Ni and Cr. Still, the ranges of these common features can vary at different locations, as the formation of soils depends not only on the nature and the abundance of the weathered parent material (e.g., Cr-bearing minerals are essentially chromite and magnetite, and to a lower extent serpentinite and pyroxene) but also on climate, topography, biota, and time [34]. Interestingly, data from Table 2 show a strong correlation between pH and Ni concentrations in soil (r = 0.975, p < 0.01).

Table 2.
Physicochemical characteristics of the soils from different ultramafic areas located in temperate regions.
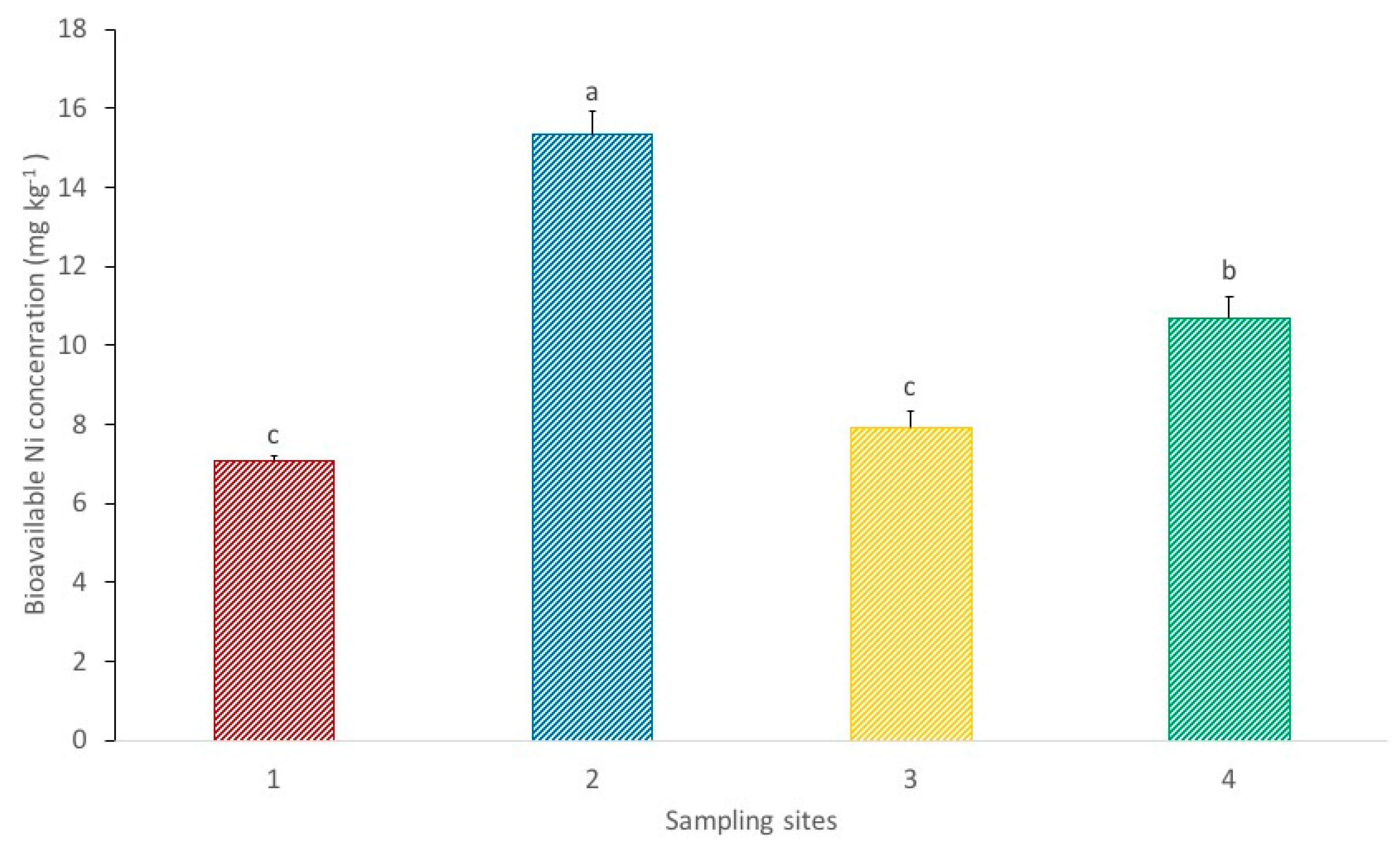
Figure 3 shows the bioavailable Ni concentrations in the soil from each sampling site. Site 2 presents a significantly higher bioavailable concentration in comparison to the other sampling sites. Nickel bioavailable concentrations between sites 1 and 3 do not show significant differences and are both significantly lower in relation to site 4. These results show that, despite the higher Ni pseudo total concentrations in the soil from site 1, soil collected at site 4 has greater Ni bioavailability. The bioavailability of metals in soil depends on various factors, including the metal pseudo-total concentrations in the soil and the pH. The soil pH affects the solubility of trace elements and therefore is considered a major factor influencing Ni bioavailability (metal bioavailability dwindles with increasing pH) [32]. According to the results presented in Table 1, site number 1 shows a significantly higher pH value in comparison to the other sampling sites, meaning the soil is less acidic and consequently Ni is less bioavailable. This is in conformity with the results presented in Figure 3. Hence, the greater bioavailability of Ni in sites 2 and 4 may be explained by the lower pH values at both points (particularly at the latter). The elevated bioavailability of Ni at site 2 in comparison to sites 1 and 3 is promoted by its lower pH and higher Ni pseudo-total contents, respectively.
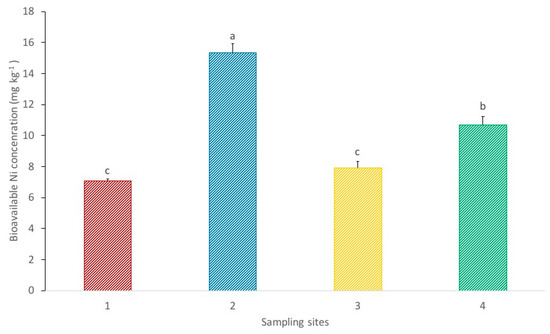
Figure 3.
Bioavailable Ni concentrations in the soils from each sampling site. Each value represents the mean of three replicate measurements ± SD. Different letters indicate significant differences between sites at p < 0.05.
The Cr bioavailability values from each sampling site cannot be discussed because the corresponding results were under the detection limits. Hence, it stands to reason that the selected extraction method (NH4Ac, pH 7.0) may not be reliable to assess Cr bioavailability in ultramafic soils where its solubility can be influenced by high pH values. Further research should consider additional extraction methods to determine Cr bioavailability, such as DTPA, NH4Ac (pH 4.5), CaCl2 and Sr(NO3)2 [35].
3.2. Plants
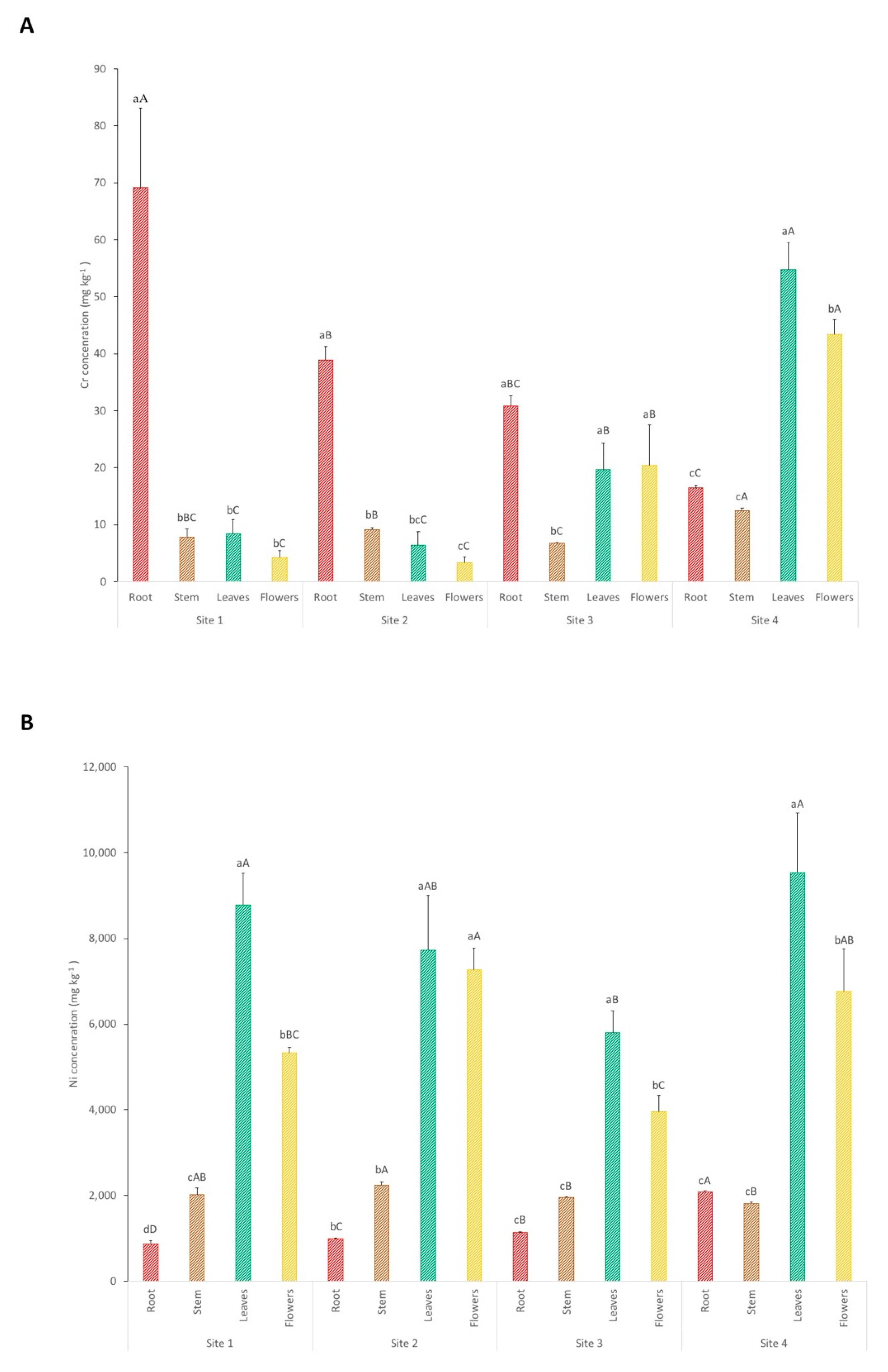
The results of the concentrations of Cr and Ni in the different plant tissues from each sampling site are presented in Figure 4. Regarding Cr concentrations in site 1, plants accumulate significantly higher amounts of Cr in the roots (69 mg kg−1) in comparison to the other tissues (Cr concentrations in stem, leaves, and flowers combined make a total of 20 mg kg−1). Likewise, site 2 accumulates 39 mg kg−1 in roots and a total concentration of 19 mg kg−1 in the other tissues. Shanker et al. reported that the maximum quantity of this element (Cr is a non-essential element for plants) is contained in roots, the reason being its immobilization and its inability to translocate to leaves and flowers for the plant to avoid toxicity [29]. However, this is not the case for plants collected in sites 3 and 4. Chromium concentrations in plant roots from site 3 (31 mg kg−1) show no significant differences in comparison with concentrations found in leaves and flowers from the same site. Plants from site 4 accumulate significantly higher Cr concentrations in leaves and flowers, 55 and 43 mg kg−1, respectively, in comparison to the amounts of Cr in roots (17 mg kg−1). Studies show that mycorrhizae and organic acids in the rhizosphere play important roles in increasing Cr translocation to shoots [36,37]. This may be the case for sites 3 and 4, since Ca values in these sites are significantly greater, and the general concentrations of heavy metals (Cr and Ni) are lower. Hence, the rhizosphere is under less environmental stress and therefore is richer with greater amounts of microorganisms and consequently a more adequate environment that allows Cr translocation in plants. It is also important to point out that the molecules responsible for Ni uptake can be the same for Cr, thus extreme concentrations of this elements in the soil may overload the molecules responsible for uptake and translocation [29].
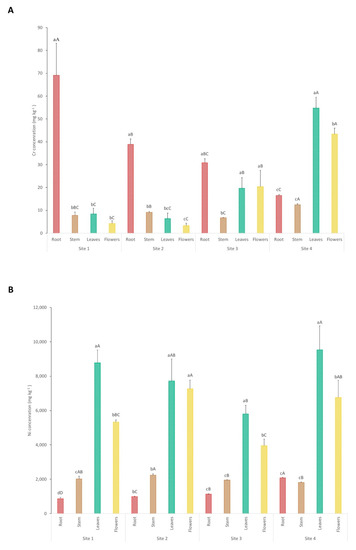
Figure 4.
Cr (A) and Ni (B) concentrations in the different plant tissues from each sampling site. Each value represents the mean of three replicate measurements ± SD. Different lowercase letters indicate significant differences between different plant parts for each point; different uppercase letters indicate significant differences between sampling sites for each plant part, p < 0.05.
Regarding Ni concentrations in plant tissues, they are always significantly higher in leaves and flowers in comparison to stems and roots. In sites 1, 3, and 4, the Ni concentrations in leaves are significantly higher, with means of 8784, 5797, and 9545 mg kg−1, respectively, in comparison to the values accumulated in flowers. The Ni concentration values in leaves are in agreement with other results reported for the same species [13]. Flowers from sites 1, 3, and 4 show Ni concentrations of 5333, 3961, and 6758 mg kg−1, respectively. Leaves and flowers from site 2 present no significant differences between them in terms of Ni accumulation, with 7723 and 7264 mg kg−1, respectively. These results are expected, since other studies show that the leaves are the main storage organ where the majority of Ni is accumulated [38]. Groeber et al. reported that flowers of hyperaccumulator plants can accumulate Ni concentrations as high as that in leaves, which is in agreement with this study [39]. The plants from sites 1, 2, and 4 show a greater Ni accumulation, which indicates that, despite site 4 having lower Ni concentrations in the soil, the bioavailability of this metal plus other soil chemical characteristics (such as higher Ca concentration and lower Cr values) makes this site equally suitable for Ni phytomining.
Table 3 shows the harvestable amounts of nickel in plants from each site as well as the translocation factor (TF) and the bioconcentration factor (BF). The BF indicates the capacity of a plant to extract metals from the soil, and the TF shows the plant competence to translocate metals from the root to the shoot [23]. The results confirm that the sites with most potential for phytomining are sites 1, 2, and 4, since the HA of Ni is significantly higher. The TF is significantly greater in sites 1 and 2 in comparison to the other sampling sites. It should be highlighted that the lower TF value observed in site 4 may be ascribed to the competition between Ni and Cr uptake, since shoot Cr levels are significantly higher at this site. The BF is greater at sites 3 and 4, suggesting that lower concentrations of metals in soils (such as Cr) make the uptake of Ni from soil by the plant more efficient.

Table 3.
Nickel harvestable amount (HA), translocation factor (TF), and bioconcentration factor (BF) in plants.
4. Conclusions
This study shows that the more appropriate sites for nickel phytomining are sites 1, 2, and 4, because the plants present the highest nickel harvestable amount values, meaning that the process could have a greater economic return in these sites. However, plants from site 3 also hyperaccumulate considerable amounts of Ni. It is important to consider that biomass yield and metal concentrations of plant shoots are crucial for phytomining, because they govern the quantity of metal to be harvested from each plant (harvestable amount) [40].
Future research will encompass further laboratory and field trials in order to assess the viability of the phytomining process at the Morais massif. More specifically, different agronomic aspects such as plant density, cropping patterns, fertilization regimes, bioaugmentation with plant growth-promoting bacteria, and application of phytohormones, to list a few, should be addressed [1,41]. If the upcoming studies show promising results concerning potential nickel yield and profitability, the implementation of a nickel agromining system in the Morais massif could represent an additional source of income to local farmers, since this serpentinite area has low productivity for food production.
Author Contributions
Conceptualization, L.A.B.N. and E.F.S.; field sampling, L.A.B.N. and E.F.S.; Laboratory analyses, L.A.B.N. and A.R.A.A.; writing—original draft preparation, A.R.A.A.; writing—review and editing, L.A.B.N. and E.F.S.; All authors have read and approved the final manuscript.
Funding
This research was funded by the ERDF Interreg Sudoe Program (PhytoSUDOE-SOE1/P5/E0189) and the Portuguese Foundation for Science and Technology (FCT; UID/GEO/04035/2019).
Acknowledgments
The authors gratefully acknowledge the support provided by the representatives of the Morais parish.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kidd, P.S.; Bani, A.; Benizri, E.; Gonnelli, C.; Hazotte, C.; Kisser, J.; Konstantinou, M.; Kuppens, T.; Kyrkas, D.; Laubie, B.; et al. Developing Sustainable Agromining Systems in Agricultural Ultramafic Soils for Nickel Recovery. Front. Environ. Sci. 2018, 6, 44. [Google Scholar] [CrossRef]
- Kruckeberg, A.R. Geology and Plant Life: The Effects of Landforms and Rock Types on Plants; University of Washington Press: Seattle, WA, USA, 2004; ISBN 9780295984520. [Google Scholar]
- Alves, S.; Trancoso, M.A.; de Lurdes Simões Gonçalves, M.; Correia dos Santos, M.M. A nickel availability study in serpentinised areas of Portugal. Geoderma 2011, 164, 155–163. [Google Scholar] [CrossRef]
- Reeves, R.D. Hyperaccumulation of nickel by serpentine plants. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept: Andover, UK, 1992; pp. 253–277. [Google Scholar]
- Sobczyk, M.K.; Smith, J.A.C.; Pollard, A.J.; Filatov, D.A. Evolution of nickel hyperaccumulation and serpentine adaptation in the Alyssum serpyllifolium species complex. Heredity 2017, 118, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Nkrumah, P.N.; Baker, A.J.M.; Chaney, R.L.; Erskine, P.D.; Echevarria, G.; Morel, J.L.; van der Ent, A. Current status and challenges in developing nickel phytomining: An agronomic perspective. Plant Soil 2016, 406, 55–69. [Google Scholar] [CrossRef]
- Brooks, R.R. Serpentine and Its Vegetation: A Multidisciplinary Approach; Dioscorides Press: Portland, OR, USA, 1988; Volume 40. [Google Scholar]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary Ecology of Plant Adaptation to Serpentine Soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Whiting, S.N.; Reeves, R.D.; Baker, A.J.M. Mining, metallophytes and land reclamation. Min. Environ. Manag. 2002, 10, 11–16. [Google Scholar]
- Alves, S.; Nabais, C.; de Lurdes Simões Gonçalves, M.; Correia dos Santos, M.M. Nickel speciation in the xylem sap of the hyperaccumulator Alyssum serpyllifolium ssp. lusitanicum growing on serpentine soils of northeast Portugal. J. Plant Physiol. 2011, 168, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Díez Lázaro, J.; Kidd, P.S.; Monterroso Martínez, C. A phytogeochemical study of the Trás-os-Montes region (NE Portugal): Possible species for plant-based soil remediation technologies. Sci. Total Environ. 2006, 354, 265–277. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Gonçalves, M.T.; Gonçalves, S.C.; Portugal, A.; Silva, S.; Sousa, J.P.; Freitas, H. Effects of nickel hyperaccumulation in Alyssum pintodasilvae on model arthropods representatives of two trophic levels. Plant Soil 2007, 293, 177–188. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Tappero, R.V.; Maugel, T.K.; Erbe, E.F.; Sparks, D.L.; Chaney, R.L. Interaction of nickel and manganese in accumulation and localization in leaves of the Ni hyperaccumulators Alyssum murale and Alyssum corsicum. Plant Soil 2009, 314, 35–48. [Google Scholar] [CrossRef]
- Goolsby, E.W.; Mason, C.M. Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.S.; Martens, S.N. The raison d’être for metal hyperaccumulation by plants. In The Vegetation of Ultra- mafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept Limited: Andover, MA, USA, 1992; pp. 279–289. [Google Scholar]
- Minguzzi, C.; Vergnano, O. Il contenuto di nichel nelle ceneri di Alyssum bertolonii. Atti della Soc. Toscana di Sci. Nat. A 1948, 55, 49–77. [Google Scholar]
- Brooks, R.R.; Lee, J.; Reeves, R.D.; Jaffre, T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 1977, 7, 49–57. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.J.M.; Brooks, R.R. Terrestrial higher plants which hyperaccumulate metallic elements—A review of their distribution, ecology and phytochemistry. Biorecovery 1989, 1, 81–126. [Google Scholar]
- Dudley, T.R. A new Portuguese subspecies of Alyssum serpyllifolium Desfontaines. Agron. Lusit. 1967, 28, 69–76. [Google Scholar]
- Pinto da Silva, A.R. A flora e a vegetação das áreas ultrabásicas do nordeste transmontano. Agron. Lusit. 1970, 30, 175–364. [Google Scholar]
- Novo, L.A.B.; Castro, P.M.L.; Alvarenga, P.; da Silva, E.F. Phytomining of Rare and Valuable Metals. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., R. Lanza, G., Newman, L., Eds.; Springer International Publishing: Cham, Germany, 2017; pp. 469–486. ISBN 978-3-319-52379-8. [Google Scholar]
- Brooks, R.R.; Chambers, M.F.; Nicks, L.J.; Robinson, B.H. Phytomining. Trends Plant Sci. 1998, 3, 359–362. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Chaney, R.L.; Anderson, C.W.N.; Meech, J.A.; Erskine, P.D.; Simonnot, M.-O.; Vaughan, J.; Morel, J.L.; et al. Agromining: Farming for Metals in the Future? Environ. Sci. Technol. 2015, 49, 4773–4780. [Google Scholar] [CrossRef]
- Ribeiro, A.; Quesada, C.; Dallmeyer, R.D. Geodynamic evolution of the Iberian Massif. In Pre-Mesozoic Geology of Iberia; Dallmeyer, R.D., Martínez-Garcia, E., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 397–410. [Google Scholar]
- Santos, F.J.; Ibarguchi, J.I.G.; Pin, C.; Paquette, J.L. Composite origin of an early Variscan transported suture: Ophiolitic units of the Morais Nappe Complex (north Portugal). Tectonics 2006, 25, 1–19. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Ghaderian, S.M.; Rodríguez-Garrido, B.; Prieto-Fernández, Á.; Kidd, P.S. Plant species-specificity and effects of bioinoculants and fertilization on plant performance for nickel phytomining. Plant Soil 2018, 425, 265–285. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Robinson, B.H.; Chiarucci, A.; Brooks, R.R.; Petit, D.; Kirkman, J.H.; Gregg, P.E.H.; De Dominicis, V. The nickel hyperaccumulator plant Alyssum bertolonii as a potential agent for phytoremediation and phytomining of nickel. J. Geochem. Explor. 1997, 59, 75–86. [Google Scholar] [CrossRef]
- Jones, J.B. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9780849302060. [Google Scholar]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Phytomining: A review. Miner. Eng. 2009, 22, 1007–1019. [Google Scholar] [CrossRef]
- Romero-Freire, A.; Olmedo-Cobo, J.; Gómez-Zotano, J. Elemental Concentration in Serpentinitic Soils over Ultramafic Bedrock in Sierra Bermeja (Southern Spain). Minerals 2018, 8, 447. [Google Scholar] [CrossRef]
- Vithanage, M.; Kumarathilaka, P.; Oze, C.; Karunatilake, S.; Seneviratne, M.; Hseu, Z.-Y.; Gunarathne, V.; Dassanayake, M.; Ok, Y.S.; Rinklebe, J. Occurrence and cycling of trace elements in ultramafic soils and their impacts on human health: A critical review. Environ. Int. 2019, 131, 104974. [Google Scholar] [CrossRef]
- van der Ent, A.; Nkrumah, P.N.; Tibbett, M.; Echevarria, G. Evaluating soil extraction methods for chemical characterization of ultramafic soils in Kinabalu Park (Malaysia). J. Geochem. Explor. 2019, 196, 235–246. [Google Scholar] [CrossRef]
- Davies, F.T.; Puryear, J.D.; Newton, R.J.; Egilla, J.N.; Saraiva Grossi, J.A. Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus). J. Plant Physiol. 2001, 158, 777–786. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhu, Z.X.; He, Z.Y. Pollution behaviour of organic Cr(III) complexes in soil–plant system. Chin. J. Appl. Ecol. 1994, 5, 187–191. [Google Scholar]
- Deng, T.-H.-B.; van der Ent, A.; Tang, Y.-T.; Sterckeman, T.; Echevarria, G.; Morel, J.-L.; Qiu, R.-L. Nickel hyperaccumulation mechanisms: A review on the current state of knowledge. Plant Soil 2018, 423, 1–11. [Google Scholar] [CrossRef]
- Groeber, S.; Przybyłowicz, W.; Echevarria, G.; Montarges-Pelletier, E.; Barnabas, A.; Mesjasz-Przybyłowicz, J. Fate of nickel and calcium in seedlings of the hyperaccumulator Berkheya coddii during germination. Biol. Plant. 2015, 59, 560–569. [Google Scholar] [CrossRef]
- Novo, A.B. Plants to harvest rhenium: Scientific and economic viability. Environ. Chem. Lett. 2015, 13, 439–445. [Google Scholar] [CrossRef]
- Bani, A.; Echevarria, G.; Sulçe, S.; Morel, J.L. Improving the Agronomy of Alyssum murale for Extensive Phytomining: A Five-Year Field Study. Int. J. Phytoremediation 2015, 17, 117–127. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).