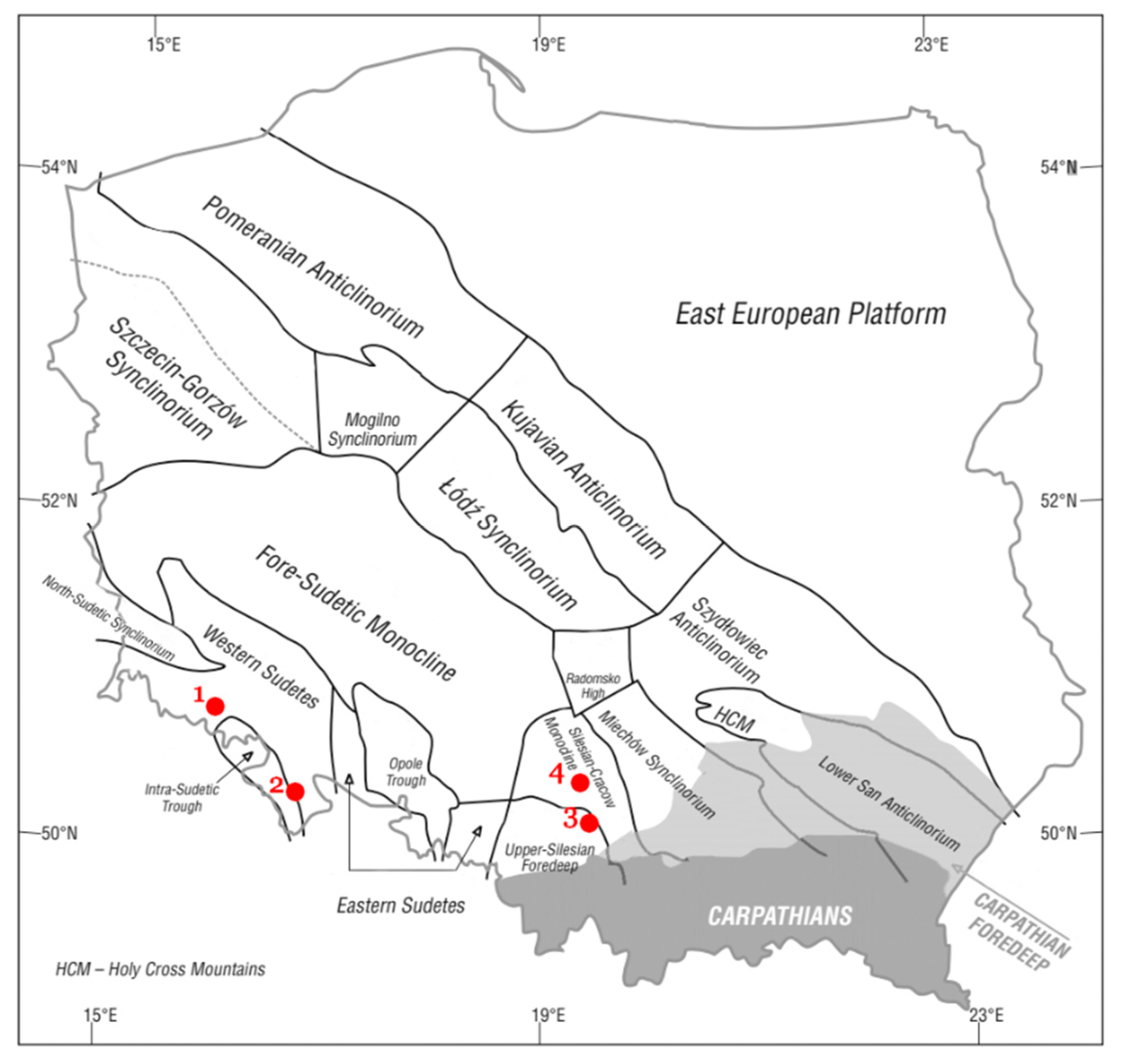
Figure 1.
The locations of dolomite deposits from which materials were collected for research on the background the main tectonic units on the sub-Cenozoic surface of the Polish [
19]. Explanations: 1—Rędziny deposit; 2—Romanowo Górne deposit; 3—Żelatowa deposit; 4—Chruszczobród II deposit.
Figure 1.
The locations of dolomite deposits from which materials were collected for research on the background the main tectonic units on the sub-Cenozoic surface of the Polish [
19]. Explanations: 1—Rędziny deposit; 2—Romanowo Górne deposit; 3—Żelatowa deposit; 4—Chruszczobród II deposit.
Figure 2.
The dolomites from the Rędziny deposit: (a) anhedral, often repeatedly twinned crystals dolomite; (b) irregular enclave filled with serpentine minerals (grey colours). Polarizing microscope, Xp.
Figure 2.
The dolomites from the Rędziny deposit: (a) anhedral, often repeatedly twinned crystals dolomite; (b) irregular enclave filled with serpentine minerals (grey colours). Polarizing microscope, Xp.
Figure 3.
The phase composition of dolomite from the Romanowo Górne deposit is shown in the diffraction pattern. Explanations: A—albite (NaAlSi3O8), D—dolomite (CaMg(CO3)2); C—Calcite (CaCO3); F—flogopite (K2(Mg,Fe2+)6[(OH,F)4/Al2Si6O20); W—vermiculite ((Mg,Ca)(Mg,Fe3+,Al)6[(OH)4/Al,Si)8O20]∙8H2O); Q—quartz (SiO2).
Figure 3.
The phase composition of dolomite from the Romanowo Górne deposit is shown in the diffraction pattern. Explanations: A—albite (NaAlSi3O8), D—dolomite (CaMg(CO3)2); C—Calcite (CaCO3); F—flogopite (K2(Mg,Fe2+)6[(OH,F)4/Al2Si6O20); W—vermiculite ((Mg,Ca)(Mg,Fe3+,Al)6[(OH)4/Al,Si)8O20]∙8H2O); Q—quartz (SiO2).
Figure 4.
The dolomites from the Chruszczobród II deposit: (a) the relics of allochemic components forming the skeleton of the rock (arrow); (b) the rock pores’ opal filling. Polarizing microscope, Ip (a), Xp (b).
Figure 4.
The dolomites from the Chruszczobród II deposit: (a) the relics of allochemic components forming the skeleton of the rock (arrow); (b) the rock pores’ opal filling. Polarizing microscope, Ip (a), Xp (b).
Figure 5.
The dolomites from the Chruszczobród II deposit: (a) the relics of carbonate bioclasts (arrows), the iron compounds of the iron oxides–hydroxides nature (brown colour), rock pores stained with blue dye; (b) the dolomite crystals of a rhombohedral habit (arrow). Polarizing microscope, Ip (a), Xp (b).
Figure 5.
The dolomites from the Chruszczobród II deposit: (a) the relics of carbonate bioclasts (arrows), the iron compounds of the iron oxides–hydroxides nature (brown colour), rock pores stained with blue dye; (b) the dolomite crystals of a rhombohedral habit (arrow). Polarizing microscope, Ip (a), Xp (b).
Figure 6.
The phase composition of dolomite from the Żelatowa deposit is shown on the diffraction pattern. Explanations: D—dolomite (CaMg(CO3)2); DFe—ferruginous dolomite Ca(Mg,Fe)(CO3)2; Q—quartz (SiO2).
Figure 6.
The phase composition of dolomite from the Żelatowa deposit is shown on the diffraction pattern. Explanations: D—dolomite (CaMg(CO3)2); DFe—ferruginous dolomite Ca(Mg,Fe)(CO3)2; Q—quartz (SiO2).
Figure 7.
The dolomites from the Żelatowa deposit. The visible recrystallized carbonate grains; (a) polarizing microscope, Xp; (b) cathodoluminescence (CL).
Figure 7.
The dolomites from the Żelatowa deposit. The visible recrystallized carbonate grains; (a) polarizing microscope, Xp; (b) cathodoluminescence (CL).
Figure 8.
The SEM images of dolomite grain from the Chruszczobród II deposit (a,c) and industrial sorbent produced from limestones (b,d) in cross-section after the sulfation process in the light of backscattered electrons. Visible: (a,c)—evenly sulfated interior of the dolomite grain; (b,d)—non-sulfated grain interior of industrial sorbent; (c,d)—the S distribution is marked in yellow (SEM/EDX).
Figure 8.
The SEM images of dolomite grain from the Chruszczobród II deposit (a,c) and industrial sorbent produced from limestones (b,d) in cross-section after the sulfation process in the light of backscattered electrons. Visible: (a,c)—evenly sulfated interior of the dolomite grain; (b,d)—non-sulfated grain interior of industrial sorbent; (c,d)—the S distribution is marked in yellow (SEM/EDX).
Figure 9.
The thermal curves of differential thermal analysis (DTA), thermogravimetry (TG), and derivative thermogravimetry (DTG) of dolomite from the Chruszczobród II deposit, showing the course of the thermal dissociation process.
Figure 9.
The thermal curves of differential thermal analysis (DTA), thermogravimetry (TG), and derivative thermogravimetry (DTG) of dolomite from the Chruszczobród II deposit, showing the course of the thermal dissociation process.
Figure 10.
The phase composition of dolomites from the Chruszczobród II deposit after the decarbonation process at the temperature of 850 °C is shown in the diffraction pattern. Explanations: A—anhydrite (CaSO4), C—calcite (CaCO3), L—lime (CaO), P—periclase (MgO), Po—portlandite (Ca(OH)2), Q—quartz (SiO2).
Figure 10.
The phase composition of dolomites from the Chruszczobród II deposit after the decarbonation process at the temperature of 850 °C is shown in the diffraction pattern. Explanations: A—anhydrite (CaSO4), C—calcite (CaCO3), L—lime (CaO), P—periclase (MgO), Po—portlandite (Ca(OH)2), Q—quartz (SiO2).
Figure 11.
The distribution of pore volume as a function of the diameters of industrial sorbent made of limestone. Explanations: natural sample; sample after the thermal dissociation process; sample after the sulfation process.
Figure 11.
The distribution of pore volume as a function of the diameters of industrial sorbent made of limestone. Explanations: natural sample; sample after the thermal dissociation process; sample after the sulfation process.
Figure 12.
The distribution of pore volume as a function of the diameters of dolomite sorbent made from the Chruszczobród II deposit. Explanations: natural sample; sample after the thermal dissociation process; sample after the sulfation process.
Figure 12.
The distribution of pore volume as a function of the diameters of dolomite sorbent made from the Chruszczobród II deposit. Explanations: natural sample; sample after the thermal dissociation process; sample after the sulfation process.
Figure 13.
The SEM image of the dolomite surface morphology from the Chruszczobród II deposit before decarbonation (a) and after decarbonation (b), in the light of backscattered electrons.
Figure 13.
The SEM image of the dolomite surface morphology from the Chruszczobród II deposit before decarbonation (a) and after decarbonation (b), in the light of backscattered electrons.
Figure 14.
The SEM images of the industrial sorbent morphology before decarbonation (a) and after decarbonation (b), in the light of backscattered electrons.
Figure 14.
The SEM images of the industrial sorbent morphology before decarbonation (a) and after decarbonation (b), in the light of backscattered electrons.
Figure 15.
The SEM images of the surface morphology of desulfurization products produced on the surface of dolomite grains from the Chruszczobród II deposit (a) and industrial sorbent (b) in the light of backscattered electrons.
Figure 15.
The SEM images of the surface morphology of desulfurization products produced on the surface of dolomite grains from the Chruszczobród II deposit (a) and industrial sorbent (b) in the light of backscattered electrons.
Figure 16.
The phase composition of the dolomite sulfation product is shown in the diffraction pattern (on the example of dolomites from the Chruszczobród II deposit). Explanations: A—anhydrite (CaSO4), CMS—calcium and magnesium sulfate (CaMg2(SO4)3), Q—quartz (SiO2).
Figure 16.
The phase composition of the dolomite sulfation product is shown in the diffraction pattern (on the example of dolomites from the Chruszczobród II deposit). Explanations: A—anhydrite (CaSO4), CMS—calcium and magnesium sulfate (CaMg2(SO4)3), Q—quartz (SiO2).
Figure 17.
The thermal curves show the TG and DTG of dolomite sulfation products from the Chruszczobród II deposit.
Figure 17.
The thermal curves show the TG and DTG of dolomite sulfation products from the Chruszczobród II deposit.
Table 1.
The list of dolomites used for the evaluation of sorption properties in relation to SO2 in the conditions of fluid combustion technology.
Table 1.
The list of dolomites used for the evaluation of sorption properties in relation to SO2 in the conditions of fluid combustion technology.
| Sample Number | 1. | 2. | 3. | 4. |
|---|
| Origin | Dolomite mined from the Rędziny deposit | Dolomite mined from the Romanowo Górne deposit | Dolomite mined from the Żelatowa deposit | Dolomite mined from the Chruszczobród II deposit |
| Age | Sylurian | Precambrian | Triassic | Triassic |
Table 2.
The reference values of the reactivity (RI) (Ca moles/S moles) and the absolute sorption (CI) (g S/1000 g of the sorbent) [
20].
Table 2.
The reference values of the reactivity (RI) (Ca moles/S moles) and the absolute sorption (CI) (g S/1000 g of the sorbent) [
20].
| The Sorption Capacity of the Sorbent | RI (kmol Ca/kmol S) | CI (g S/1 kg of Sorbent) |
|---|
| Excellent | <2.5 | >120 |
| Very good | 2.5–3.0 | 100–120 |
| Good | 3.0–4.0 | 80–00 |
| Sufficient | 4.0–5.0 | 60–80 |
| Low quality | >5.0 | <60 |
Table 3.
Chemical composition of the tested dolomites and industrial sorbent (% wt.).
Table 3.
Chemical composition of the tested dolomites and industrial sorbent (% wt.).
| Component | Dolomite from the Rędziny Deposit (1) | Dolomite from the Żelatowa Deposit (2) | Dolomite from the Romanowo Górne Deposit (3) | Dolomite from the Chruszczobród II Deposit (4) | Industrial Sorbent (5) |
|---|
| SiO2 | 0.15 | 0.32 | 2.87 | 0.82 | 0.53 |
| TiO2 | >0.001 | 0.050 | >0.001 | 0.006 | >0.001 |
| Al2O3 | 0.25 | 0.22 | 0.13 | 0.06 | 0.28 |
| Fe2O3 | 0.48 | 0.86 | 0.09 | 0.53 | 0.24 |
| CaO | 27.54 | 32.01 | 31.57 | 26.94 | 54.58 |
| MgO | 23.56 | 19.28 | 19.12 | 23.06 | 0.33 |
| MnO | 0.07 | 0.08 | 0.04 | 0.023 | 0.011 |
| Na2O | 0.04 | 0.06 | 0.14 | 0.035 | 0.014 |
| K2O | 0.007 | 0.01 | 0.08 | 0.006 | 0.005 |
| P2O5 | 0.01 | 0.02 | 0.01 | 0.055 | 0.028 |
| Ignition loss | 47.87 | 47.07 | 45.59 | 48.16 | 43.78 |
| SUM | 99.99 | 99.98 | 99.64 | 99.59 | 99.80 |
| CaCO3 | 49.02 | 56.99 | 56.19 | 48.06 | 97.37 |
| MgCO3 | 49.00 | 40.10 | 39.78 | 47.97 | 0.68 |
| Sum of carbonate | 98.03 | 97.08 | 95.96 | 96.03 | 98.05 |
Table 4.
The values of the absolute sorption (CI) (g S/1 kg sorbent) and reactivity (RI) (kmol Ca/kmol S) indicators of the dolomites and industrial sorbent tested.
Table 4.
The values of the absolute sorption (CI) (g S/1 kg sorbent) and reactivity (RI) (kmol Ca/kmol S) indicators of the dolomites and industrial sorbent tested.
| Indicator | Dolomite from Rędziny Deposit (1) | Dolomite from Żelatowa Deposit (2) | Dolomite from Romanowo Górne Deposit (3) | Dolomite from Chruszczobród II Deposit (4) | Industrial Sorbent (5) |
|---|
| CI | 197 | 180 | 168 | 174 | 130 |
| RI | 1.52 | 2.05 | 2.10 | 1.80 | 2.35 |
Table 5.
The thermal effects occurring during the heating of the dolomite in the temperature range up to 1000 °C on the example of the dolomite from the Chruszczobród II deposit.
Table 5.
The thermal effects occurring during the heating of the dolomite in the temperature range up to 1000 °C on the example of the dolomite from the Chruszczobród II deposit.
| Type of Process | Type of Reaction | Process/Reaction Temperature | Change of Weight |
|---|
| Beginning | End |
|---|
| (°C) | (°C) | (% by wt.) |
|---|
| Evaporation of surface water and hygroscopic moisture | – | 25 | 600 | – |
| Decomposition of CaMg(CO3)2 | endothermic | 600 | 790 | 21 |
| Formation of secondary CaCO3 | exothermic | 790 | 840 | 40 |
| Decomposition of CaCO3 | endothermic | 840 | 890 | 47 |
| – | – | 890 | 1000 | - |
Table 6.
Weight loss during heating of the tested dolomites (% wt.).
Table 6.
Weight loss during heating of the tested dolomites (% wt.).
| Temperature Range | Dolomite from Deposit |
|---|
| Rędziny (1) | Żelatowa (2) | Romanowo Górne (3) | Chruszczobród II (4) |
|---|
| 25–850 °C | 43.22 | 38.40 | 42.02 | 45.92 |
| 25–1000 °C | 44.40 | 41.84 | 44.51 | 47.02 |
The heating
850 °C for 0.5 h | 44.81 | 39.51 | 43.12 | 45.58 |
Table 7.
The parameters of the porous texture of dolomite from the Chruszczobród II deposit and industrial sorbent produced from limestone, determined with the use of mercury porosimetry.
Table 7.
The parameters of the porous texture of dolomite from the Chruszczobród II deposit and industrial sorbent produced from limestone, determined with the use of mercury porosimetry.
| Sample Symbol | The Total Pore Volume VPOR (cm3) | The Average Pore Diameter DPOR (μm) | The Specific Surface SPOR (m2/g) | The Effective Porosity PPOR (%) |
|---|
| Dolomite form the Chruszczobród II deposit |
| 1 | 0.57 | 3.77 | 0.47 | 54.87 * | 9.61 ** |
| 2 | 1.97 | 0.61 | 18.57 | 82.32 * | 42.76 ** |
| 3 | 0.46 | 9.02 | 0.21 | 55.39 * | 2.98 ** |
| Industrial sorbent |
| 1 | 0.34 | 4.98 | 0.24 | 43.02 * | 1.00 ** |
| 2 | 0.93 | 0.45 | 3.14 | 70.17 * | 28.16 ** |
| 3 | 0.35 | 0.56 | 0.52 | 32.80 * | 4.18 ** |
Table 8.
The characteristic sintering (TS), softening (TA), melting (TB), and flowing (TC) temperatures of dolomite sulfation products presented on the example of dolomites from the Chruszczobród II deposit (°C).
Table 8.
The characteristic sintering (TS), softening (TA), melting (TB), and flowing (TC) temperatures of dolomite sulfation products presented on the example of dolomites from the Chruszczobród II deposit (°C).
| Characteristic Temperature | Atmosphere |
|---|
| Oxidizing | Reducing |
|---|
| TS | 1150 | 1130 |
| TA | 1390 | >1500 |
| TB | >1500 | >1500 |
| TC | >1500 | >1500 |