D-Amino Acids as a Biomarker in Schizophrenia
Abstract
1. Introduction
2. D-Amino Acids in Brain
3. Relationship between D-Amino Acids and Schizophrenia
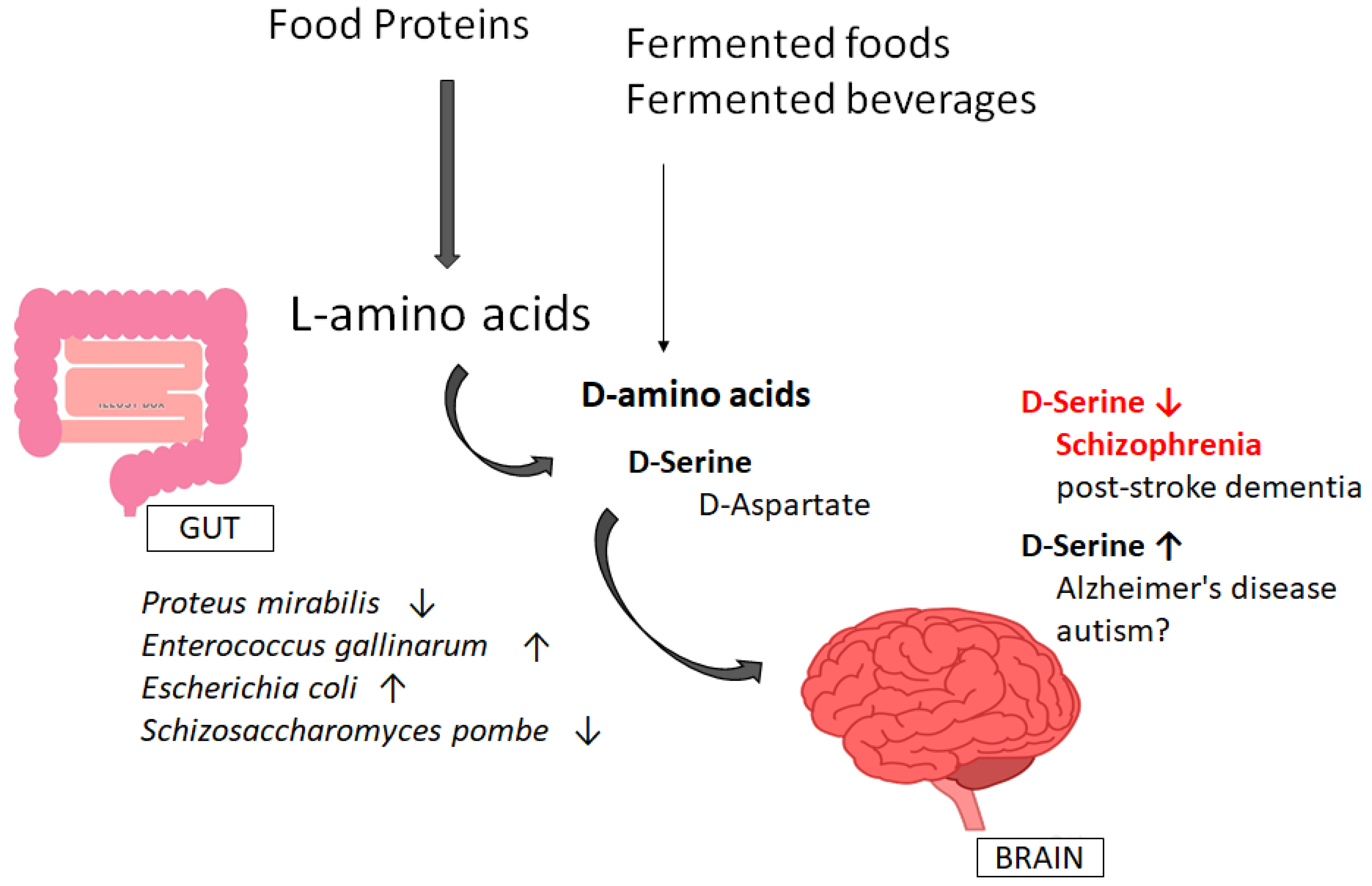
4. Involvement of Gut–Brain Axis via the Production of D-Amino Acids
5. D-amino Acids as a Useful Biomarker
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALS | amyotrophic lateral sclerosis |
| ASD | autism spectrum disorders |
| ATP | adenosine triphosphate |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DAO | D-amino acids oxidase |
| DAOA | DAO activator |
| DDO | D-Aspartate oxidase |
| DNA | deoxyribonucleic acid |
| LTP | long-term potentiation |
| miRNA | microRNA |
| mTOR | mammalian target of rapamycin |
| NMDA | N-methyl-D-aspartate |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Uda, K.; Abe, K.; Dehara, Y.; Mizobata, K.; Sogawa, N.; Akagi, Y.; Saigan, M.; Radkov, A.D.; Moe, L.A. Distribution and evolution of the serine/aspartate racemase family in invertebrates. Amino Acids 2016, 48, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Ritz-Timme, S.; Laumeier, I.; Collins, M.J. Aspartic acid racemization: Evidence for marked longevity of elastin in human skin. Br. J. Dermatol. 2003, 149, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, T.; Miroballo, M.; Casamassa, A.; Mancini, A.; Gaetani, L.; Nisticò, R.; Eusebi, P.; Katane, M.; Homma, H.; Calabresi, P.; et al. Cerebrospinal fluid and serum d-serine concentrations are unaltered across the whole clinical spectrum of Alzheimer’s disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140537. [Google Scholar] [CrossRef] [PubMed]
- Mutaguchi, Y.; Ohmori, T.; Akano, H.; Doi, K.; Ohshima, T. Distribution of D-amino acids in vinegars and involvement of lactic acid bacteria in the production of D-amino acids. SpringerPlus 2013, 27, 691. [Google Scholar] [CrossRef]
- Genchi, G. An overview on D-amino acids. Amino Acids 2017, 49, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Sekine-Hayakawa, Y.; Okiyama, A.; Ninomiya, Y.; Kawai, M. Gustatory sensation of (L)- and (D)-amino acids in humans. Amino Acids 2012, 43, 2349–2358. [Google Scholar]
- Kobayashi, J. d-Amino Acids and Lactic Acid Bacteria. Microorganisms 2019, 12, 690. [Google Scholar] [CrossRef]
- Friedman, M. Origin, microbiology, nutrition, and pharmacology of D-amino acids. Chem. Biodivers. 2010, 7, 1491–1530. [Google Scholar] [CrossRef]
- Sasabe, J.; Miyoshi, Y.; Rakoff-Nahoum, S.; Zhang, T.; Mita, M.; Davis, B.M.; Hamase, K.; Waldor, M.K. Interplay between microbial d-amino acids and host d-amino acid oxidase modifies murine mucosal defence and gut microbiota. Nat. Microbiol. 2016, 1, 16125. [Google Scholar] [CrossRef]
- Radkov, A.D.; Moe, L.A. Bacterial synthesis of D-amino acids. Appl. Microbiol. Biotechnol. 2014, 98, 5363–5374. [Google Scholar] [CrossRef]
- Hernández, S.B.; Cava, F. Environmental roles of microbial amino acid racemases. Environ. Microbiol. 2016, 18, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New Insights Into the Mechanisms and Biological Roles of D-Amino Acids in Complex Eco-Systems. Front. Microbiol. 2018, 6, 683. [Google Scholar] [CrossRef] [PubMed]
- Zilm, P.S.; Butnejski, V.; Rossi-Fedele, G.; Kidd, S.P.; Edwards, S.; Vasilev, K. D-amino acids reduce Enterococcus faecalis biofilms in vitro and in the presence of antimicrobials used for root canal treatment. PLoS ONE 2017, 12, e0170670. [Google Scholar] [CrossRef] [PubMed]
- Semenza, E.R.; Harraz, M.M.; Abramson, E.; Malla, A.P.; Vasavda, C.; Gadalla, M.M.; Kornberg, M.D.; Snyder, S.H.; Roychaudhuri, R. D-cysteine is an endogenous regulator of neural progenitor cell dynamics in the mammalian brain. Proc. Natl. Acad. Sci. USA 2021, 118, e2110610118. [Google Scholar] [CrossRef] [PubMed]
- Seckler, J.M.; Lewis, S.J. Advances in D-Amino Acids in Neurological Research. Int. J. Mol. Sci. 2020, 21, 7325. [Google Scholar] [CrossRef]
- Stuchlik, A.; Sumiyoshi, T. Cognitive deficits in schizophrenia and other neuropsychiatric disorders: Convergence of preclinical and clinical evidence. Front. Behav. Neurosci. 2014, 8, 444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsu, P.C.; Yang, U.C.; Shih, K.H.; Liu, C.M.; Liu, Y.L.; Hwu, H.G. A protein interaction based model for schizophrenia study. BMC Bioinform. 2008, 9 (Suppl. 12), S23. [Google Scholar] [CrossRef] [PubMed]
- Gnegy, M.E. Ca2+/calmodulin signaling in NMDA-induced synaptic plasticity. Crit. Rev. Neurobiol. 2000, 14, 91–129. [Google Scholar] [CrossRef]
- Sparey, T.; Abeywickrema, P.; Almond, S.; Brandon, N.; Byrne, N.; Campbell, A.; Hutson, P.H.; Jacobson, M.; Jones, B.; Munshi, S.; et al. The discovery of fused pyrrole carboxylic acids as novel, potent D-amino acid oxidase (DAO) inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 3386–3391. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, P.K.; Chang, Y.C.; Chuo, L.J.; Chen, Y.S.; Tsai, G.E.; Lane, H.Y. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: A randomized, double-blind, placebo-controlled trial. Biol. Psychiatry 2014, 75, 678–685. [Google Scholar] [CrossRef]
- Ikeda, H.; Nagasawa, M.; Yamaguchi, T.; Minaminaka, K.; Goda, R.; Chowdhury, V.S.; Yasuo, S.; Furuse, M. Disparities in activity levels and learning ability between Djungarian hamster (Phodopus sungorus) and Roborovskii hamster (Phodopus roborovskii). Anim. Sci. J. 2017, 88, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.A.; Berger, R.; Klomp, L.W.; de Koning, T.J. D-amino acids in the central nervous system in health and disease. Mol. Genet. Metab. 2005, 85, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qiao, H.; Wen, L.; Zhou, W.; Zhang, Y. D-serine enhances impaired long-term potentiation in CA1 subfield of hippocampal slices from aged senescence-accelerated mouse prone/8. Neurosci. Lett. 2005, 379, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Qiu, T.A.; Sweedler, J.V. d-Alanine: Distribution, origin, physiological relevance, and implications in disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140482. [Google Scholar] [CrossRef]
- Hamase, K.; Homma, H.; Takigawa, Y.; Fukushima, T.; Santa, T.; Imai, K. Regional distribution and postnatal changes of D-amino acids in rat brain. Biochim. Biophys. Acta 1997, 1334, 214–222. [Google Scholar] [CrossRef]
- Demuyser, T.; Bentea, E.; Deneyer, L.; Albertini, G.; Massie, A.; Smolders, I. Disruption of the HPA-axis through corticosterone-release pellets induces robust depressive-like behavior and reduced BDNF levels in mice. Neurosci. Lett. 2016, 626, 119–125. [Google Scholar] [CrossRef]
- D’Ascenzo, M.; Podda, M.V.; Grassi, C. The role of D-serine as co-agonist of NMDA receptors in the nucleus accumbens: Relevance to cocaine addiction. Front. Synaptic Neurosci. 2014, 6, 16. [Google Scholar]
- Suzuki, M.; Imanishi, N.; Mita, M.; Hamase, K.; Aiso, S.; Sasabe, J. Heterogeneity of D-Serine Distribution in the Human Central Nervous System. ASN Neuro 2017, 9, 1759091417713905. [Google Scholar] [CrossRef]
- Koga, R.; Miyoshi, Y.; Sakaue, H.; Hamase, K.; Konno, R. Mouse d-Amino-Acid Oxidase: Distribution and Physiological Substrates. Front. Mol. Biosci. 2017, 4, 82. [Google Scholar] [CrossRef]
- Paul, P.; de Belleroche, J. The role of D-amino acids in amyotrophic lateral sclerosis pathogenesis: A review. Amino Acids 2012, 43, 1823–1831. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Konno, R.; Sasabe, J.; Ueno, K.; Tojo, Y.; Mita, M.; Aiso, S.; Hamase, K. Alteration of intrinsic amounts of D-serine in the mice lacking serine racemase and D-amino acid oxidase. Amino Acids 2012, 43, 1919–1931. [Google Scholar] [CrossRef]
- Wolosker, H. The Neurobiology of d-Serine Signaling. Adv. Pharmacol. 2018, 82, 325–348. [Google Scholar]
- Coyle, J.T.; Balu, D.T. The Role of Serine Racemase in the Pathophysiology of Brain Disorders. Adv. Pharmacol. 2018, 82, 35–56. [Google Scholar]
- Yang, Y.; Ge, W.; Chen, Y.; Zhang, Z.; Shen, W.; Wu, C.; Poo, M.; Duan, S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. USA 2003, 100, 15194–15199. [Google Scholar] [CrossRef]
- Shimazaki, T.; Kaku, A.; Chaki, S. D-Serine and a glycine transporter-1 inhibitor enhance social memory in rats. Psychopharmacology 2010, 209, 263–270. [Google Scholar] [CrossRef]
- D’Aniello, S.; Somorjai, I.; Garcia-Fernàndez, J.; Topo, E.; D’Aniello, A. D-Aspartic acid is a novel endogenous neurotransmitter. FASEB J. 2011, 25, 1014–1027. [Google Scholar] [CrossRef]
- Roshanzamir, F.; Safavi, S.M. The putative effects of D-Aspartic acid on blood testosterone levels: A systematic review. Int. J. Reprod. Biomed. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Errico, F.; Napolitano, F.; Nisticò, R.; Usiello, A. New insights on the role of free D-aspartate in the mammalian brain. Amino Acids 2012, 43, 1861–1871. [Google Scholar] [CrossRef]
- Hashimoto, A.; Kumashiro, S.; Nishikawa, T.; Oka, T.; Takahashi, K.; Mito, T.; Takashima, S.; Doi, N.; Mizutani, Y.; Yamazaki, T.; et al. Embryonic development and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J. Neurochem. 1993, 61, 348–351. [Google Scholar] [CrossRef]
- Katane, M.; Kawata, T.; Nakayama, K.; Saitoh, Y.; Kaneko, Y.; Matsuda, S.; Saitoh, Y.; Miyamoto, T.; Sekine, M.; Homma, H. Characterization of the enzymatic and structural properties of human D-aspartate oxidase and comparison with those of the rat and mouse enzymes. Biol. Pharm. Bull. 2015, 38, 298–305. [Google Scholar] [CrossRef]
- Sun, G.C.; Lee, Y.J.; Lee, Y.C.; Yu, H.F.; Wang, D.C. Exercise prevents the impairment of learning and memory in prenatally phthalate-exposed male rats by improving the expression of plasticity-related proteins. Behav. Brain Res. 2021, 413, 113444. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, R.; Weinberger, D.R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017, 18, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Lancet 2018, 392, 1789–1858.
- Olney, J.W.; Farber, N.B. Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry 1995, 52, 998–1007. [Google Scholar] [CrossRef]
- Beneyto, M.; Kristiansen, L.V.; Oni-Orisan, A.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 2007, 32, 1888–1902. [Google Scholar] [CrossRef]
- Errico, F.; Mothet, J.P.; Usiello, A. D-Aspartate: An endogenous NMDA receptor agonist enriched in the developing brain with potential involvement in schizophrenia. J. Pharm. Biomed. Anal. 2015, 116, 7–17. [Google Scholar] [CrossRef]
- D’Aniello, A. D-Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef]
- Choi, K.H.; Wykes, T.; Kurtz, M.M. Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: Meta-analytical investigation of efficacy. Br. J. Psychiatry 2013, 203, 172–178. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wang, S.C.; Hwu, H.G.; Fann, C.S.; Yang, U.C.; Yang, W.C.; Hsu, P.C.; Chang, C.C.; Wen, C.C.; Tsai-Wu, J.J.; et al. Haplotypes of the D-Amino Acid Oxidase Gene Are Significantly Associated with Schizophrenia and Its Neurocognitive Deficits. PLoS ONE 2016, 11, e0150435. [Google Scholar] [CrossRef] [PubMed]
- Verrall, L.; Burnet, P.W.; Betts, J.F.; Harrison, P.J. The neurobiology of D-amino acid oxidase and its involvement in schizophrenia. Mol. Psychiatry 2010, 15, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Burnet, P.W.; Eastwood, S.L.; Bristow, G.C.; Godlewska, B.R.; Sikka, P.; Walker, M.; Harrison, P.J. D-amino acid oxidase activity and expression are increased in schizophrenia. Mol. Psychiatry 2008, 13, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Lim, K.S.; Cheng, A.; Garrick, T.; Kapoor, V. Preliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1). Brain Res. 2006, 1106, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Orzylowski, M.; Fujiwara, E.; Mousseau, D.D.; Baker, G.B. An Overview of the Involvement of D-Serine in Cognitive Impairment in Normal Aging and Dementia. Front. Psychiatry 2021, 12, 754032. [Google Scholar] [CrossRef] [PubMed]
- Errico, F.; Napolitano, F.; Squillace, M.; Vitucci, D.; Blasi, G.; de Bartolomeis, A.; Bertolino, A.; D’Aniello, A.; Usiello, A. Decreased levels of D-aspartate and NMDA in the prefrontal cortex and striatum of patients with schizophrenia. J. Psychiatr. Res. 2013, 47, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, T.; Sakai, Y.; Maeshima, H.; Hatano, T.; Hanzawa, R.; Abe, S.; Kida, S.; Shibata, N.; Suzuki, T.; Arai, H. Changes in plasma glycine, L-serine, and D-serine levels in patients with schizophrenia as their clinical symptoms improve: Results from the Juntendo University Schizophrenia Projects (JUSP). Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1905–1912. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, C.H.; Chang, Y.C.; Huang, Y.J.; Chen, P.W.; Yang, H.T.; Lane, H.Y. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Psychiatry 2018, 84, 422–432. [Google Scholar] [CrossRef]
- Harai, T.; Inoue, R.; Fujita, Y.; Tanaka, A.; Horio, M.; Hashimoto, K.; Hongou, K.; Miyawaki, T.; Mori, H. Decreased susceptibility to seizures induced by pentylenetetrazole in serine racemase knockout mice. Epilepsy Res. 2012, 102, 180–187. [Google Scholar] [CrossRef]
- Kim, S.H.; Shishido, Y.; Sogabe, H.; Rachadech, W.; Yorita, K.; Kato, Y.; Fukui, K. Age- and gender-dependent D-amino acid oxidase activity in mouse brain and peripheral tissues: Implication for aging and neurodegeneration. J. Biochem. 2019, 166, 187–196. [Google Scholar] [CrossRef]
- Taniguchi, K.; Ikeda, Y.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Implications of Gut-Brain axis in the pathogenesis of Psychiatric disorders. AIMS Bioeng. 2021, 8, 243–256. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kunisawa, A.; Hattori, T.; Kawana, S.; Kitada, Y.; Tamada, H.; Kawano, S.; Hayakawa, Y.; Iida, J.; Fukusaki, E. Free D-amino acids produced by commensal bacteria in the colonic lumen. Sci. Rep. 2018, 8, 17915. [Google Scholar] [CrossRef] [PubMed]
- Cava, F.; Lam, H.; de Pedro, M.A.; Waldor, M.K. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell. Mol. Life Sci. 2011, 68, 817–831. [Google Scholar] [CrossRef]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef]
- Kawase, T.; Nagasawa, M.; Ikeda, H.; Yasuo, S.; Koga, Y.; Furuse, M. Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br. J. Nutr. 2017, 117, 775–783. [Google Scholar] [CrossRef]
- Alam, R.; Abdolmaleky, H.M.; Zhou, J.R. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 651–660. [Google Scholar] [CrossRef]
- Tomasik, J.; Yolken, R.H.; Bahn, S.; Dickerson, F.B. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark. Insights 2015, 10, 47–54. [Google Scholar] [CrossRef]
- Nemani, K.; Hosseini Ghomi, R.; McCormick, B.; Fan, X. Schizophrenia and the gut-brain axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 155–160. [Google Scholar] [CrossRef]
- Hantsoo, L.; Zemel, B.S. Stress gets into the belly: Early life stress and the gut microbiome. Behav. Brain Res. 2021, 414, 113474. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, J.; Ke, P.; Kong, L.; Lei, B.; Zhou, J.; Huang, Y.; Li, H.; Li, G.; Chen, J.; et al. The gut microbiome is associated with brain structure and function in schizophrenia. Sci. Rep. 2021, 11, 9743. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Kuo, P.H.; Hsu, C.Y.; Chiu, Y.H.; Liu, Y.W.; Lu, M.L.; Chen, C.H. Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients 2021, 13, 3731. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Marwaha, S.; Birchwood, M. Depression and Schizophrenia: Cause, Consequence, or Trans-diagnostic Issue? Schizophr. Bull. 2017, 43, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Stilling, R.M.; Moloney, G.M.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome 2017, 5, 102. [Google Scholar] [CrossRef]
- Murtas, G.; Sacchi, S.; Tedeschi, G.; Maffioli, E.; Notomista, E.; Cafaro, V.; Abbondi, M.; Mothet, J.P.; Pollegioni, L. Antimicrobial D-amino acid oxidase-derived peptides specify gut microbiota. Cell. Mol. Life Sci. 2021, 78, 3607–3620. [Google Scholar] [CrossRef]
- Takagi, S.; Balu, D.T.; Coyle, J.T. Factors regulating serine racemase and d-amino acid oxidase expression in the mouse striatum. Brain Res. 2021, 1751, 147202. [Google Scholar] [CrossRef]
- Kong, L.; Fan, D.; Zhou, L.; Wei, S. The influence of modified molecular (D/L-serine) chirality on the theragnostics of PAMAM-based nanomedicine for acute kidney injury. J. Mater. Chem. B. 2021, 9, 9023–9030. [Google Scholar] [CrossRef]
- Nakade, Y.; Iwata, Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R.; Miyake, T.; Sakai, N.; Kitajima, S.; Toyama, T.; et al. Gut microbiota-derived D-serine protects against acute kidney injury. JCI Insight 2018, 3, e97957. [Google Scholar] [CrossRef]
- Brauer, A.L.; White, A.N.; Learman, B.S.; Johnson, A.O.; Armbruster, C.E. d-Serine Degradation by Proteus mirabilis Contributes to Fitness during Single-Species and Polymicrobial Catheter-Associated Urinary Tract Infection. mSphere 2019, 4, e00020-19. [Google Scholar] [CrossRef]
- Arias, C.A.; Weisner, J.; Blackburn, J.M.; Reynolds, P.E. Serine and alanine racemase activities of VanT: A protein necessary for vancomycin resistance in Enterococcus gallinarum BM4174. Microbiology 2000, 146, 1727–1734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arias, C.A.; Peña, J.; Panesso, D.; Reynolds, P. Role of the transmembrane domain of the VanT serine racemase in resistance to vancomycin in Enterococcus gallinarum BM4174. J. Antimicrob. Chemother. 2003, 51, 557–564. [Google Scholar] [CrossRef][Green Version]
- Meziane-Cherif, D.; Stogios, P.J.; Evdokimova, E.; Egorova, O.; Savchenko, A.; Courvalin, P. Structural and Functional Adaptation of Vancomycin Resistance VanT Serine Racemases. mBio 2015, 6, e00806. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Okada, H.; Abe, K.; Kera, Y. D-amino acid-induced expression of D-amino acid oxidase in the yeast Schizosaccharomyces pombe. Curr. Microbiol. 2012, 65, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Weickert, C.S.; Weickert, T.W.; Pillai, A.; Buckley, P.F. Biomarkers in schizophrenia: A brief conceptual consideration. Dis. Markers 2013, 35, 3–9. [Google Scholar] [CrossRef]
- Harris, L.W.; Pietsch, S.; Cheng, T.M.; Schwarz, E.; Guest, P.C.; Bahn, S. Comparison of peripheral and central schizophrenia biomarker profiles. PLoS ONE 2012, 7, e46368. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Scarr, E.; Udawela, M.; Everall, I.; Chen, W.J.; Dean, B. Biomarkers in schizophrenia: A focus on blood based diagnostics and theranostics. World J. Psychiatry 2016, 6, 102–117. [Google Scholar] [CrossRef]
- Hamase, K.; Morikawa, A.; Etoh, S.; Tojo, Y.; Miyoshi, Y.; Zaitsu, K. Analysis of small amounts of D-amino acids and the study of their physiological functions in mammals. Anal. Sci. 2009, 25, 961–968. [Google Scholar] [CrossRef]
- Bendikov, I.; Nadri, C.; Amar, S.; Panizzutti, R.; De Miranda, J.; Wolosker, H.; Agam, G. A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr. Res. 2007, 90, 41–51. [Google Scholar] [CrossRef]
- Hashimoto, K.; Engberg, G.; Shimizu, E.; Nordin, C.; Lindström, L.H.; Iyo, M. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 767–769. [Google Scholar] [CrossRef]
- Tsai, G.; Yang, P.; Chung, L.C.; Lange, N.; Coyle, J.T. D-serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 1998, 44, 1081–1089. [Google Scholar] [CrossRef]
- Ishiwata, S.; Hattori, K.; Sasayama, D.; Teraishi, T.; Miyakawa, T.; Yokota, Y.; Matsumura, R.; Yoshida, F.; Nishikawa, T.; Kunugi, H. Plasma and cerebrospinal fluid G72 protein levels in schizophrenia and major depressive disorder. Psychiatry Res. 2017, 254, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Corvin, A.; McGhee, K.A.; Murphy, K.; Donohoe, G.; Nangle, J.M.; Schwaiger, S.; Kenny, N.; Clarke, S.; Meagher, D.; Quinn, J.; et al. Evidence for association and epistasis at the DAOA/G30 and D-amino acid oxidase loci in an Irish schizophrenia sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, S.; Binelli, G.; Pollegioni, L. G72 primate-specific gene: A still enigmatic element in psychiatric disorders. Cell. Mol. Life Sci. 2016, 73, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Lin, C.H.; Hung, C.C.; Lane, H.Y. An Ensemble Approach to Predict Schizophrenia Using Protein Data in the N-methyl-D-Aspartate Receptor (NMDAR) and Tryptophan Catabolic Pathways. Front. Bioeng. Biotechnol. 2020, 8, 569. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chou, W.H.; Tsou, H.H.; Fang, C.P.; Liu, T.H.; Tsao, H.H.; Hsu, W.C.; Weng, Y.C.; Wang, Y.; Liu, Y.L. A Post-hoc Study of D-Amino Acid Oxidase in Blood as an Indicator of Post-stroke Dementia. Front. Neurol. 2019, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yang, H.T.; Chiu, C.C.; Lane, H.Y. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci. Rep. 2017, 7, 14849. [Google Scholar] [CrossRef]
- Madeira, C.; Lourenco, M.V.; Vargas-Lopes, C.; Suemoto, C.K.; Brandão, C.O.; Reis, T.; Leite, R.E.; Laks, J.; Jacob-Filho, W.; Pasqualucci, C.A.; et al. d-serine levels in Alzheimer’s disease: Implications for novel biomarker development. Transl. Psychiatry 2015, 5, e561. [Google Scholar] [CrossRef]
- Piubelli, L.; Murtas, G.; Rabattoni, V.; Pollegioni, L. The Role of D-Amino Acids in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 80, 475–492. [Google Scholar] [CrossRef]
- Tomiyama, T.; Asano, S.; Furiya, Y.; Shirasawa, T.; Endo, N.; Mori, H. Racemization of Asp23 residue affects the aggregation properties of Alzheimer amyloid beta protein analogues. J. Biol. Chem. 1994, 269, 10205–10208. [Google Scholar] [CrossRef]
- Nuzzo, T.; Sekine, M.; Punzo, D.; Miroballo, M.; Katane, M.; Saitoh, Y.; Galbusera, A.; Pasqualetti, M.; Errico, F.; Gozzi, A.; et al. Dysfunctional d-aspartate metabolism in BTBR mouse model of idiopathic autism. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140531. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, J.; Suzuki, M. Emerging Role of D-Amino Acid Metabolism in the Innate Defense. Front. Microbiol. 2018, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Burt, M.A.; Tse, Y.C.; Boksa, P.; Wong, T.P. Prenatal immune activation interacts with stress and corticosterone exposure later in life to modulate N-methyl-D-aspartate receptor synaptic function and plasticity. Int. J. Neuropsychopharmacol. 2013, 16, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Homma, H. Detection and quantification of d-amino acid residues in peptides and proteins using acid hydrolysis. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Rosini, E.; D’Antona, P.; Pollegioni, L. Biosensors for D-Amino Acids: Detection Methods and Applications. Int. J. Mol. Sci. 2020, 21, 4574. [Google Scholar] [CrossRef]
- MacKay, M.B.; Kravtsenyuk, M.; Thomas, R.; Mitchell, N.D.; Dursun, S.M.; Baker, G.B. D-Serine: Potential Therapeutic Agent and/or Biomarker in Schizophrenia and Depression? Front. Psychiatry 2019, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Yang, H.T.; Lane, H.Y. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 2019, 185, 172760. [Google Scholar] [CrossRef]
- Hashimoto, K.; Malchow, B.; Falkai, P.; Schmitt, A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 367–377. [Google Scholar] [CrossRef]
- Choi, S.R.; Roh, D.H.; Yoon, S.Y.; Choi, H.S.; Kang, S.Y.; Han, H.J.; Beitz, A.J.; Lee, J.H. Astrocyte D-serine modulates the activation of neuronal NOS leading to the development of mechanical allodynia in peripheral neuropathy. Mol. Pain 2019, 15, 1744806919843046. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. D-Amino Acids as a Biomarker in Schizophrenia. Diseases 2022, 10, 9. https://doi.org/10.3390/diseases10010009
Taniguchi K, Sawamura H, Ikeda Y, Tsuji A, Kitagishi Y, Matsuda S. D-Amino Acids as a Biomarker in Schizophrenia. Diseases. 2022; 10(1):9. https://doi.org/10.3390/diseases10010009
Chicago/Turabian StyleTaniguchi, Kurumi, Haruka Sawamura, Yuka Ikeda, Ai Tsuji, Yasuko Kitagishi, and Satoru Matsuda. 2022. "D-Amino Acids as a Biomarker in Schizophrenia" Diseases 10, no. 1: 9. https://doi.org/10.3390/diseases10010009
APA StyleTaniguchi, K., Sawamura, H., Ikeda, Y., Tsuji, A., Kitagishi, Y., & Matsuda, S. (2022). D-Amino Acids as a Biomarker in Schizophrenia. Diseases, 10(1), 9. https://doi.org/10.3390/diseases10010009





