Hemophagocytic Lymphohistiocytosis in the Setting of Therapy-Induced Acute Myeloid Leukemia: An Autopsy Report
Abstract
:1. Introduction
2. Case Presentation
2.1. Clinical History
2.2. Autopsy Findings
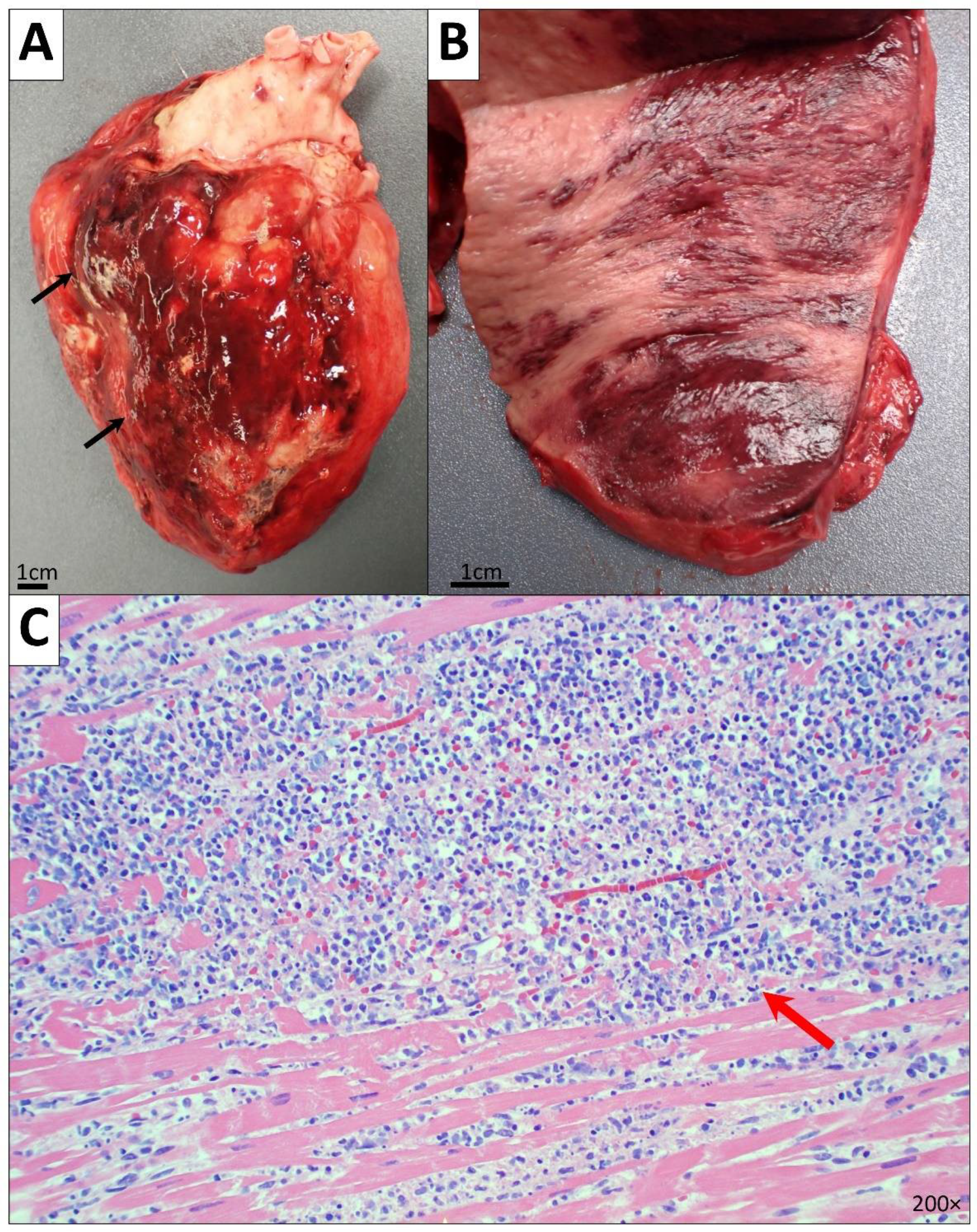
2.2.1. Cardiovascular System
2.2.2. Respiratory System
2.2.3. Gastrointestinal System
2.2.4. Genitourinary System
2.2.5. Hematopoietic System
2.2.6. Endocrine System
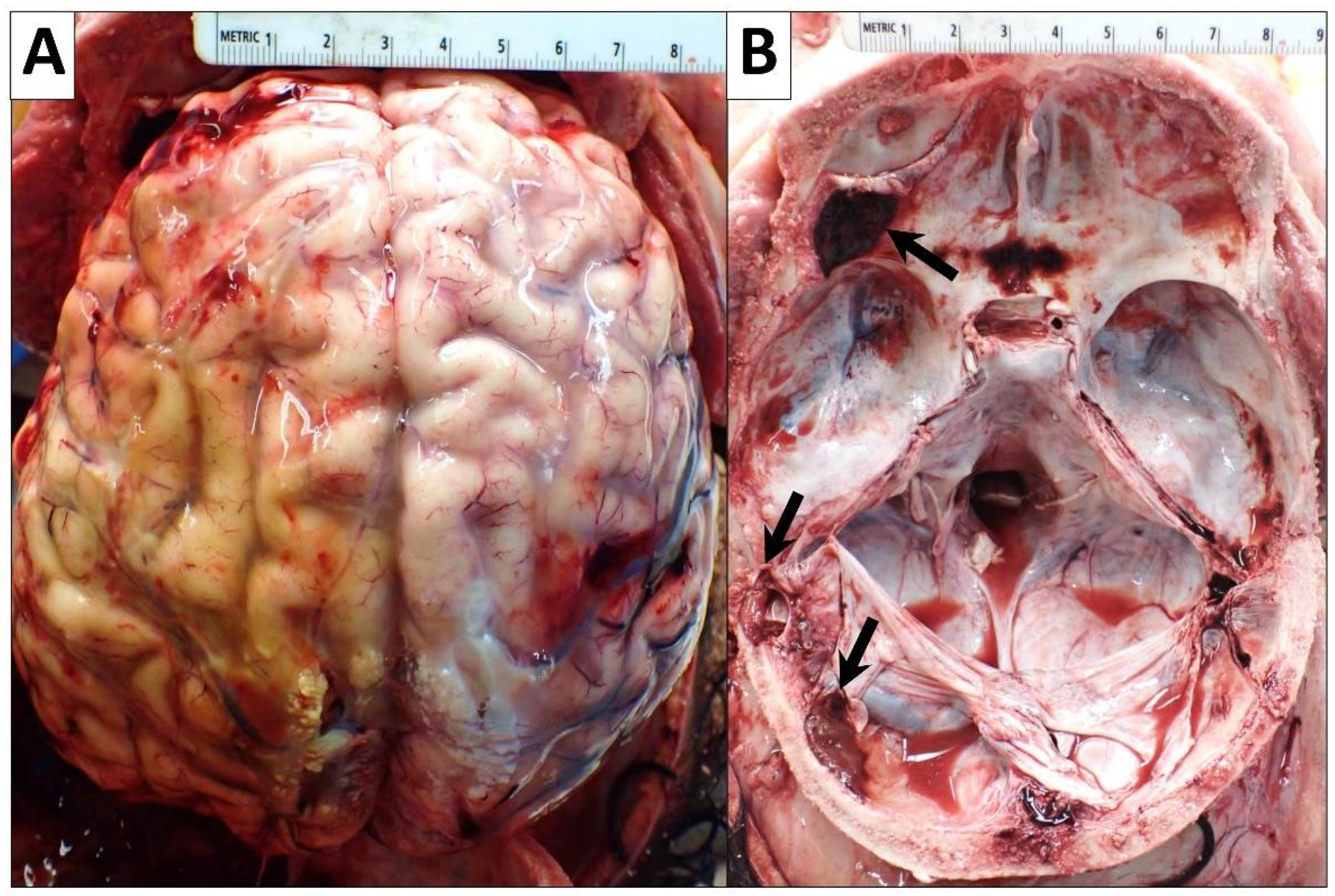
2.2.7. Central Nervous System
2.2.8. Cultures
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McClain, K.L. How I treat hemophagocytic lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef]
- Strenger, V.; Merth, G.; Lackner, H.; Aberle, S.W.; Kessler, H.H.; Seidel, M.G.; Schwinger, W.; Sperl, D.; Sovinz, P.; Karastaneva, A.; et al. Malignancy and chemotherapy induced haemophagocytic lymphohistiocytosis in children and adolescents—A single centre experience of 20 years. Ann. Hematol. 2018, 97, 989–998. [Google Scholar] [CrossRef]
- Gosh, J.B.; Roy, M.; Bala, A. Infection Associated with Hemophagocytic Lymphohisticytosis triggered by nosocomial Infection. Oman Med. J. 2009, 24, 223–225. [Google Scholar] [CrossRef]
- Karlsson, T. Secondary haemophagocytic lymphohistiocytosis: Experience from the Uppsala University Hospital. Upsala J. Med. Sci. 2015, 120, 257–262. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, L.; Tang, W.; Zhou, Y.; Li, F. A systematic review of malignancy-associated hemophagocytic lymphohistiocytosis that needs more attentions. Oncotarget 2017, 8, 59977–59985. [Google Scholar] [CrossRef]
- Molina, D.K.; Pinneri, K.; Stash, J.A.; Li, L.; Vance, K.; Cross, C. Organ Weight Reference Ranges for Ages 0 to 12 Years. Am. J. Forensic Med. Pathol. 2019, 40, 318–328. [Google Scholar] [CrossRef]
- Scott, R.B.; Robb-Smith, A. Histiocytic Medullary Reticulosis. Lancet 1939, 234, 194–198. [Google Scholar] [CrossRef]
- Egeler, R.M.; Shapiro, R.; Loechelt, B.; Filipovich, A. Characteristic Immune Abnormalities in Hemophagocytic Lymphohistiocytosis. J. Pediatr. Hematol. Oncol. 1996, 18, 340–345. [Google Scholar] [CrossRef]
- Vrotsos, E.; Soaita, M.; Khatib, Z.; Brathwaite, C.; Filipovich, A.; Robinson, M.J.; Castellano-Sanchez, A.A. Atypical clinical presentation of primary hemophagocytic lymphohistiocytosis with a novel perforin1 gene mutation. J. Hematop. 2013, 6, 105–108. [Google Scholar] [CrossRef]
- Clarke, R.T.; Van den Bruel, A.; Bankhead, C.; Mitchell, C.D.; Phillips, B.; Thompson, M.J. Clinical presentation of childhood leukaemia: A systematic review and meta-analysis. Arch. Dis. Child. 2016, 101, 894–901. [Google Scholar] [CrossRef]
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Roberts, K.G. Genetics and prognosis of ALL in children vs adults. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 137–145. [Google Scholar] [CrossRef]
- Onciu, M. Acute Lymphoblastic Leukemia. Hematol. Clin. N. Am. 2009, 23, 655–674. [Google Scholar] [CrossRef]
- Ribera, J.-M.; Oriol, A. Acute Lymphoblastic Leukemia in Adolescents and Young Adults. Hematol. Oncol. Clin. N. Am. 2009, 23, 1033–1042. [Google Scholar] [CrossRef]
- Kroll, M.E.; Stiller, C.A.; Murphy, M.F.G.; Carpenter, L.M. Childhood leukaemia and socioeconomic status in England and Wales 1976–2005: Evidence of higher incidence in relatively affluent communities persists over time. Br. J. Cancer 2011, 105, 1783–1787. [Google Scholar] [CrossRef]
- Feng, Q.; de Smith, A.J.; Vergara-Lluri, M.; Muskens, I.S.; McKean-Cowdin, R.; Kogan, S.; Brynes, R.; Wiemels, J.L. Trends in Acute Lymphoblastic Leukemia Incidence in the United States by Race/Ethnicity From 2000 to 2016. Am. J. Epidemiol. 2021, 190, 519–527. [Google Scholar] [CrossRef]
- Greaves, M. Infection, immune responses and the aetiology of childhood leukaemia. Nat. Rev. Cancer 2006, 6, 193–203. [Google Scholar] [CrossRef]
- Krestinina, L.; Davis, F.G.; Schonfeld, S.; Preston, D.L.; Degteva, M.; Epifanova, S.; Akleyev, A.V. Leukaemia incidence in the Techa River Cohort: 1953–2007. Br. J. Cancer 2013, 109, 2886–2893. [Google Scholar] [CrossRef]
- Urayama, K.Y.; Ma, X.; Selvin, S.; Metayer, C.; Chokkalingam, A.P.; Wiemels, J.L.; Does, M.; Chang, J.; Wong, A.; Trachtenberg, E.; et al. Early life exposure to infections and risk of childhood acute lymphoblastic leukemia. Int. J. Cancer 2011, 128, 1632–1643. [Google Scholar] [CrossRef]
- Boothe, V.L.; Boehmer, T.K.; Wendel, A.M.; Yip, F.Y. Residential traffic exposure and childhood leukemia: A systematic review and meta-analysis. Am. J. Prev. Med. 2014, 46, 413–422. [Google Scholar] [CrossRef]
- Buitenkamp, T.D.; Izraeli, S.; Zimmermann, M.; Forestier, E.; Heerema, N.A.; van den Heuvel-Eibrink, M.M.; Pieters, R.; Korbijn, C.M.; Silverman, L.B.; Schmiegelow, K.; et al. Acute lymphoblastic leukemia in children with Down syndrome: A retrospective analysis from the Ponte di Legno study group. Blood 2014, 123, 70–77. [Google Scholar] [CrossRef]
- Chen, J.; Glasser, C. New and Emerging Targeted Therapies for Pediatric Acute Myeloid Leukemia (AML). Children 2020, 7, 12. [Google Scholar] [CrossRef]
- Meyers, C.A.; Albitar, M.; Estey, E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 2005, 104, 788–793. [Google Scholar] [CrossRef]
- Bhatia, S.; Neglia, J. Epidemiology of Childhood Acute Myelogenous Leukemia. J. Pediatr. Hematol. Oncol. 1995, 17, 94–100. [Google Scholar] [CrossRef]
- Puumala, S.E.; Ross, J.A.; Aplenc, R.; Spector, L.G. Epidemiology of childhood acute myeloid leukemia. Pediatr. Blood Cancer 2013, 60, 728–733. [Google Scholar] [CrossRef]
- Granfeldt Østgård, L.S.; Medeiros, B.C.; Sengeløv, H.; Nørgaard, M.; Andersen, M.K.; Dufva, I.H.; Friis, L.S.; Kjeldsen, E.; Marcher, C.W.; Preiss, B.; et al. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J. Clin. Oncol. 2015, 33, 3641–3649. [Google Scholar] [CrossRef]
- Sardou-Cezar, I.; Lopes, B.A.; Andrade, F.G.; Fonseca, T.C.C.; Fernandez, T.D.S.; Larghero, P.; de Souza, R.Q.; Loth, G.; Ribeiro, L.L.; Bonfim, C.; et al. Therapy-related acute myeloid leukemia with KMT2A-SNX9 gene fusion associated with a hyperdiploid karyotype after hemophagocytic lymphohistiocytosis. Cancer Genet. 2021, 256–257, 86–90. [Google Scholar] [CrossRef]
- Tebbi, C.K.; London, W.B.; Friedman, D.; Villaluna, D.; De Alarcon, P.A.; Constine, L.S.; Mendenhall, N.P.; Sposto, R.; Chauvenet, A.; Schwartz, C.L. Dexrazoxane-Associated Risk for Acute Myeloid Leukemia/Myelodysplastic Syndrome and Other Secondary Malignancies in Pediatric Hodgkin’s Disease. J. Clin. Oncol. 2007, 25, 493–500. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, J.; Philip, P. Two different classes of therapy-related and de-novo acute myeloid leukemia? Cancer Genet. Cytogenet. 1991, 55, 119–124. [Google Scholar] [CrossRef]
- Palazzi, D.L.; McClain, K.; Kaplan, S.L. Hemophagocytic Syndrome in Children: An Important Diagnostic Consideration in Fever of Unknown Origin. Clin. Infect. Dis. 2003, 36, 306–312. [Google Scholar] [CrossRef]
- Fukaya, S.; Yasuda, S.; Hashimoto, T.; Oku, K.; Kataoka, H.; Horita, T.; Atsumi, T.; Koike, T. Clinical features of haemophagocytic syndrome in patients with systemic autoimmune diseases: Analysis of 30 cases. Rheumatology 2008, 47, 1686–1691. [Google Scholar] [CrossRef]
- Favara, B.E. Histopathology of the Liver in Histiocytosis Syndromes. Pediatr. Pathol. Lab. Med. 1996, 16, 413–433. [Google Scholar] [CrossRef]
- Kerguenec, C.; Hillaire, S.; Molinie, V.; Gardin, C.; Degott, C.; Erlinger, S.; Valla, D. Hepatic manifestations of hemophagocytic syndrome: A study of 30 cases. Am. J. Gastroenterol. 2001, 96, 852–857. [Google Scholar] [CrossRef]
- Jovanovic, A.; Kuzmanovic, M.; Kravljanac, R.; Micic, D.; Jovic, M.; Gazikalovic, S.; Pasic, S. Central Nervous System Involvement in Hemophagocytic Lymphohistiocytosis: A Single-Center Experience. Pediatr. Neurol. 2014, 50, 233–237. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Mostaza-Fernández, J.L.; Guerra Laso, J.; Carriedo Ule, D.; Ruiz de Morales, J.M. Hemophagocytic lymphohistiocytosis associated with viral infections: Diagnostic challenges and therapeutic dilemmas. Rev. Clin. Esp. 2014, 214, 320–327. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Bosch, X.; Pérez-De-Lis, M.; Pérez-Álvarez, R.; Fraile, G.; Gheitasi, H.; Retamozo, S.; Bové, A.; Monclús, E.; Escoda, O.; et al. Infection is the major trigger of hemophagocytic syndrome in adult patients treated with biological therapies. Semin. Arthritis Rheum. 2016, 45, 391–399. [Google Scholar] [CrossRef]
- Parikh, S.A.; Kapoor, P.; Letendre, L.; Kumar, S.; Wolanskyj, A.P. Prognostic Factors and Outcomes of Adults With Hemophagocytic Lymphohistiocytosis. Mayo Clin. Proc. 2014, 89, 484–492. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Eby, C.S. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am. J. Hematol. 2015, 90, 220–224. [Google Scholar] [CrossRef]
- Schram, A.M.; Comstock, P.; Campo, M.; Gorovets, D.; Mullally, A.; Bodio, K.; Arnason, J.; Berliner, N. Haemophagocytic lymphohistiocytosis in adults: A multicentre case series over 7 years. Br. J. Haematol. 2016, 172, 412–419. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Kostov, B.; Moral-Moral, P.; Martínez-Zapico, A.; Díaz-Pedroche, C.; Fraile, G.; Pérez-Guerrero, P.; Fonseca, E.; Robles, A.; Vaquero-Herrero, M.P.; et al. Prognostic Factors of Death in 151 Adults With Hemophagocytic Syndrome: Etiopathogenically Driven Analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 2018, 2, 267–276. [Google Scholar] [CrossRef]





| Diagnosis Is Established If One of Either (I) or (II) Is Fulfilled |
|---|
| (I) Molecular diagnosis consistent with HLH |
| (II) Clinicopathologic criteria for HLH fulfilled (5 out of the 8 criteria shown below) |
| 1. Fever ≥ 38.5 °C for ≥ 7 days |
| 2. Splenomegaly ≥ 3 finger breadth below the left subcostal margin |
| 3. Cytopenias affecting ≥ 2 of 3 lineages in peripheral blood |
| • Hemoglobin < 9 g/L |
| • Platelets < 100 × 109/L |
| • Absolute neutrophil count < 1.0 × 109/L |
| 4. Hypertriglyceridemia and/or hypofibrinogenemia |
| • Fasting triglycerides ≥ 265 mg/dL, Fibrinogen ≥ 1.5 g/L |
| 5. Hemophagocytosis in the bone marrow or spleen or lymph node |
| 6. Low or absent NK cell activity (according to the local laboratory reference) |
| 7. Ferritin ≥ 500 μg/L |
| 8. Soluble CD25 (sIL-2 receptor) ≥ 2400 U/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahmad, H.F.; Gogola, S.; Elajami, M.K.; Brathwaite, C.; Castellano-Sánchez, A.A.; Sriganeshan, V.; Omarzai, Y. Hemophagocytic Lymphohistiocytosis in the Setting of Therapy-Induced Acute Myeloid Leukemia: An Autopsy Report. Diseases 2022, 10, 54. https://doi.org/10.3390/diseases10030054
Bahmad HF, Gogola S, Elajami MK, Brathwaite C, Castellano-Sánchez AA, Sriganeshan V, Omarzai Y. Hemophagocytic Lymphohistiocytosis in the Setting of Therapy-Induced Acute Myeloid Leukemia: An Autopsy Report. Diseases. 2022; 10(3):54. https://doi.org/10.3390/diseases10030054
Chicago/Turabian StyleBahmad, Hisham F., Samantha Gogola, Mohamad K. Elajami, Carole Brathwaite, Amilcar A. Castellano-Sánchez, Vathany Sriganeshan, and Yumna Omarzai. 2022. "Hemophagocytic Lymphohistiocytosis in the Setting of Therapy-Induced Acute Myeloid Leukemia: An Autopsy Report" Diseases 10, no. 3: 54. https://doi.org/10.3390/diseases10030054
APA StyleBahmad, H. F., Gogola, S., Elajami, M. K., Brathwaite, C., Castellano-Sánchez, A. A., Sriganeshan, V., & Omarzai, Y. (2022). Hemophagocytic Lymphohistiocytosis in the Setting of Therapy-Induced Acute Myeloid Leukemia: An Autopsy Report. Diseases, 10(3), 54. https://doi.org/10.3390/diseases10030054








