Novel ATM Gene c.5644 C > T (p.Arg1882*) Variant Detected in a Patient with Pancreatic Adenocarcinoma and Two Primary Non-Small Cell Lung Adenocarcinomas: A Case Report
Abstract
1. Introduction
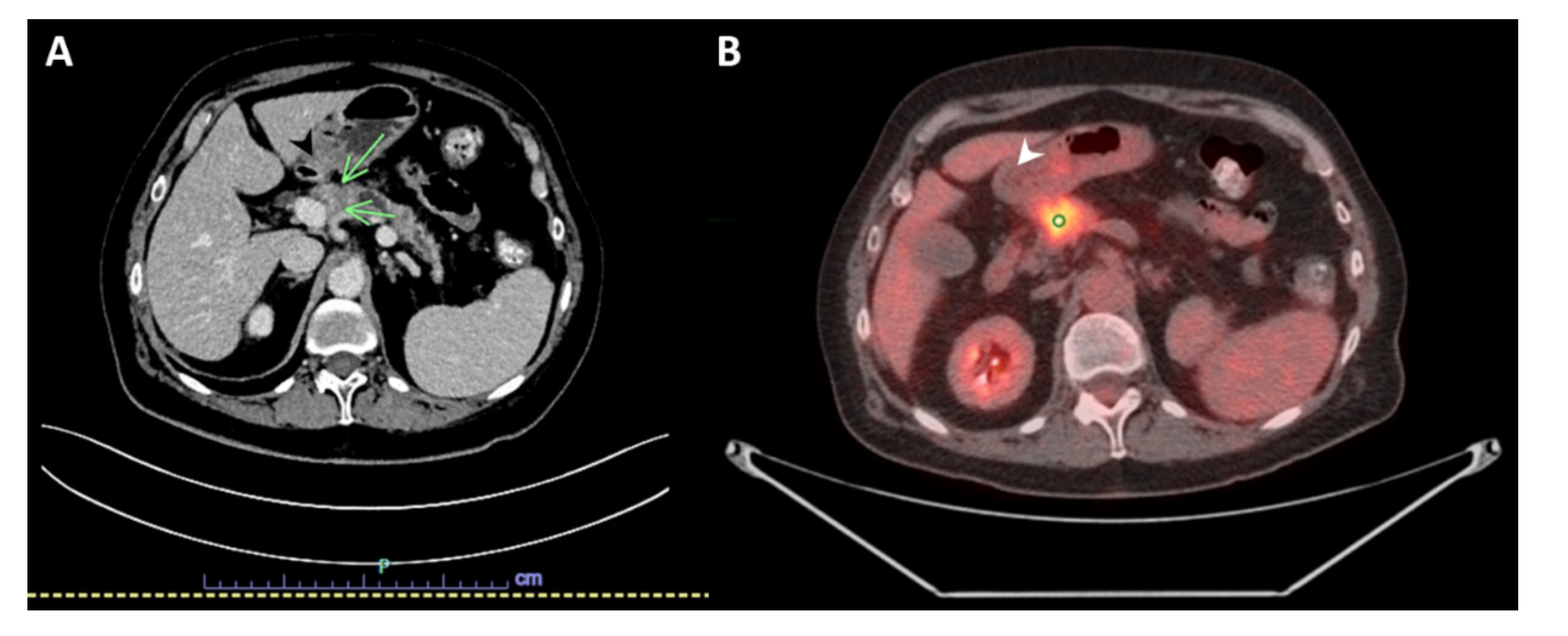
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia telangiectasia: A review. Orphanet J. Rare Dis. 2016, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, G.; Mitui, M.; Campbell, C.; Costa Carvalho, B.T.; Nahas, S.; Sun, X.; Huo, Y.; Lai, C.H.; Thorstenson, Y.; Tanouye, R.; et al. Five haplotypes account for fifty-five percent of ATM mutations in Brazilian patients with ataxia telangiectasia: Seven new mutations. Am. J. Med. Genet. A 2004, 126a, 33–40. [Google Scholar] [CrossRef]
- Buzin, C.H.; Gatti, R.A.; Nguyen, V.Q.; Wen, C.Y.; Mitui, M.; Sanal, O.; Chen, J.S.; Nozari, G.; Mengos, A.; Li, X.; et al. Comprehensive scanning of the ATM gene with DOVAM-S. Hum. Mutat. 2003, 21, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Stucci, L.S.; Internò, V.; Tucci, M.; Perrone, M.; Mannavola, F.; Palmirotta, R.; Porta, C. The ATM Gene in breast cancer: Its relevance in clinical practice. Genes 2021, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Rotman, G.; Shiloh, Y. ATM: From gene to function. Hum. Mol. Genet. 1998, 7, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Tong, X.; Ma, Y.; Liu, S.; Yang, L.; Yang, X.; Yang, X.; Bai, M.; Fan, H. Association between ATM gene polymorphisms, lung cancer susceptibility and radiation-induced pneumonitis: A meta-analysis. BMC Pulm. Med. 2017, 17, 205. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Schultz, C.W.; Azimi-Sadjadi, A.; Brody, J.R.; Pishvaian, M.J. ATM dysfunction in pancreatic adenocarcinoma and associated therapeutic implications. Mol. Cancer Ther. 2019, 18, 1899–1908. [Google Scholar] [CrossRef]
- Shen, L.; Yin, Z.H.; Wan, Y.; Zhang, Y.; Li, K.; Zhou, B.S. Association between ATM polymorphisms and cancer risk: A meta-analysis. Mol. Biol. Rep. 2012, 39, 5719–5725. [Google Scholar] [CrossRef]
- Ye, F.; Chai, W.; Yang, M.; Xie, M.; Yang, L. Ataxia-telangiectasia with a novel ATM gene mutation and Burkitt leukemia: A case report. Mol. Clin. Oncol. 2018, 9, 493–498. [Google Scholar] [CrossRef]
- McKinnon, P.J. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu. Rev. Pathol. 2012, 7, 303–321. [Google Scholar] [CrossRef]
- Martino, C.; Pandya, D.; Lee, R.; Levy, G.; Lo, T.; Lobo, S.; Frank, R.C. ATM-Mutated pancreatic cancer: Clinical and molecular response to gemcitabine/nab-paclitaxel after genome-based therapy resistance. Pancreas 2020, 49, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. Jama 2018, 319, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Jeggo, P.A. ATM’s role in the repair of DNA double-strand breaks. Genes 2021, 12, 1370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Zhao, Y.; He, S.; Zhao, R.; Song, Y.; Cheng, J.; Gong, Y.; Xie, J.; Wang, Y.; et al. Caspase-3 knockout attenuates radiation-induced tumor repopulation via impairing the ATM/p53/Cox-2/PGE(2) pathway in non-small cell lung cancer. Aging 2020, 12, 21758–21776. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hart, S.N.; Bamlet, W.R.; Moore, R.M.; Nandakumar, K.; Eckloff, B.W.; Lee, Y.K.; Petersen, G.M.; McWilliams, R.R.; Couch, F.J. Prevalence of Pathogenic Mutations in Cancer Predisposition Genes among Pancreatic Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2016, 25, 207–211. [Google Scholar] [CrossRef]
- Na, R.; Zheng, S.L.; Han, M.; Yu, H.; Jiang, D.; Shah, S.; Ewing, C.M.; Zhang, L.; Novakovic, K.; Petkewicz, J.; et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur. Urol. 2017, 71, 740–747. [Google Scholar] [CrossRef]
- Pilié, P.G.; Johnson, A.M.; Hanson, K.L.; Dayno, M.E.; Kapron, A.L.; Stoffel, E.M.; Cooney, K.A. Germline genetic variants in men with prostate cancer and one or more additional cancers. Cancer 2017, 123, 3925–3932. [Google Scholar] [CrossRef]
- Sriramulu, S.; Ramachandran, M.; Subramanian, S.; Kannan, R.; Gopinath, M.; Sollano, J.; Bissi, L.; Banerjee, A.; Marotta, F.; Pathak, S. A review on role of ATM gene in hereditary transfer of colorectal cancer. Acta Biomed. 2019, 89, 463–469. [Google Scholar] [CrossRef]
- Lilyquist, J.; LaDuca, H.; Polley, E.; Davis, B.T.; Shimelis, H.; Hu, C.; Hart, S.N.; Dolinsky, J.S.; Couch, F.J.; Goldgar, D.E. Frequency of mutations in a large series of clinically ascertained ovarian cancer cases tested on multi-gene panels compared to reference controls. Gynecol. Oncol. 2017, 147, 375–380. [Google Scholar] [CrossRef]
- van Os, N.J.; Roeleveld, N.; Weemaes, C.M.; Jongmans, M.C.; Janssens, G.O.; Taylor, A.M.; Hoogerbrugge, N.; Willemsen, M.A. Health risks for ataxia-telangiectasia mutated heterozygotes: A systematic review, meta-analysis and evidence-based guideline. Clin. Genet. 2016, 90, 105–117. [Google Scholar] [CrossRef]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef]
- Jeddane, L.; Ailal, F.; Dubois-d’Enghien, C.; Abidi, O.; Benhsaien, I.; Kili, A.; Chaouki, S.; Kriouile, Y.; El Hafidi, N.; Fadil, H.; et al. Molecular defects in moroccan patients with ataxia-telangiectasia. Neuromol. Med. 2013, 15, 288–294. [Google Scholar] [CrossRef]
- Mitui, M.; Campbell, C.; Coutinho, G.; Sun, X.; Lai, C.H.; Thorstenson, Y.; Castellvi-Bel, S.; Fernandez, L.; Monros, E.; Carvalho, B.T.; et al. Independent mutational events are rare in the ATM gene: Haplotype prescreening enhances mutation detection rate. Hum. Mutat. 2003, 22, 43–50. [Google Scholar] [CrossRef]
- Micol, R.; Ben Slama, L.; Suarez, F.; Le Mignot, L.; Beauté, J.; Mahlaoui, N.; Dubois d’Enghien, C.; Laugé, A.; Hall, J.; Couturier, J.; et al. Morbidity and mortality from ataxia-telangiectasia are associated with ATM genotype. J. Allergy Clin. Immunol. 2011, 128, 382–389. [Google Scholar] [CrossRef]
- Tefferi, A.; Vardiman, J.W. Classification and diagnosis of myeloproliferative neoplasms: The 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 2008, 22, 14–22. [Google Scholar] [CrossRef]
- Nielsen, C.; Bojesen, S.E.; Nordestgaard, B.G.; Kofoed, K.F.; Birgens, H.S. JAK2V617F somatic mutation in the general population: Myeloproliferative neoplasm development and progression rate. Haematologica 2014, 99, 1448–1455. [Google Scholar] [CrossRef]
- Klavanian, J.; Zakalik, D.; Konde, A.S.; Rangarajan, T. ATM mutation carriers and family history of pancreatic cancer. J. Clin. Oncol. 2020, 38, 1529. [Google Scholar] [CrossRef]
- Roberts, N.J.; Jiao, Y.; Yu, J.; Kopelovich, L.; Petersen, G.M.; Bondy, M.L.; Gallinger, S.; Schwartz, A.G.; Syngal, S.; Cote, M.L.; et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov. 2012, 2, 41–46. [Google Scholar] [CrossRef]
- Lee, H.-W.; Park, S.-H.; Weng, M.-w.; Wang, H.-T.; Huang, W.C.; Lepor, H.; Wu, X.-R.; Chen, L.-C.; Tang, M.-s. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1560–E1569. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Daouk, R.; Hassane, M.; Bahmad, H.F.; Sinjab, A.; Fujimoto, J.; Abou-Kheir, W.; Kadara, H. Genome-Wide and phenotypic evaluation of stem cell progenitors derived from Gprc5a-deficient murine lung adenocarcinoma with somatic kras mutations. Front. Oncol. 2019, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Greulich, H. The genomics of lung adenocarcinoma: Opportunities for targeted therapies. Genes Cancer 2010, 1, 1200–1210. [Google Scholar] [CrossRef]
- Scoccianti, C.; Vesin, A.; Martel, G.; Olivier, M.; Brambilla, E.; Timsit, J.F.; Tavecchio, L.; Brambilla, C.; Field, J.K.; Hainaut, P. Prognostic value of TP53, KRAS and EGFR mutations in nonsmall cell lung cancer: The EUELC cohort. Eur. Respir. J. 2012, 40, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Aran, V.; Zalis, M.; Montella, T.; de Sousa, C.A.M.; Ferrari, B.L.; Gil Ferreira, C. Evaluation of KRAS concomitant mutations in advanced lung adenocarcinoma patients. Medicina 2021, 57, 1039. [Google Scholar] [CrossRef] [PubMed]
- König, D.; Savic Prince, S.; Rothschild, S.I. Targeted therapy in advanced and metastatic non-small cell lung cancer. an update on treatment of the most important actionable oncogenic driver alterations. Cancers 2021, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Weng, X.; Xu, D.; Cai, S.; Lou, H.; Ding, L. Non-small cell lung cancer cells with deficiencies in homologous recombination genes are sensitive to PARP inhibitors. Biochem. Biophys. Res. Commun. 2020, 522, 121–126. [Google Scholar] [CrossRef]
- Scheffler, M.; Ihle, M.A.; Hein, R.; Merkelbach-Bruse, S.; Scheel, A.H.; Siemanowski, J.; Brägelmann, J.; Kron, A.; Abedpour, N.; Ueckeroth, F.; et al. K-ras mutation subtypes in NSCLC and associated co-occuring mutations in other oncogenic pathways. J. Thorac. Oncol. 2019, 14, 606–616. [Google Scholar] [CrossRef]



| Gene | Variant | Zygosity | Variant Classification |
|---|---|---|---|
| ATM | c.5644C > T (p.Arg1882*) | Heterozygous | Pathogenic |
| Detected Alteration(s)/Biomarker(s) | Associated FDA-Approved Therapies | Clinical Trial Availability | % cfDNA or Amplification |
|---|---|---|---|
| ATM R1882* | Niraparib, Olaparib, Rucaparib, Talazoparib | Yes | 43.6% |
| JAK2 V617F | Ruxolitinib | Yes | 7.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljamal, A.A.; Elajami, M.K.; Mansour, E.H.; Bahmad, H.F.; Medina, A.M.; Cusnir, M. Novel ATM Gene c.5644 C > T (p.Arg1882*) Variant Detected in a Patient with Pancreatic Adenocarcinoma and Two Primary Non-Small Cell Lung Adenocarcinomas: A Case Report. Diseases 2022, 10, 115. https://doi.org/10.3390/diseases10040115
Aljamal AA, Elajami MK, Mansour EH, Bahmad HF, Medina AM, Cusnir M. Novel ATM Gene c.5644 C > T (p.Arg1882*) Variant Detected in a Patient with Pancreatic Adenocarcinoma and Two Primary Non-Small Cell Lung Adenocarcinomas: A Case Report. Diseases. 2022; 10(4):115. https://doi.org/10.3390/diseases10040115
Chicago/Turabian StyleAljamal, Abed A., Mohamad K. Elajami, Ephraim H. Mansour, Hisham F. Bahmad, Ana Maria Medina, and Mike Cusnir. 2022. "Novel ATM Gene c.5644 C > T (p.Arg1882*) Variant Detected in a Patient with Pancreatic Adenocarcinoma and Two Primary Non-Small Cell Lung Adenocarcinomas: A Case Report" Diseases 10, no. 4: 115. https://doi.org/10.3390/diseases10040115
APA StyleAljamal, A. A., Elajami, M. K., Mansour, E. H., Bahmad, H. F., Medina, A. M., & Cusnir, M. (2022). Novel ATM Gene c.5644 C > T (p.Arg1882*) Variant Detected in a Patient with Pancreatic Adenocarcinoma and Two Primary Non-Small Cell Lung Adenocarcinomas: A Case Report. Diseases, 10(4), 115. https://doi.org/10.3390/diseases10040115








