Gadofosveset-Trinatrium-Enhanced MR Angiography and MR Venography in the Diagnosis of Venous Thromboembolic Disease: A Single-Center Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
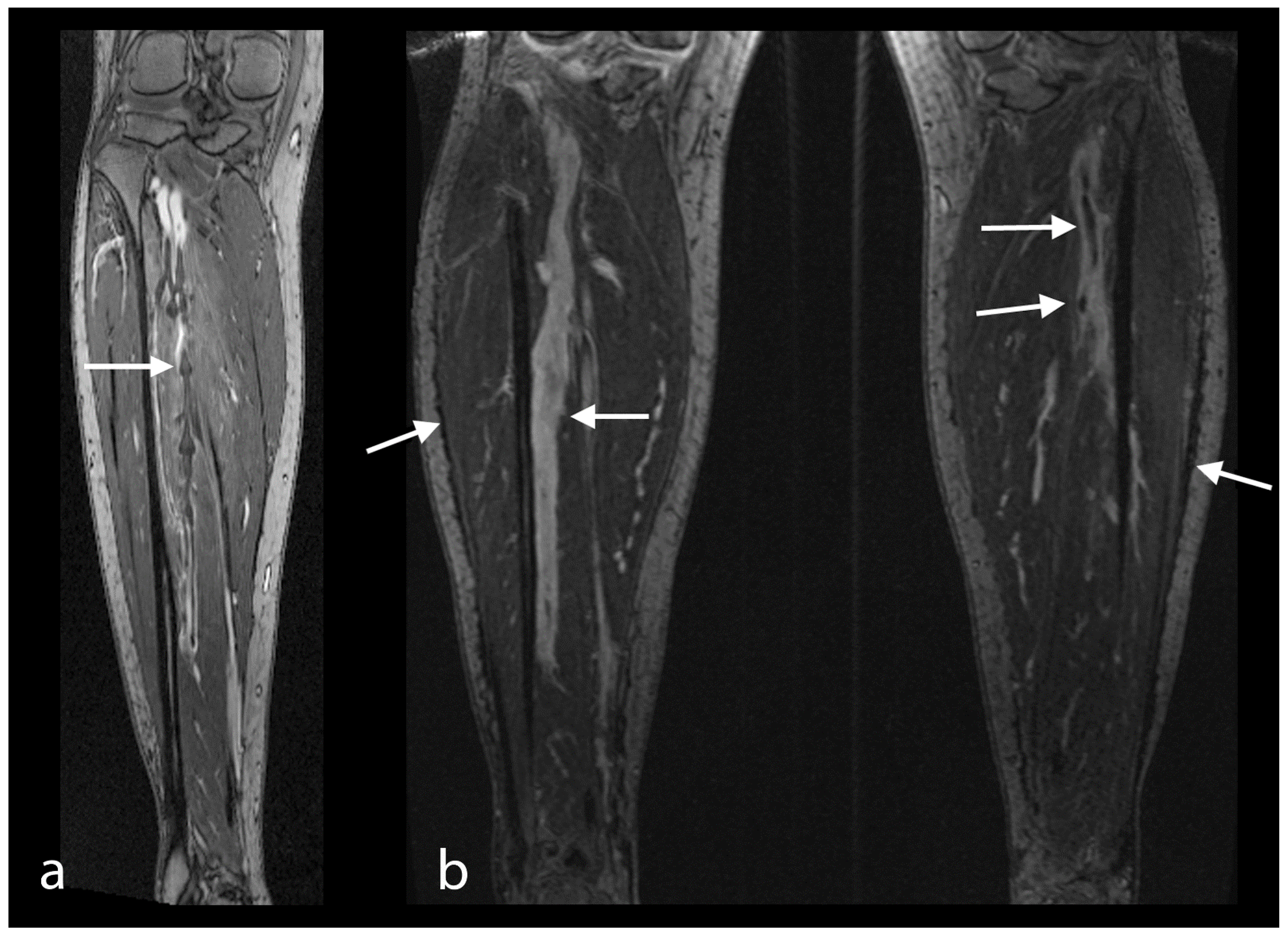
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andia, M.E.; Saha, P.; Jenkins, J.; Modarai, B.; Wiethoff, A.J.; Phinikaridou, A.; Grover, S.P.; Patel, A.S.; Schaeffter, T.; Smith, A.; et al. Fibrin-targeted magnetic resonance imaging allows in vivo quantification of thrombus fibrin content and identifies thrombi amenable for thrombolysis. Arter. Thromb. Vasc. Biol. 2014, 34, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochegger, B.; Ley-Zaporozhan, J.; Marchiori, E.; Irion, K.; Soares Souza, A., Jr.; Moreira, J.; Kauczor, H.U.; Ley, S. Magnetic resonance imaging findings in acute pulmonary embolism. Br. J. Radiol. 2011, 84, 282–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obernosterer, A.; Aschauer, M.; Portugaller, H.; Köppel, H.; Lipp, R.W. Three-dimensional gadolinium-enhanced magnetic resonance angiography used as a “one-stop shop” imaging procedure for venous thromboembolism: A pilot study. Angiology 2005, 56, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.; Rodríguez, C.; León, F.; Jara–Palomares, L.; López-Reyes, R.; Ruiz-Artacho, P.; Elías, T.; Otero, R.; García-Ortega, A.; Rivas-Guerrero, A.; et al. Randomised controlled trial of a prognostic assessment and management pathway to reduce the length of hospital stay in normotensive patients with acute pulmonary embolism. Eur. Respir. J. 2021, 59, 2100412. [Google Scholar] [CrossRef] [PubMed]
- Winer-Muram, H.T. Pulmonary Emboli. In Diagnostic Imaging Chest, Part II, Section 4: Pulmonary Vasculature, 1st ed.; Gurney, J.W., Ed.; Amyrsys: Salt Lake City, UT, USA, 2006; pp. 50–53. [Google Scholar]
- Liberman, A.L.; Daruwalla, V.J.; Collins, J.D.; Maas, M.B.; Botelho, M.P.; Ayache, J.B.; Carr, J.; Ruff, I.; Bernstein, R.A.; Alberts, M.J.; et al. Diagnostic yield of pelvic magnetic resonance venography in patients with cryptogenic stroke and patent foramen ovale. Stroke 2014, 45, 2324–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blättler, W.; Gerlach, H.; Hach-Wunderle, V.; Konstantinides, S.; Noppeney, T.; Pillny, M.; Riess, H.; Schellong, S.; Stiegler, H.; Wildberger, J.E. Bein- und Beckenvenenthrombose (TVT). Vasa Suppl. 2010, 78, 1–9. [Google Scholar]
- Meany, J.F.M.; Ridgway, J.P.; Chakraverty, S.; Robertson, I.; Kessel, D.; Radjenovic, A.; Kouwenhoven, M.; Kassner, A.; Smith, M.A. Stepping-table gadolinium-enhanced digital subtraction MR angiography of the aorta and lower extremity arteries: Preliminary experience. Radiology 1992, 211, 59–67. [Google Scholar] [CrossRef]
- Hansch, A.; Betge, S.; Poehlmann, G.; Neumann, S.; Baltzer, P.; Pfeil, A.; Waginger, M.; Boettcher, J.; Kaiser, W.A.; Wolf, G.; et al. Combined magnetic resonance imaging of deep venous thrombosis and pulmonary arteries after a single injection of a blood pool contrast agent. Eur. Radiol. 2011, 21, 318–325. [Google Scholar] [CrossRef]
- Tsuchiya, N.; van Beek, E.J.R.; Ohno, Y.; Hatabu, H.; Kanczor, H.U.; Swift, A.; Vogel-Claussen, J.; Biederer, J.; Wild, J.; Wielpütz, M.O.; et al. Magnetic resonance angiograpy for the primary diagnosis of pulmonary embolism: A review from the international workshop for pulmonary functional imaging. World J. Radiol. 2018, 10, 52–64. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Michaely, H.J.; Aschauer, M.A.; Deutschmann, H.; Bongartz, G.; Gutberlet, M.; Woitek, R.; Ertl-Wagner, B.; Kucharczyk, W.; Hammerstingl, R.; De Cobelli, F.; et al. Gadobutrol in renally impaired patients—Results of the GRIP study. Investig. Radiol. 2017, 52, 55–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, P.D.; Chenevert, T.L.; Fowler, S.E.; Goodman, L.R.; Gottschalk, A.; Hales, C.A.; Hull, R.D.; Jablonski, K.A.; Leeper KVJr Naidich, D.P.; Sak, D.J.; et al. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: A multicenter prospective study (PIOPED III). Ann. Intern. Med. 2010, 152, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Liu, D.; Kong, X.; Lei, Z. Combined MR imaging for pulmonary embolism and deep venous thrombosis by contrast-enhanced MR volume interpolated body examination. Curr. Med. Sci. 2020, 40, 192–198. [Google Scholar] [CrossRef]
- Ruehm, S.G. MR Venography. In Magnetic Resonance Angiography; Schneider, G., Prince, M.R., Meaney, J.F.M., Ho, V.B., Eds.; Springer: Berlin, Germany, 2005; pp. 331–340. [Google Scholar]
- Zollikofer, C.L.; Antonucci, F.; Stuckmann, G.; Mattias, P.; Brühlmann, W.F.; Salomonowitz, E.K. Use of Wallstent in the venous system including hemodialysis-related stenoses. Cardiovasc. Interv. Radiol. 1992, 15, 334–341. [Google Scholar] [CrossRef]
- Leiner, T.; Kessels, A.G.; Nelemans, P.J.; Vasbinder, G.B.; de Haan, M.W.; Kitslaar, P.E.; Ho, K.Y.; Tordoir, J.H.; van Engelshoven, J.M. Peripheral arterial disease: Comparison of color duplex US and contrast-enhanced MR angiography for diagnosis. Radiology 2005, 235, 699–708. [Google Scholar] [CrossRef]
- Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galiè, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.; Ferreira, D.; Janssens, U.; et al. Guidelines on the diagnosis and management of acute pulmonary embolism. The task force for diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2008, 29, 2276–2285. [Google Scholar] [PubMed] [Green Version]
- Sostman, D.H.; Jablonski, K.A.; Wooddard, P.K.; Stein, P.D.; Naidich, D.P.; Chenevert, T.L.; Weg, J.G.; Hales, C.A.; Hull, R.D.; Goodman, L.R.; et al. Factors in the technical quality of gadolinium enhanced magnetic resonance angiography for pulmonary embolism in PIOPED III. Int. J. Cardiovasc. Imag. 2012, 28, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Woodard, P.K.; Chenevert, T.L.; Dirk Sostman, H.; Jablonski, K.A.; Stein, P.D.; Goodman, L.R.; Londy, F.J.; Narra, V.; Hales, C.A.; Hull, R.D.; et al. Signal quality of single dose gadobenate dimeglumine pulmonary MRA examinations exceeds quality of MRA performed with double dose gadopentetate dimeglumine. Int. J. Cardiovasc. Imag. 2012, 28, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.; Rief, M.; Asbach, P.; Vogtmann, T.; Huppertz, A.; Beling, M.; Butler, C.; Laule, M.; Warmuth, C.; Taupitz, M.; et al. Gadofosveset trisodium-enhanced magnetic resonance angiography of the left atrium—A feasibility study. Eur. J. Radiol. 2010, 75, 166–172. [Google Scholar] [CrossRef]
- Goyen, M. Gadofosveset-enhanced magnetic resonance angiography. Vasc. Health Risk Manag. 2008, 4, 1–9. [Google Scholar] [CrossRef]
- McGregor, R.; Vymazal, J.; Martinez-Lopez, M.; Neuwirth, J.; Salgado, P.; Beregi, J.P.; Peduto, A.; de la Pena-Almaguer, E.; Slater, G.J.; Shamsi, K.; et al. A multi-center, comparative, phase 3 study to determine the efficacy of gadofosveset-enhanced magnetic resonance angiography for evaluation of renal artery disease. Eur. J. Radiol. 2008, 65, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Pasowicz, M.; Vymazal, J.; Seidl, Z.; Aschauer, M.; Konopka, M.; Bilecen, D.; Iezzi, R.; Ballarati, C. Gadobenate Dimenglumine and Gadofosveset Trisodium for MR Angiography of the renal arteries: Multicenter intraindividual crossover comparison. AJR Am. J. Roentgenol. 2010, 195, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Vogt, F.M.; Herborn, C.U.; Parsons, E.C., Jr.; Barkhausen, J.; Kröger, K.; Goyen, M. Diagnostic performance of contrast-enhanced angiography of the aortoiliac arteries with the blood pool agent Vasovist: Initial results in comparison to arterial DAS. Rofo 2007, 179, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Hecht, E.M.; Rosenkrantz, A. Pulmonary MR angiography techniques and applications. Magn. Reson. Imaging Clin. N. Am. 2009, 17, 101–131. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.; Ley-Zaporozhan, J.; Pitton, M.B.; Schneider, J.; Wirth, G.M.; Mayer, E.; Düber, C.; Kreitner, K.F. Diagnostic performance of state-of-the-art imaging techniques for morphology assessment of vascular abnormalities in patients with chronic thromboembolic pulmonary hypertension (CTEPH). Eur. Radiol. 2011, 22, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Haage, P.; Krimgs, T.; Schmitz-Rode, T. Nontraumatic vascular emergencies: Imaging and intervention in acute venous occlusion. Eur. Radiol. 2002, 12, 2627–2633. [Google Scholar] [CrossRef]
- Enden, T.; Storås, T.H.; Negård, A.; Haig, Y.; Sandvik, L.; Gjesdal, K.I.; Sandset, P.M.; Kløw, N.E. Visualization of deep veins and detection of deep vein thrombosis (DVT) with balancec turbo field echo (b-TFE) and contrast-enhanced T1 fast field echo (CE-FFE) using a blood pool agent (BPA). J. Magn. Reson. Imaging 2010, 31, 416–424. [Google Scholar] [CrossRef]
- Schaefer-Prokop, C.; Prokop, M. MDCT for the diagnosis of acute pulmonary embolism. Eur. Radiol. 2005, 15, D37–D41. [Google Scholar] [CrossRef]
- Fink, C. MR Angiography of the Pulmonary Vasculature. In MRI of the Lung; Kauczor, H.U., Ed.; Springer: Berlin, Germany, 2009; pp. 17–23. [Google Scholar]
- Aschauer, M.; Deutschmann, H.A.; Stollberger, R.; Hausegger, K.A.; Obernosterer, A.; Schöllnast, H.; Ebner, F. Value of a blood pool contrast agent in MR venography of the lower extremities and pelvis: Preliminary results in 12 patients. Magn. Reson. Med. 2003, 50, 993–1002. [Google Scholar] [CrossRef]
- Kucher, N.; Stuck, A.K. CardioPulse: Interventional treatment of venous thromboembolism: A review and update of treatments in 2014. Eur. Heart J. 2015, 36, 587–589. [Google Scholar] [PubMed]
- Prince, M.R.; Sostman, H.D. MR Venography: Unsung and Underutilized. Radiology 2003, 226, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Mathevosian, S.; Takegawa, Y.; Hassani, C.; Jalili, M.H.; Finn, J.P. Evaluation of aortic stent endoleaks using ferumoxytol enhanced MR angiography. In Proceedings of the 34th Annual International Conference of SMRA, Los Angeles, CA, USA, 23–26 August 2022; Yuan, C., Li, D., Eds.; SMRA: London, ON, Canada, 2022; p. 67. Available online: https://society4mra.org/index.php/smra-2022-annual-meeting (accessed on 17 November 2022).





| TR (ms) | TE (ms) | Flip Angle (Degree) | THK (mm) | FOV | Matrix | Duration (s) | |
|---|---|---|---|---|---|---|---|
| PMRA | 2.4 | 1.04 | 20 | 3 | 400–500 | 320 × 240 | 20 (4 dynamic sequences) |
| MRV * (3FOV) | 4.3 | 1.34 | 34 | 1.5 | 450–500 | 320 × 256 | 21,20,24 |
| HR-MRV (3FOV) | 6.21 | 2.08 | 24 | 1.3–1.1 | 500 | 384 × 384–512 × 512 | 123,225,286 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aschauer, M.A.; Keeling, I.M.; Salvan-Schaschl, C.V.; Knez, I.; Binder, B.; Raggam, R.B.; Trantina-Yates, A.E. Gadofosveset-Trinatrium-Enhanced MR Angiography and MR Venography in the Diagnosis of Venous Thromboembolic Disease: A Single-Center Cohort Study. Diseases 2022, 10, 122. https://doi.org/10.3390/diseases10040122
Aschauer MA, Keeling IM, Salvan-Schaschl CV, Knez I, Binder B, Raggam RB, Trantina-Yates AE. Gadofosveset-Trinatrium-Enhanced MR Angiography and MR Venography in the Diagnosis of Venous Thromboembolic Disease: A Single-Center Cohort Study. Diseases. 2022; 10(4):122. https://doi.org/10.3390/diseases10040122
Chicago/Turabian StyleAschauer, Manuela A., Ingeborg M. Keeling, Carmen V. Salvan-Schaschl, Igor Knez, Barbara Binder, Reinhard B. Raggam, and Ameli E. Trantina-Yates. 2022. "Gadofosveset-Trinatrium-Enhanced MR Angiography and MR Venography in the Diagnosis of Venous Thromboembolic Disease: A Single-Center Cohort Study" Diseases 10, no. 4: 122. https://doi.org/10.3390/diseases10040122
APA StyleAschauer, M. A., Keeling, I. M., Salvan-Schaschl, C. V., Knez, I., Binder, B., Raggam, R. B., & Trantina-Yates, A. E. (2022). Gadofosveset-Trinatrium-Enhanced MR Angiography and MR Venography in the Diagnosis of Venous Thromboembolic Disease: A Single-Center Cohort Study. Diseases, 10(4), 122. https://doi.org/10.3390/diseases10040122





