Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Comprehensive Overview
Abstract
1. Introduction
2. Thrombosis
3. Pathophysiology and Risk Factors for Thrombosis
3.1. Introduction
3.2. Genetic Predisposition
3.3. Inflammation’s Role in Thrombosis
3.4. Homocysteine Risk Triad
3.5. Venous Thrombotic Events
3.6. Spontaneous Platelet Aggregation
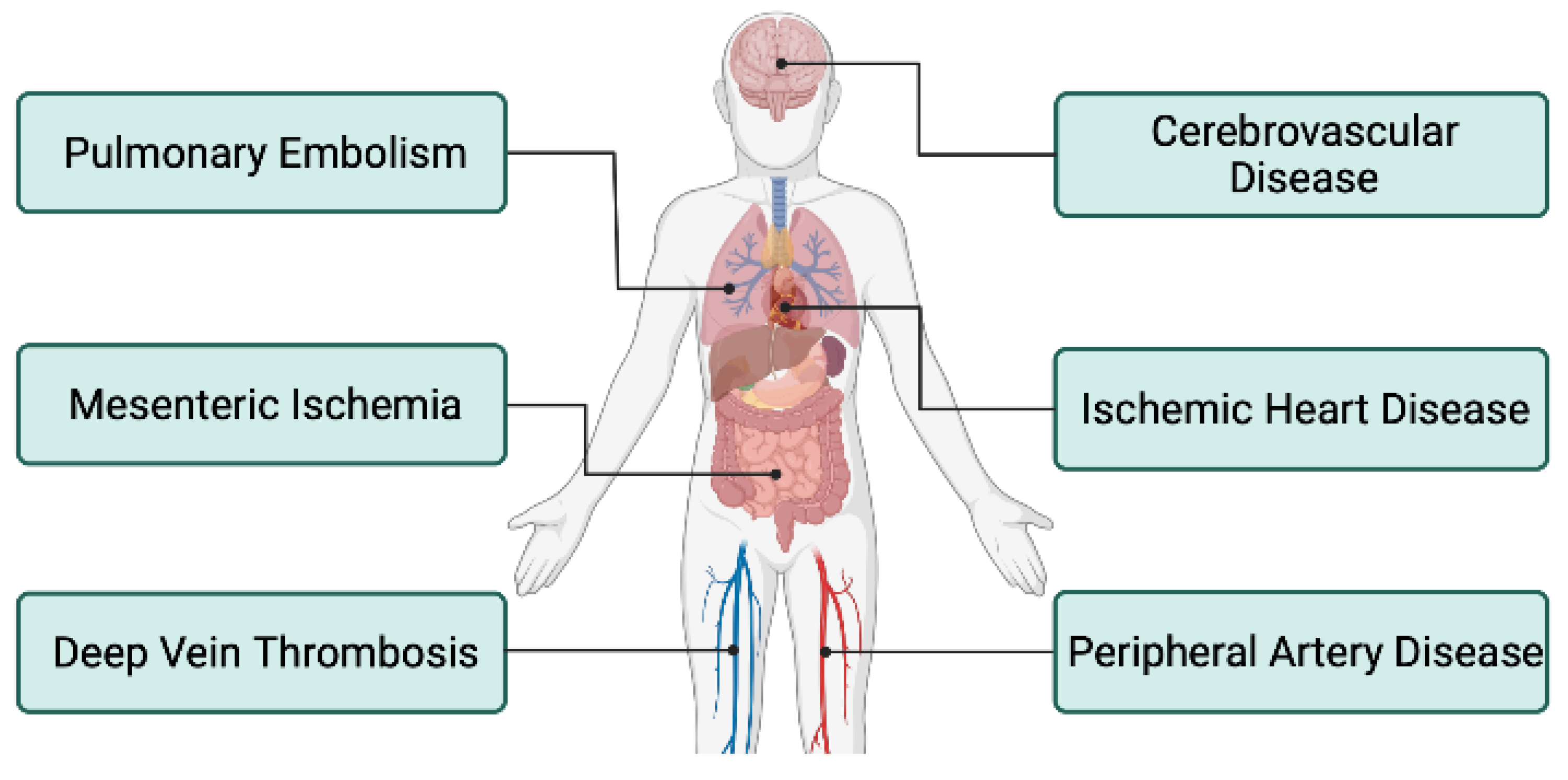
4. Thromboembolic Events in Patients with IBD
4.1. Introduction
4.2. Deep Vein Thrombosis and Pulmonary Embolism
4.3. Peripheral Artery Disease
4.4. Cerebrovascular
4.5. Ischemic Heart Disease
4.6. Mesenteric Ischemia
5. Treatment for IBD and Its Effect on Thromboembolic Risk
6. Treatment Recommendations for IBD to Reduce Risk
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Basu, D.; Lopez, I.; Kulkarni, A.; Sellin, J.H. Impact of race and ethnicity on inflammatory bowel disease. Am. J. Gastroenterol. 2005, 100, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Harries, A.D.; Baird, A.; Rhodes, J. Non-smoking: A feature of ulcerative colitis. Br. Med. J. Clin. Res. Ed. 1982, 284, 706. [Google Scholar] [CrossRef]
- Amre, D.K.; D’Souza, S.; Morgan, K.; Seidman, G.; Lambrette, P.; Grimard, G.; Israel, D.; Mack, D.; Ghadirian, P.; Deslandres, C.; et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am. J. Gastroenterol. 2007, 102, 2016–2025. [Google Scholar] [CrossRef]
- Weinstock, J.V.; Elliott, D.E. Helminths and the IBD hygiene hypothesis. Inflamm. Bowel Dis. 2009, 15, 128–133. [Google Scholar] [CrossRef]
- Levine, J.S.; Burakoff, R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 235–241. [Google Scholar]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Intrinsic Pathway of Coagulation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Bombeli, T.; Spahn, D.R. Updates in perioperative coagulation: Physiology and management of thromboembolism and haemorrhage. Br. J. Anaesth. 2004, 93, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; Hanlin, E.; Glurich, I.; Mazza, J.J.; Yale, S.H. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin. Med. Res. 2010, 8, 168–172. [Google Scholar] [CrossRef]
- Brotman, D.J.; Deitcher, S.R.; Lip, G.Y.; Matzdorff, A.C. Virchow’s triad revisited. South Med. J. 2004, 97, 213–214. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Ogura, Y.; Inohara, N.; Benito, A.; Chen, F.F.; Yamaoka, S.; Nunez, G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 2001, 276, 4812–4818. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ladhoff, A.; Pernthaler, A.; Swidsinski, S.; Loening-Baucke, V.; Ortner, M.; Weber, J.; Hoffmann, U.; Schreiber, S.; Dietel, M.; et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002, 122, 44–54. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Dietrich, G.; Hugot, J.P.; Barreau, F. Nod2: The intestinal gate keeper. PLoS Pathog. 2017, 13, e1006177. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Sina, C.; Gavrilova, O.; Hasler, R.; Ott, S.; Baines, J.F.; Schreiber, S.; Rosenstiel, P. Nod2 is essential for temporal development of intestinal microbial communities. Gut 2011, 60, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Hamm, C.M.; Gulati, A.S.; Sartor, R.B.; Chen, H.; Wu, X.; Zhang, T.; Rohlf, F.J.; Zhu, W.; Gu, C.; et al. Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS ONE 2012, 7, e26284. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kitani, A.; Murray, P.J.; Strober, W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 2004, 5, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Salzman, N.H.; Porter, E.; Nuding, S.; Weichenthal, M.; Petras, R.E.; Shen, B.; Schaeffeler, E.; Schwab, M.; Linzmeier, R.; et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 18129–18134. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cezard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef]
- Speckmann, C.; Ehl, S. XIAP deficiency is a mendelian cause of late-onset IBD. Gut 2014, 63, 1031–1032. [Google Scholar] [CrossRef]
- Uhlig, H.H. Monogenic diseases associated with intestinal inflammation: Implications for the understanding of inflammatory bowel disease. Gut 2013, 62, 1795–1805. [Google Scholar] [CrossRef]
- Shim, J.O.; Hwang, S.; Yang, H.R.; Moon, J.S.; Chang, J.Y.; Ko, J.S.; Park, S.S.; Kang, G.H.; Kim, W.S.; Seo, J.K. Interleukin-10 receptor mutations in children with neonatal-onset Crohn’s disease and intractable ulcerating enterocolitis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1235–1240. [Google Scholar] [CrossRef]
- Cadwell, K.; Liu, J.Y.; Brown, S.L.; Miyoshi, H.; Loh, J.; Lennerz, J.K.; Kishi, C.; Kc, W.; Carrero, J.A.; Hunt, S.; et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008, 456, 259–263. [Google Scholar] [CrossRef]
- Gunther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.J.; Hedrick, S.M.; Tenzer, S.; Neurath, M.F.; et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Khosravi, A.; Kusumawardhani, I.P.; Kwon, A.H.; Vasconcelos, A.C.; Cunha, L.D.; Mayer, A.E.; Shen, Y.; Wu, W.L.; Kambal, A.; et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science 2016, 352, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Anver, M.R.; Haines, D.C.; Melhorn, J.M.; Gorelick, P.; Yan, L.; Fox, J.G. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab. Anim. Sci. 1996, 46, 15–20. [Google Scholar] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Nenci, A.; Becker, C.; Wullaert, A.; Gareus, R.; van Loo, G.; Danese, S.; Huth, M.; Nikolaev, A.; Neufert, C.; Madison, B.; et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007, 446, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Chamouard, P.; Grunebaum, L.; Wiesel, M.L.; Frey, P.L.; Wittersheim, C.; Sapin, R.; Baumann, R.; Cazenave, J.P. Prothrombin fragment 1 + 2 and thrombin-antithrombin III complex as markers of activation of blood coagulation in inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 1995, 7, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Bisoendial, R.J.; Kastelein, J.J.; Levels, J.H.; Zwaginga, J.J.; van den Bogaard, B.; Reitsma, P.H.; Meijers, J.C.; Hartman, D.; Levi, M.; Stroes, E.S. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ. Res. 2005, 96, 714–716. [Google Scholar] [CrossRef]
- Thompson, N.P.; Wakefield, A.J.; Pounder, R.E. Inherited disorders of coagulation appear to protect against inflammatory bowel disease. Gastroenterology 1995, 108, 1011–1015. [Google Scholar] [CrossRef]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics 2012, 130, e794–e803. [Google Scholar] [CrossRef]
- Bhatia, P.; Singh, N. Homocysteine excess: Delineating the possible mechanism of neurotoxicity and depression. Fundam. Clin. Pharmacol. 2015, 29, 522–528. [Google Scholar] [CrossRef]
- Danese, S.; Sgambato, A.; Papa, A.; Scaldaferri, F.; Pola, R.; Sans, M.; Lovecchio, M.; Gasbarrini, G.; Cittadini, A.; Gasbarrini, A. Homocysteine triggers mucosal microvascular activation in inflammatory bowel disease. Am. J. Gastroenterol. 2005, 100, 886–895. [Google Scholar] [CrossRef]
- Danese, S.; Papa, A.; Saibeni, S.; Repici, A.; Malesci, A.; Vecchi, M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am. J. Gastroenterol. 2007, 102, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Topal, G.; Brunet, A.; Millanvoye, E.; Boucher, J.L.; Rendu, F.; Devynck, M.A.; David-Dufilho, M. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radic. Biol. Med. 2004, 36, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Brozek, J.; Szczeklik, A. Homocysteine and thrombosis: From basic science to clinical evidence. Thromb. Haemost. 2005, 94, 907–915. [Google Scholar] [CrossRef]
- Ingrosso, D.; Cimmino, A.; Perna, A.F.; Masella, L.; De Santo, N.G.; De Bonis, M.L.; Vacca, M.; D’Esposito, M.; D’Urso, M.; Galletti, P.; et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 2003, 361, 1693–1699. [Google Scholar] [CrossRef]
- Lentz, S.R.; Sadler, J.E. Inhibition of thrombomodulin surface expression and protein C activation by the thrombogenic agent homocysteine. J. Clin. Investig. 1991, 88, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.J.; Coleman, M.; Innerfield, I. Letter: Antithrombin activity in homocystinuria. Lancet 1975, 1, 1094. [Google Scholar] [CrossRef]
- Lentz, S.R.; Sobey, C.G.; Piegors, D.J.; Bhopatkar, M.Y.; Faraci, F.M.; Malinow, M.R.; Heistad, D.D. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J. Clin. Investig. 1996, 98, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, G.; Davi, G.; Margaglione, M.; Cirillo, F.; Grandone, E.; Ciabattoni, G.; Catalano, I.; Strisciuglio, P.; Andria, G.; Patrono, C.; et al. Abnormally high thromboxane biosynthesis in homozygous homocystinuria. Evidence for platelet involvement and probucol-sensitive mechanism. J. Clin. Investig. 1993, 92, 1400–1406. [Google Scholar] [CrossRef]
- Coppola, A.; Davi, G.; De Stefano, V.; Mancini, F.P.; Cerbone, A.M.; Di Minno, G. Homocysteine, coagulation, platelet function, and thrombosis. Semin. Thromb. Hemost. 2000, 26, 243–254. [Google Scholar] [CrossRef]
- Davi, G.; Di Minno, G.; Coppola, A.; Andria, G.; Cerbone, A.M.; Madonna, P.; Tufano, A.; Falco, A.; Marchesani, P.; Ciabattoni, G.; et al. Oxidative stress and platelet activation in homozygous homocystinuria. Circulation 2001, 104, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Fryer, R.H.; Wilson, B.D.; Gubler, D.B.; Fitzgerald, L.A.; Rodgers, G.M. Homocysteine, a risk factor for premature vascular disease and thrombosis, induces tissue factor activity in endothelial cells. Arterioscler. Thromb. 1993, 13, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, B.H.; de Bastos, M.; Rosendaal, F.R.; van Hylckama Vlieg, A.; Helmerhorst, F.M.; Stijnen, T.; Dekkers, O.M. Different combined oral contraceptives and the risk of venous thrombosis: Systematic review and network meta-analysis. BMJ 2013, 347, f5298. [Google Scholar] [CrossRef] [PubMed]
- Melis, F.; Vandenbrouke, J.P.; Buller, H.R.; Colly, L.P.; Bloemenkamp, K.W. Estimates of risk of venous thrombosis during pregnancy and puerperium are not influenced by diagnostic suspicion and referral basis. Am. J. Obs. Gynecol. 2004, 191, 825–829. [Google Scholar] [CrossRef]
- Weng, M.T.; Park, S.H.; Matsuoka, K.; Tung, C.C.; Lee, J.Y.; Chang, C.H.; Yang, S.K.; Watanabe, M.; Wong, J.M.; Wei, S.C. Incidence and Risk Factor Analysis of Thromboembolic Events in East Asian Patients with Inflammatory Bowel Disease, a Multinational Collaborative Study. Inflamm. Bowel Dis. 2018, 24, 1791–1800. [Google Scholar] [CrossRef]
- Heo, C.M.; Kim, T.J.; Kim, E.R.; Hong, S.N.; Chang, D.K.; Yang, M.; Kim, S.; Kim, Y.H. Risk of venous thromboembolism in Asian patients with inflammatory bowel disease: A nationwide cohort study. Sci. Rep. 2021, 11, 2025. [Google Scholar] [CrossRef]
- van Zaane, B.; Nur, E.; Squizzato, A.; Gerdes, V.E.; Buller, H.R.; Dekkers, O.M.; Brandjes, D.P. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J. Thromb. Haemost. 2010, 8, 2483–2493. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.D.; Skup, M.; Mulani, P.M.; Lin, J.; Chao, J. Increased risk of venous thromboembolic events with corticosteroid vs. biologic therapy for inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2015, 13, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Webberley, M.J.; Hart, M.T.; Melikian, V. Thromboembolism in inflammatory bowel disease: Role of platelets. Gut 1993, 34, 247–251. [Google Scholar] [CrossRef]
- van Wersch, J.W.; Houben, P.; Rijken, J. Platelet count, platelet function, coagulation activity and fibrinolysis in the acute phase of inflammatory bowel disease. J. Clin. Chem. Clin. Biochem. 1990, 28, 513–517. [Google Scholar] [CrossRef]
- Danese, S.; Fiocchi, C. Platelet activation and the CD40/CD40 ligand pathway: Mechanisms and implications for human disease. Crit. Rev. Immunol. 2005, 25, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Rampton, D.S.; Rogers, J.; Williams, N.S. Platelet aggregation and neutrophil sequestration in the mesenteric circulation in inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 1997, 9, 1213–1217. [Google Scholar] [PubMed]
- Klinger, M.H. Platelets and inflammation. Anat. Embryol. 1997, 196, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; de la Motte, C.; Sturm, A.; Vogel, J.D.; West, G.A.; Strong, S.A.; Katz, J.A.; Fiocchi, C. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology 2003, 124, 1249–1264. [Google Scholar] [CrossRef]
- Power, C.A.; Clemetson, J.M.; Clemetson, K.J.; Wells, T.N. Chemokine and chemokine receptor mRNA expression in human platelets. Cytokine 1995, 7, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, A.H.; Kosmidou, M.; Giannopoulos, S.; Katsanos, K.H.; Tsivgoulis, G.; Kyritsis, A.P.; Tsianos, E.V. Cerebral arterial infarction in inflammatory bowel diseases. Eur. J. Intern. Med. 2014, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, J.H.; Sharpe, J.A.; Sutton, D.M. Cerebral and retinal vascular complications of inflammatory bowel disease. Ann. Neurol. 1979, 5, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.M.; Alstead, E.M.; Greaves, R.R.; Feakins, R.M.; Pollok, R.C.; Rampton, D.S. Acute mesenteric infarction: An important cause of abdominal pain in ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Prior, A.; Strang, F.A.; Whorwell, P.J. Internal carotid artery occlusion in association with Crohn’s disease. Dig. Dis. Sci. 1987, 32, 1047–1050. [Google Scholar] [CrossRef]
- Mutlu, B.; Ermeydan, C.M.; Enc, F.; Fotbolcu, H.; Demirkol, O.; Bayrak, F.; Basaran, Y. Acute myocardial infarction in a young woman with severe ulcerative colitis. Int. J. Cardiol. 2002, 83, 183–185. [Google Scholar] [CrossRef]
- Novacek, G.; Haumer, M.; Schima, W.; Muller, C.; Miehsler, W.; Polterauer, P.; Vogelsang, H. Aortic mural thrombi in patients with inflammatory bowel disease: Report of two cases and review of the literature. Inflamm. Bowel Dis. 2004, 10, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Haumer, M.; Teml, A.; Dirisamer, A.; Vogelsang, H.; Koppensteiner, R.; Novacek, G. Severe ulcerative colitis complicated by an arterial thrombus in the brachiocephalic trunk. Inflamm. Bowel Dis. 2007, 13, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Fumery, M.; Xiaocang, C.; Dauchet, L.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Colombel, J.F. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: A meta-analysis of observational studies. J. Crohns Colitis 2014, 8, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.K.; Nguyen, G.C. Venous thromboembolism in inflammatory bowel disease: An epidemiological review. Am. J. Gastroenterol. 2011, 106, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Blanchard, J.F.; Houston, D.S.; Wajda, A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: A population-based cohort study. Thromb. Haemost. 2001, 85, 430–434. [Google Scholar]
- Huerta, C.; Johansson, S.; Wallander, M.A.; Rodriguez, L.A.G. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch. Intern. Med. 2007, 167, 935–943. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Horvath-Puho, E.; Sandler, R.S.; Rubin, D.T.; Ullman, T.A.; Pedersen, L.; Baron, J.A.; Sorensen, H.T. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: A population-based nationwide study. Gut 2011, 60, 937–943. [Google Scholar] [CrossRef]
- Grainge, M.J.; West, J.; Card, T.R. Venous thromboembolism during active disease and remission in inflammatory bowel disease: A cohort study. Lancet 2010, 375, 657–663. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Nabalamba, A. Hospitalization-based major comorbidity of inflammatory bowel disease in Canada. Can. J. Gastroenterol. 2007, 21, 507–511. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Sam, J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am. J. Gastroenterol. 2008, 103, 2272–2280. [Google Scholar] [CrossRef]
- Sridhar, A.R.; Parasa, S.; Navaneethan, U.; Crowell, M.D.; Olden, K. Comprehensive study of cardiovascular morbidity in hospitalized inflammatory bowel disease patients. J. Crohns Colitis 2011, 5, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Boudreau, H.; Harris, M.L.; Maxwell, C.V. Outcomes of obstetric hospitalizations among women with inflammatory bowel disease in the United States. Clin. Gastroenterol. Hepatol. 2009, 7, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Wallaert, J.B.; De Martino, R.R.; Marsicovetere, P.S.; Goodney, P.P.; Finlayson, S.R.; Murray, J.J.; Holubar, S.D. Venous thromboembolism after surgery for inflammatory bowel disease: Are there modifiable risk factors? Data from ACS NSQIP. Dis. Colon Rectum 2012, 55, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Novacek, G.; Weltermann, A.; Sobala, A.; Tilg, H.; Petritsch, W.; Reinisch, W.; Mayer, A.; Haas, T.; Kaser, A.; Feichtenschlager, T.; et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology 2010, 139, 779–787.e771. [Google Scholar] [CrossRef] [PubMed]
- Yuhara, H.; Steinmaus, C.; Corley, D.; Koike, J.; Igarashi, M.; Suzuki, T.; Mine, T. Meta-analysis: The risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 37, 953–962. [Google Scholar] [CrossRef]
- Papay, P.; Miehsler, W.; Tilg, H.; Petritsch, W.; Reinisch, W.; Mayer, A.; Haas, T.; Kaser, A.; Feichtenschlager, T.; Fuchssteiner, H.; et al. Clinical presentation of venous thromboembolism in inflammatory bowel disease. J. Crohns Colitis 2013, 7, 723–729. [Google Scholar] [CrossRef]
- Duricova, D.; Pedersen, N.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Overall and cause-specific mortality in Crohn’s disease: A meta-analysis of population-based studies. Inflamm. Bowel Dis. 2010, 16, 347–353. [Google Scholar] [CrossRef]
- O’Connor, O.J.; Cahill, R.A.; Kirwan, W.O.; Redmond, H.P. The incidence of postoperative venous thrombosis among patients with ulcerative colitis. Ir. J. Med. Sci. 2005, 174, 20–22. [Google Scholar] [CrossRef]
- Merrill, A.; Millham, F. Increased risk of postoperative deep vein thrombosis and pulmonary embolism in patients with inflammatory bowel disease: A study of National Surgical Quality Improvement Program patients. Arch. Surg. 2012, 147, 120–124. [Google Scholar] [CrossRef]
- Talbot, R.W.; Heppell, J.; Dozois, R.R.; Beart, R.W., Jr. Vascular complications of inflammatory bowel disease. Mayo. Clin. Proc. 1986, 61, 140–145. [Google Scholar] [CrossRef]
- Solem, C.A.; Loftus, E.V.; Tremaine, W.J.; Sandborn, W.J. Venous thromboembolism in inflammatory bowel disease. Am. J. Gastroenterol. 2004, 99, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Cagan, A.; Gainer, V.S.; Cheng, S.C.; Cai, T.; Scoville, E.; Konijeti, G.G.; Szolovits, P.; Shaw, S.Y.; Churchill, S.; et al. Thromboprophylaxis is associated with reduced post-hospitalization venous thromboembolic events in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, H.; Loftus, E.V., Jr.; Pardi, D.S. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 382–393.e1. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chen, Y.G.; Lin, C.L.; Huang, W.S.; Kao, C.H. Inflammatory Bowel Disease Increases the Risk of Peripheral Arterial Disease: A Nationwide Cohort Study. Medicine 2015, 94, e2381. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Beaugerie, L.; Carrat, F.; Andersen, N.N.; Jess, T.; Schwarzinger, M.; BERENICE Study Group. Increased risk of acute arterial events in young patients and severely active IBD: A nationwide French cohort study. Gut 2018, 67, 1261–1268. [Google Scholar] [CrossRef]
- Scheid, R.; Teich, N. Neurologic manifestations of ulcerative colitis. Eur. J. Neurol. 2007, 14, 483–493. [Google Scholar] [CrossRef]
- Andersohn, F.; Waring, M.; Garbe, E. Risk of ischemic stroke in patients with Crohn’s disease: A population-based nested case-control study. Inflamm. Bowel Dis. 2010, 16, 1387–1392. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Wajda, A.; Blanchard, J.F. The incidence of arterial thromboembolic diseases in inflammatory bowel disease: A population-based study. Clin. Gastroenterol. Hepatol. 2008, 6, 41–45. [Google Scholar] [CrossRef]
- Casella, G.; Tontini, G.E.; Bassotti, G.; Pastorelli, L.; Villanacci, V.; Spina, L.; Baldini, V.; Vecchi, M. Neurological disorders and inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 8764–8782. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Jensen, G.V.; Torp-Pedersen, C.; Nielsen, O.H.; Gislason, G.H.; Hansen, P.R. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death–a Danish nationwide cohort study. PLoS ONE 2013, 8, e56944. [Google Scholar] [CrossRef]
- Xiao, Z.; Pei, Z.; Yuan, M.; Li, X.; Chen, S.; Xu, L. Risk of Stroke in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Stroke Cerebrovasc. Dis. 2015, 24, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jia, F.; Zhang, B.; Zhang, P. Risk of cardiovascular disease in inflammatory bowel disease. Exp. Ther. Med. 2017, 13, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.; Magowan, S.; Accortt, N.A.; Chen, J.; Stone, C.D. Risk of arterial thrombotic events in inflammatory bowel disease. Am. J. Gastroenterol. 2009, 104, 1445–1451. [Google Scholar] [CrossRef]
- Yarur, A.J.; Deshpande, A.R.; Pechman, D.M.; Tamariz, L.; Abreu, M.T.; Sussman, D.A. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am. J. Gastroenterol. 2011, 106, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, S.; Vasudevan, A.; Langenberg, D.V. Inflammatory bowel disease and superior mesenteric artery thromboembolism. Intest. Res. 2020, 18, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.; Rivero, E.; Montoro, M.A.; Garcia-Rodriguez, L.A. Risk factors for intestinal ischaemia among patients registered in a UK primary care database: A nested case-control study. Aliment. Pharmacol. Ther. 2011, 33, 969–978. [Google Scholar] [CrossRef]
- Qu, C.; Cao, J.; Liu, K.; Tan, B.; Zhu, C.; Li, K.; Qu, L. Crohn’s Disease Complicated With Extensive Thrombosis of Limbs and Mesenteric Arteries: A Case Report and Literature Review. Ann. Vasc. Surg. 2019, 58, 382.e15–382.e19. [Google Scholar] [CrossRef]
- Ng, S.C.; Kamm, M.A. Therapeutic strategies for the management of ulcerative colitis. Inflamm. Bowel Dis. 2009, 15, 935–950. [Google Scholar] [CrossRef]
- Kozuch, P.L.; Hanauer, S.B. Treatment of inflammatory bowel disease: A review of medical therapy. World J. Gastroenterol. 2008, 14, 354–377. [Google Scholar] [CrossRef]
- Travis, S.P.; Stange, E.F.; Lemann, M.; Oresland, T.; Bemelman, W.A.; Chowers, Y.; Colombel, J.F.; D’Haens, G.; Ghosh, S.; Marteau, P.; et al. European evidence-based Consensus on the management of ulcerative colitis: Current management. J. Crohns Colitis 2008, 2, 24–62. [Google Scholar] [CrossRef]
- Kuhbacher, T.; Folsch, U.R. Practical guidelines for the treatment of inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Stange, E.F.; Travis, S.P.; Vermeire, S.; Reinisch, W.; Geboes, K.; Barakauskiene, A.; Feakins, R.; Flejou, J.F.; Herfarth, H.; Hommes, D.W.; et al. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J. Crohns Colitis 2008, 2, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, A.; Sachar, D.B.; Practice Parameters Committee of the American College of G. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2004, 99, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Cahill, M.R.; Newland, A.C.; Rampton, D.S. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology 1994, 106, 840–845. [Google Scholar] [CrossRef]
- Collins, C.E.; Rampton, D.S. Platelet dysfunction: A new dimension in inflammatory bowel disease. Gut 1995, 36, 5–8. [Google Scholar] [CrossRef]
- Andoh, A.; Yoshida, T.; Yagi, Y.; Bamba, S.; Hata, K.; Tsujikawa, T.; Kitoh, K.; Sasaki, M.; Fujiyama, Y. Increased aggregation response of platelets in patients with inflammatory bowel disease. J. Gastroenterol. 2006, 41, 47–54. [Google Scholar] [CrossRef]
- Yan, S.L.; Russell, J.; Harris, N.R.; Senchenkova, E.Y.; Yildirim, A.; Granger, D.N. Platelet abnormalities during colonic inflammation. Inflamm. Bowel Dis. 2013, 19, 1245–1253. [Google Scholar] [CrossRef]
- Schurmann, G.M.; Bishop, A.E.; Facer, P.; Vecchio, M.; Lee, J.C.; Rampton, D.S.; Polak, J.M. Increased expression of cell adhesion molecule P-selectin in active inflammatory bowel disease. Gut 1995, 36, 411–418. [Google Scholar] [CrossRef]
- Fagerstam, J.P.; Whiss, P.A.; Strom, M.; Andersson, R.G. Expression of platelet P-selectin and detection of soluble P-selectin, NPY and RANTES in patients with inflammatory bowel disease. Inflamm. Res. 2000, 49, 466–472. [Google Scholar] [CrossRef]
- Hanauer, S.B. Inflammatory bowel disease. N. Engl. J. Med. 1996, 334, 841–848. [Google Scholar] [CrossRef]
- Carty, E.; MacEy, M.; Rampton, D.S. Inhibition of platelet activation by 5-aminosalicylic acid in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2000, 14, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Flourie, B.; Hagege, H.; Tucat, G.; Maetz, D.; Hebuterne, X.; Kuyvenhoven, J.P.; Tan, T.G.; Pierik, M.J.; Masclee, A.A.; Dewit, O.; et al. Randomised clinical trial: Once- vs. twice-daily prolonged-release mesalazine for active ulcerative colitis. Aliment. Pharmacol. Ther. 2013, 37, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Ozsoylu, S.; Strauss, H.S.; Diamond, L.K. Effects of corticosteroids on coagulation of the blood. Nature 1962, 195, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Johannesdottir, S.A.; Horvath-Puho, E.; Dekkers, O.M.; Cannegieter, S.C.; Jorgensen, J.O.; Ehrenstein, V.; Vandenbroucke, J.P.; Pedersen, L.; Sorensen, H.T. Use of glucocorticoids and risk of venous thromboembolism: A nationwide population-based case-control study. JAMA Intern. Med. 2013, 173, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Ordas, I.; Cabre, E.; Garcia-Sanchez, V.; Bastida, G.; Penalva, M.; Gomollon, F.; Garcia-Planella, E.; Merino, O.; Gutierrez, A.; et al. Safety of thiopurine therapy in inflammatory bowel disease: Long-term follow-up study of 3931 patients. Inflamm. Bowel Dis. 2013, 19, 1404–1410. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Renna, S.; Maida, M.; Dimarco, M.; Sapienza, C.; Affronti, M.; Orlando, E.; Rizzuto, G.; Orlando, R.; Ventimiglia, M.; et al. Tolerability profile of thiopurines in inflammatory bowel disease: A prospective experience. Scand J. Gastroenterol. 2017, 52, 981–987. [Google Scholar] [CrossRef]
- Seinen, M.L.; Ponsioen, C.Y.; de Boer, N.K.; Oldenburg, B.; Bouma, G.; Mulder, C.J.; van Bodegraven, A.A. Sustained clinical benefit and tolerability of methotrexate monotherapy after thiopurine therapy in patients with Crohn’s disease. Clin. Gastroenterol. Hepatol. 2013, 11, 667–672. [Google Scholar] [CrossRef]
- van der Poll, T.; Buller, H.R.; ten Cate, H.; Wortel, C.H.; Bauer, K.A.; van Deventer, S.J.; Hack, C.E.; Sauerwein, H.P.; Rosenberg, R.D.; ten Cate, J.W. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N. Engl. J. Med. 1990, 322, 1622–1627. [Google Scholar] [CrossRef]
- Page, M.J.; Bester, J.; Pretorius, E. The inflammatory effects of TNF-alpha and complement component 3 on coagulation. Sci. Rep. 2018, 8, 1812. [Google Scholar] [CrossRef]
- deFonseka, A.M.; Tuskey, A.; Conaway, M.R.; Behm, B.W. Antitumor Necrosis Factor-alpha Therapy Is Associated With Reduced Risk of Thromboembolic Events in Hospitalized Patients With Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2016, 50, 578–583. [Google Scholar] [CrossRef]
- Bollen, L.; Vande Casteele, N.; Peeters, M.; Bessonov, K.; Van Steen, K.; Rutgeerts, P.; Ferrante, M.; Hoylaerts, M.F.; Vermeire, S.; Gils, A. Short-term effect of infliximab is reflected in the clot lysis profile of patients with inflammatory bowel disease: A prospective study. Inflamm. Bowel Dis. 2015, 21, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Detrez, I.; Thomas, D.; Van Steen, K.; Ballet, V.; Peeters, M.; Hoylaerts, M.F.; Van Assche, G.; Vermeire, S.; Ferrante, M.; Gils, A. Successful Infliximab Treatment is Associated With Reversal of Clotting Abnormalities in Inflammatory Bowel Disease Patients. J. Clin. Gastroenterol. 2020, 54, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Heit, J.A. Venous thromboembolism epidemiology: Implications for prevention and management. Semin. Thromb. Hemost. 2002, 28 (Suppl. 2), 3–13. [Google Scholar] [CrossRef]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Schunemann, H.J.; Cushman, M.; Burnett, A.E.; Kahn, S.R.; Beyer-Westendorf, J.; Spencer, F.A.; Rezende, S.M.; Zakai, N.A.; Bauer, K.A.; Dentali, F.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018, 2, 3198–3225. [Google Scholar] [CrossRef]
- Geerts, W.H.; Bergqvist, D.; Pineo, G.F.; Heit, J.A.; Samama, C.M.; Lassen, M.R.; Colwell, C.W. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008, 133, 381S–453S. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Sharma, S. Feasibility of venous thromboembolism prophylaxis during inflammatory bowel disease flares in the outpatient setting: A decision analysis. Inflamm. Bowel Dis. 2013, 19, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Bernstein, C.N.; Bitton, A.; Chan, A.K.; Griffiths, A.M.; Leontiadis, G.I.; Geerts, W.; Bressler, B.; Butzner, J.D.; Carrier, M.; et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology 2014, 146, 835–848.e836. [Google Scholar] [CrossRef] [PubMed]
- Ra, G.; Thanabalan, R.; Ratneswaran, S.; Nguyen, G.C. Predictors and safety of venous thromboembolism prophylaxis among hospitalized inflammatory bowel disease patients. J. Crohns Colitis 2013, 7, e479–e485. [Google Scholar] [CrossRef] [PubMed]
- Leizorovicz, A.; Cohen, A.T.; Turpie, A.G.; Olsson, C.G.; Vaitkus, P.T.; Goldhaber, S.Z.; Group, P.M.T.S. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004, 110, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ran, Z.H.; Tong, J.L.; Xiao, S.D. Meta-analysis: The utility and safety of heparin in the treatment of active ulcerative colitis. Aliment. Pharmacol. Ther. 2007, 26, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Michell, N.P.; Lalor, P.; Langman, M.J. Heparin therapy for ulcerative colitis? Effects and mechanisms. Eur. J. Gastroenterol. Hepatol. 2001, 13, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Underwood, F.E.; Coward, S.; Agrawal, M.; Ungaro, R.C.; Brenner, E.J.; Gearry, R.B.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; et al. The Multiple Waves of COVID-19 in Patients With Inflammatory Bowel Disease: A Temporal Trend Analysis. Inflamm. Bowel Dis. 2022, izab339. [Google Scholar] [CrossRef] [PubMed]
- Anikhindi, S.A.; Kumar, A.; Arora, A. COVID-19 in patients with inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1187–1193. [Google Scholar] [CrossRef]
- Siegel, C.A.; Christensen, B.; Kornbluth, A.; Rosh, J.R.; Kappelman, M.D.; Ungaro, R.C.; Johnson, D.F.; Chapman, S.; Wohl, D.A.; Mantzaris, G.J. Guidance for Restarting Inflammatory Bowel Disease Therapy in Patients Who Withheld Immunosuppressant Medications During COVID-19. J. Crohns Colitis 2020, 14, S769–S773. [Google Scholar] [CrossRef]
- Din, S.; Kent, A.; Pollok, R.C.; Meade, S.; Kennedy, N.A.; Arnott, I.; Beattie, R.M.; Chua, F.; Cooney, R.; Dart, R.J.; et al. Adaptations to the British Society of Gastroenterology guidelines on the management of acute severe UC in the context of the COVID-19 pandemic: A RAND appropriateness panel. Gut 2020, 69, 1769–1777. [Google Scholar] [CrossRef]

| Author (Year) | Findings |
|---|---|
| Yuhara (2013) [85] | This study found an RR of 2.2 (95% CI 1.83–2.65) when comparing the risk of VTE among subjects with and without IBD with similar results after adjusting for obesity and smoking. |
| Fumery (2014) [73] | The overall risk of VTE in an IBD population was increased by 96% in this study compared to the general population, (RR = 1.96, 95% CI = 1.67–2.30) with no statistical difference between UC and CD subgroups. |
| Papay (2013) [86] | This study found that 90% of VTE’s were DVT’s and PE’s among patients with IBD. |
| Bernstein (2007) [75] | VTE occurrence among hospitalized patients with IBD was significantly higher compared to hospitalized patients without IBD (IRR: 4.5 (UC) and 9.6 (CD)). |
| Novacek (2010) [84] | The probability of recurrence of VTE 5 years after cessation of anticoagulant medication was elevated among patients with IBD in comparison to patients without IBD (33.4%; 95% confidence interval [CI]: 21.8–45.0 vs. 21.7%; 95% CI: 18.8–24.6; p = 0.01). |
| Grainge (2010) [78] | This study found an RR of 3.0 (CI 1.7–6.3) when comparing VTE occurrence in hospitalized patients with IBD to those without. |
| Nguyen (2008) [80] | This study reported a significantly higher risk of VTE in patients with IBD discharges compared to non-IBD discharges. (OR 1.85 for UC and OR 1.48 for CD). Additionally, VTE was associated with increased mortality, longer hospital stays (by an average of 5 days) and a higher healthcare cost ($47,515 vs. $21,499) (OR 2.5) |
| Author | Findings |
|---|---|
| Kristensen (2013) [100] | Patients with IBD with flares or persistent disease had an increased risk (RR 1.17, 95% CI 1.05–1.31) of myocardial infarction compared to control patients. Patients with IBD with flares or persistent disease had an elevated risk (RR 1.35, 95% CI 1.25–1.45) of cardiovascular death compared to control patients. In patients with ongoing IBD flares this study reported an increased risk of myocardial infarction (RR 1.49, 95% CI 1.16–1.93) when compared to control patients. In patients with persistent IBD this study reported an elevated risk of myocardial infarction (RR 2.05, 95% CI 1.58–2.65) when compared to control patients. |
| Ha (2009) [103] | This study found that patients with IBD overall did not have an elevated risk of ischemic heart disease compared to controls but found that women over 40 years of age with IBD had a higher risk of myocardial infarction (HR = 1.6, p = 0.003) with similar risk between Crohn’s disease and ulcerative. |
| Yarur (2011) [104] | The incidence of coronary artery disease was significantly elevated in patients with IBD (HR 4.08, CI 2.49–6.70) compared to control patients even after adjusting for concurrent risk factors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gala, D.; Newsome, T.; Roberson, N.; Lee, S.M.; Thekkanal, M.; Shah, M.; Kumar, V.; Bandaru, P.; Gayam, V. Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Comprehensive Overview. Diseases 2022, 10, 73. https://doi.org/10.3390/diseases10040073
Gala D, Newsome T, Roberson N, Lee SM, Thekkanal M, Shah M, Kumar V, Bandaru P, Gayam V. Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Comprehensive Overview. Diseases. 2022; 10(4):73. https://doi.org/10.3390/diseases10040073
Chicago/Turabian StyleGala, Dhir, Taylor Newsome, Nicole Roberson, Soo Min Lee, Marvel Thekkanal, Mili Shah, Vikash Kumar, Praneeth Bandaru, and Vijay Gayam. 2022. "Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Comprehensive Overview" Diseases 10, no. 4: 73. https://doi.org/10.3390/diseases10040073
APA StyleGala, D., Newsome, T., Roberson, N., Lee, S. M., Thekkanal, M., Shah, M., Kumar, V., Bandaru, P., & Gayam, V. (2022). Thromboembolic Events in Patients with Inflammatory Bowel Disease: A Comprehensive Overview. Diseases, 10(4), 73. https://doi.org/10.3390/diseases10040073








