Hypertension, Anxiety and Obstructive Sleep Apnea in Cardiovascular Disease and COVID-19: Mediation by Dietary Salt
Abstract
:1. Introduction
2. Method
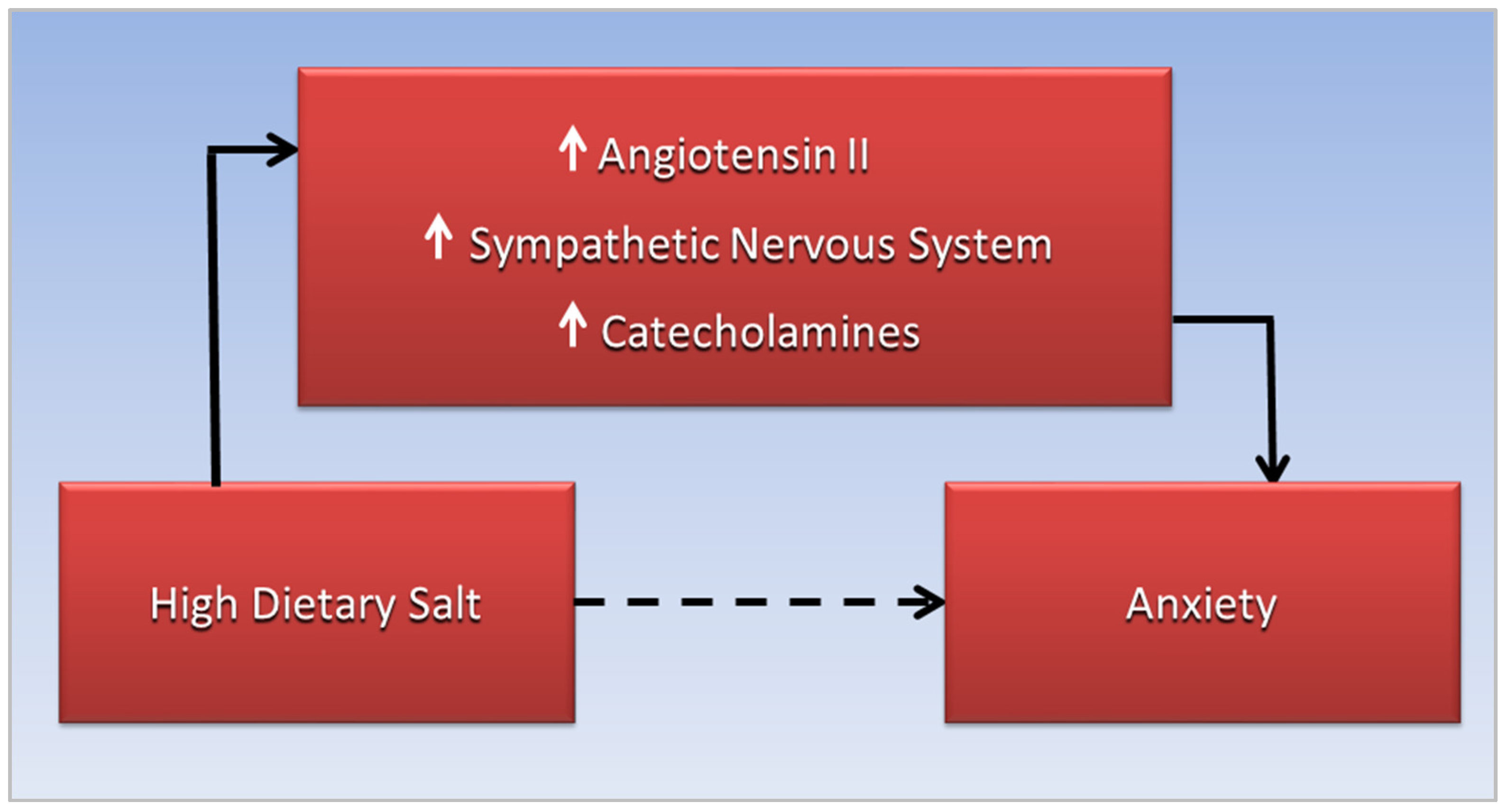
3. Anxiety, CVD, and Sodium Toxicity
4. OSA, Hypertension, and the Renin-Angiotensin-Aldosterone System
5. OSA, Anxiety, and Angiotensin II
6. Future Directions
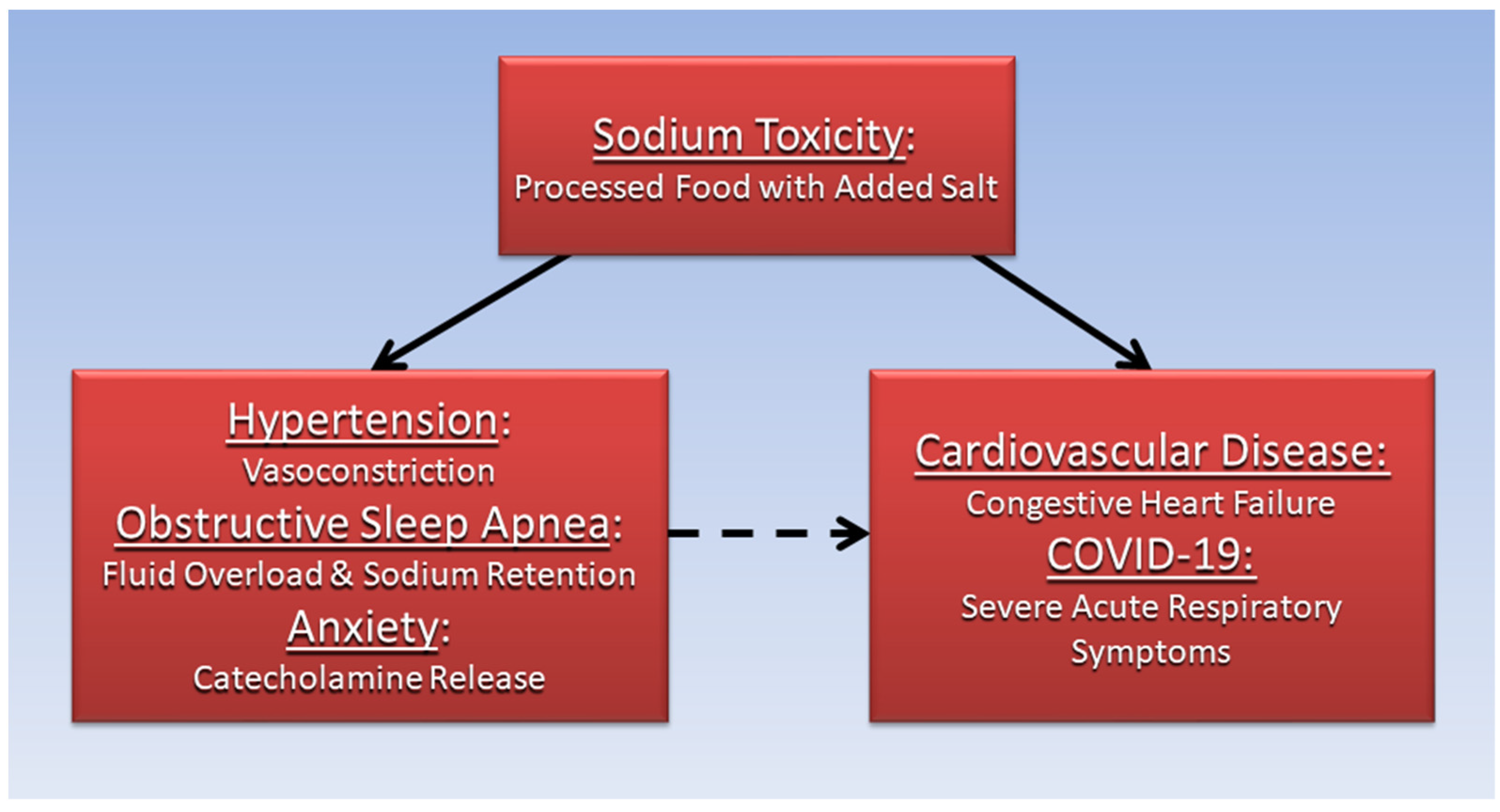
7. Conclusions
Funding
Conflicts of Interest
References
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hu, H.Y.; Chou, Y.J.; Huang, N.; Chou, Y.C.; Li, C.P. High Blood Pressure and All-Cause and Cardiovascular Disease Mortalities in Community-Dwelling Older Adults. Medicine 2015, 94, e2160. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Guyton, A.C.; Coleman, T.G.; Mizelle, H.L.; Woods, L.L. Regulation of arterial pressure: Role of pressure natriuresis and diuresis. Fed. Proc. 1986, 45, 2897–2903. [Google Scholar] [PubMed]
- Who.Int. Salt Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 30 August 2022).
- Choi, H.Y.; Park, H.C.; Ha, S.K. Salt Sensitivity and Hypertension: A Paradigm Shift from Kidney Malfunction to Vascular Endothelial Dysfunction. Electrolytes Blood Press. 2015, 13, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium intake and hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rust, P.; Ekmekcioglu, C. Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension. In Hypertension: From Basic Research to Clinical Practice; Islam, M.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 61–84. [Google Scholar] [CrossRef]
- Luzardo, L.; Noboa, O.; Boggia, J. Mechanisms of Salt-Sensitive Hypertension. Curr. Hypertens. Rev. 2015, 11, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Mugavero, K.; Bowman, B.A.; Frieden, T.R. Dietary Sodium and Cardiovascular Disease Risk—Measurement Matters. N. Engl. J. Med. 2016, 375, 580–586. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Xu, P.; Wang, Y.; Song, T.J.; Luo, N.; Zhao, L.J. Prevalence of and risk factors for anxiety after coronary heart disease: Systematic review and meta-analysis. Medicine 2019, 98, e16973. [Google Scholar] [CrossRef] [PubMed]
- Allgulander, C. Anxiety as a risk factor in cardiovascular disease. Curr. Opin. Psychiatry 2016, 29, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, H.R.; Saksvik-Lehouillier, I.; Stone, K.L.; Schernhammer, E.; Yaffe, K.; Langvik, E. Anxiety as a risk factor for cardiovascular disease independent of depression: A prospective examination of community-dwelling men (the MrOS study). Psychol. Health 2021, 36, 148–163. [Google Scholar] [CrossRef]
- De Hert, M.; Detraux, J.; Vancampfort, D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin. Neurosci. 2018, 20, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Takov, V.; Coletti, V.A. Generalized Anxiety Disorder. StatPearls [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441870/ (accessed on 7 October 2022).
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Who.Int. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 7 October 2022).
- Karlsen, H.R.; Matejschek, F.; Saksvik-Lehouillier, I.; Langvik, E. Anxiety as a risk factor for cardiovascular disease independent of depression: A narrative review of current status and conflicting findings. Health Psychol. Open 2021, 8, 2055102920987462. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Slowik, J.M.; Sankari, A.; Collen, J.F. Obstructive Sleep Apnea. StatPearls [Internet]. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459252/ (accessed on 7 October 2022).
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.; Herrmann, M.J.; Grize, L.; Hostettler, K.E.; Bassetti, S.; Siegemund, M.; Khanna, N.; Sava, M.; Sommer, G.; Tamm, M.; et al. Is sleep disordered breathing a risk factor for COVID-19 or vice versa? ERJ Open Res. 2022, 8, 00034-2022. [Google Scholar] [CrossRef]
- Kang, H.H.; Kim, J.H.; Kang, B.J.; Lee, T.; Ra, S.W.; Seo, K.W.; Jegal, Y.; Ahn, J.J. Undiagnosed Obstructive Sleep Apnea and Acute COVID-19 Infection—A Case Series. Chronobiol. Med. 2021, 3, 167–170. [Google Scholar] [CrossRef]
- Iannella, G.; Vicini, C.; Lechien, J.R.; Ravaglia, C.; Poletti, V.; di Cesare, S.; Amicarelli, E.; Gardelli, L.; Grosso, C.; Patacca, A.; et al. Association Between Severity of COVID-19 Respiratory Disease and Risk of Obstructive Sleep Apnea. Ear Nose Throat J. 2021, 1455613211029783. [Google Scholar] [CrossRef]
- Pena Orbea, C.; Wang, L.; Shah, V.; Jehi, L.; Milinovich, A.; Foldvary-Schaefer, N.; Chung, M.K.; Mashaqi, S.; Aboussouan, L.; Seidel, K.; et al. Association of Sleep-Related Hypoxia With Risk of COVID-19 Hospitalizations and Mortality in a Large Integrated Health System. JAMA Netw. Open 2021, 4, e2134241. [Google Scholar] [CrossRef]
- Acet Öztürk, N.A.; Aydın Güçlü, Ö.; Alkan, S.; Şengören Dikiş, Ö.; Sali, M.; Yılmaz, D.; Taşbaş, E.; Ertem Cengiz, A.; Bahçetepe, D.; Aydın, A. High-risk obstructive sleep apnea is related to longer hospital stay in COVID-19 patients. Eurasian J. Pulmonol. 2022, 24, 95. [Google Scholar] [CrossRef]
- Hwang, D.; Shi, J.; Chen, A.; Arguelles, J.; Becker, K.A.; Kim, J.B.; Woodrum, R.R.; Valentine, K.; Benjafield, A. Impact of Obstructive Sleep Apnea and Positive Airway Pressure Therapy on COVID-19 Outcomes. Am. J. Respir. Crit. Care Med. 2021, A1108. [Google Scholar]
- Labarca, G.; Henríquez-Beltrán, M.; Lamperti, L.; Nova-Lamperti, E.; Sanhueza, S.; Cabrera, C.; Quiroga, R.; Antilef, B.; Ormazábal, V.; Zúñiga, F.; et al. Impact of Obstructive Sleep Apnea (OSA) in COVID-19 Survivors, Symptoms Changes Between 4-Months and 1 Year After the COVID-19 Infection. Front. Med. 2022, 9, 884218. [Google Scholar] [CrossRef]
- Voncken, S.F.J.; Feron, T.M.H.; Laven, S.; Karaca, U.; Beerhorst, K.; Klarenbeek, P.; Straetmans, J.; de Vries, G.J.; Kolfoort-Otte, A.A.B.; de Kruif, M.D. Impact of obstructive sleep apnea on clinical outcomes in patients hospitalized with COVID-19. Sleep Breath. 2022, 26, 1399–1407. [Google Scholar] [CrossRef]
- Maas, M.B.; Kim, M.; Malkani, R.G.; Abbott, S.M.; Zee, P.C. Obstructive Sleep Apnea and Risk of COVID-19 Infection, Hospitalization and Respiratory Failure. Sleep Breath. 2021, 25, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Rögnvaldsson, K.G.; Eyþórsson, E.S.; Emilsson, Ö.I.; Eysteinsdóttir, B.; Pálsson, R.; Gottfreðsson, M.; Guðmundsson, G.; Steingrímsson, V. Obstructive sleep apnea is an independent risk factor for severe COVID-19: A population-based study. Sleep 2022, 45, zsab272. [Google Scholar] [CrossRef]
- Hu, M.; Han, X.; Ren, J.; Wang, Y.; Yang, H. Significant association of obstructive sleep apnoea with increased risk for fatal COVID-19: A quantitative meta-analysis based on adjusted effect estimates. Sleep Med. Rev. 2022, 63, 101624. [Google Scholar] [CrossRef] [PubMed]
- Cade, B.E.; Dashti, H.S.; Hassan, S.M.; Redline, S.; Karlson, E.W. Sleep Apnea and COVID-19 Mortality and Hospitalization. Am. J. Respir. Crit. Care Med. 2020, 202, 1462–1464. [Google Scholar] [CrossRef] [PubMed]
- Strausz, S.; Kiiskinen, T.; Broberg, M.; Ruotsalainen, S.; Koskela, J.; Bachour, A.; Palotie, A.; Palotie, T.; Ripatti, S.; Ollila, H.M. Sleep apnoea is a risk factor for severe COVID-19. BMJ Open Respir. Res. 2021, 8, e000845. [Google Scholar] [CrossRef]
- Schwarzl, G.; Hayden, M.; Limbach, M.; Schultz, K. The prevalence of Obstructive Sleep Apnea (OSA) in patients recovering from COVID-19. ERJ Open Res. 2021, 7, 24. [Google Scholar] [CrossRef]
- WHO. Hypertension and COVID-19: Scientific brief, 17 June 2021; World Health Organization: Geneva, Switzerland, 2021.
- Muhamad, S.-A.; Ugusman, A.; Kumar, J.; Skiba, D.; Hamid, A.A.; Aminuddin, A. COVID-19 and Hypertension: The What, the Why, and the How. Front. Physiol. 2021, 12, 665064. [Google Scholar] [CrossRef]
- Akpek, M. Does COVID-19 Cause Hypertension? Angiology 2022, 73, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.; Driver, M.; Joung, S.; Tran, T.; Barajas, D.; Wu, M.; Botting, P.; Navarrette, J.; Sun, N.; Cheng, S. Hypertension and Excess Risk for Severe COVID-19 Illness Despite Booster Vaccination. Hypertension 2022, 79, e132–e134. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Qin, J.; Ruan, C.; Zeng, X.; Xu, A.; Yang, R.; Li, J.; Cai, H.; Zhang, Z. Hypertension as an independent risk factor for severity and mortality in patients with COVID-19: A retrospective study. Postgrad. Med. J. 2022, 98, 515–522. [Google Scholar] [CrossRef]
- Bepouka, B.; Situakibanza, H.; Sangare, M.; Mandina, M.; Mayasi, N.; Longokolo, M.; Odio, O.; Mangala, D.; Isekusu, F.; Kayembe, J.M.; et al. Mortality associated with COVID-19 and hypertension in sub-Saharan Africa. A systematic review and meta-analysis. J. Clin. Hypertens. 2022, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.; Uehara, S. Systemic arterial hypertension as a risk factor for the severe form of covid-19: Scoping review. Rev. De Saúde Pública 2022, 56, 20. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Li, K.; Du, K.; Huang, X.; Zhou, Z.; Ma, Y.; Guo, S.; Hou, Y.; Li, Q.; et al. Retrospective Study of Aging and Sex-Specific Risk Factors of COVID-19 with Hypertension in China. Cardiovasc. Ther. 2022, 2022, 5978314. [Google Scholar] [CrossRef]
- Swamy, S.; Koch, C.A.; Hannah-Shmouni, F.; Schiffrin, E.L.; Klubo-Gwiezdzinska, J.; Gubbi, S. Hypertension and COVID-19: Updates from the era of vaccines and variants. J. Clin. Transl. Endocrinol. 2022, 27, 100285. [Google Scholar] [CrossRef]
- Savoia, C.; Volpe, M.; Kreutz, R. Hypertension, a Moving Target in COVID-19. Circ. Res. 2021, 128, 1062–1079. [Google Scholar] [CrossRef]
- Clark, C.E.; McDonagh, S.T.J.; McManus, R.J.; Martin, U. COVID-19 and hypertension: Risks and management. A scientific statement on behalf of the British and Irish Hypertension Society. J. Hum. Hypertens. 2021, 35, 304–307. [Google Scholar] [CrossRef]
- Tadic, M.; Saeed, S.; Grassi, G.; Taddei, S.; Mancia, G.; Cuspidi, C. Hypertension and COVID-19: Ongoing Controversies. Front. Cardiovasc. Med. 2021, 8, 639222. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, N.; Zha, W.; Lv, Y. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 745–755. [Google Scholar] [CrossRef]
- Xia, F.; Zhang, M.; Cui, B.; An, W.; Chen, M.; Yang, P.; Qin, T.; Zhou, X.; Liao, Y.; Xu, X.; et al. COVID-19 patients with hypertension are at potential risk of worsened organ injury. Sci. Rep. 2021, 11, 3779. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Ren, L.; Shao, Y.; Tao, W.; Dai, X.J. Preexisting Mental Disorders Increase the Risk of COVID-19 Infection and Associated Mortality. Front. Public Health 2021, 9, 684112. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, Y.; Wang, L.; Yin, X.; Li, Y.; Liu, Y.; Dai, Q.; Tong, A.; Li, D.; Zhang, L.; et al. Anxiety, home blood pressure monitoring, and cardiovascular events among older hypertension patients during the COVID-19 pandemic. Hypertens. Res. 2022, 45, 856–865. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]
- Li, T.; Sun, S.; Liu, B.; Wang, J.; Zhang, Y.; Gong, C.; Duan, J. Prevalence and Risk Factors for Anxiety and Depression in Patients With COVID-19 in Wuhan, China. Psychosom. Med. 2021, 83, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Zhang, J.; Chen, S.; Olatosi, B.; Hardeman, S.; Narasimhan, M.; Bruner, L.; Diedhiou, A.; Scott, C.; Mansaray, A.; et al. How Different Pre-existing Mental Disorders and Their Co-occurrence Affects COVID-19 Clinical Outcomes? A Real-World Data Study in the Southern United States. Front. Public Health 2022, 10, 831189. [Google Scholar] [CrossRef]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Krause, T.M.; Ghosh, L.; Shahani, L.; Machado-Vieira, R.; Lane, S.D.; Boerwinkle, E.; Soares, J.C. Analysis of COVID-19 Infection and Mortality Among Patients With Psychiatric Disorders, 2020. JAMA Netw. Open 2021, 4, e2134969. [Google Scholar] [CrossRef]
- Luykx, J.J.; Lin, B.D. Are psychiatric disorders risk factors for COVID-19 susceptibility and severity? a two-sample, bidirectional, univariable, and multivariable Mendelian Randomization study. Transl. Psychiatry 2021, 11, 210. [Google Scholar] [CrossRef]
- Metheny, N.A.; Krieger, M.M. Salt Toxicity: A Systematic Review and Case Reports. J. Emerg. Nurs. 2020, 46, 428–439. [Google Scholar] [CrossRef]
- Agócs, R.; Sugár, D.; Szabó, A.J. Is too much salt harmful? Yes. Pediatr. Nephrol. 2020, 35, 1777–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.B. Sodium Toxicity in the Nutritional Epidemiology and Nutritional Immunology of COVID-19. Medicina 2021, 57, 739. [Google Scholar] [CrossRef] [PubMed]
- Wolfswinkel, J.F.; Furtmueller, E.; Wilderom, C.P.M. Using grounded theory as a method for rigorously reviewing literature. Eur. J. Inf. Syst. 2013, 22, 45–55. [Google Scholar] [CrossRef]
- Janszky, I.; Ahnve, S.; Lundberg, I.; Hemmingsson, T. Early-Onset Depression, Anxiety, and Risk of Subsequent Coronary Heart Disease: 37-Year Follow-Up of 49,321 Young Swedish Men. J. Am. Coll. Cardiol. 2010, 56, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Roest, A.M.; Martens, E.J.; de Jonge, P.; Denollet, J. Anxiety and risk of incident coronary heart disease: A meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Batelaan, N.M.; Seldenrijk, A.; Bot, M.; van Balkom, A.J.; Penninx, B.W. Anxiety and new onset of cardiovascular disease: Critical review and meta-analysis. Br. J. Psychiatry 2016, 208, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Reiner, I.C.; Tibubos, A.N.; Werner, A.M.; Ernst, M.; Brähler, E.; Wiltink, J.; Michal, M.; Schulz, A.; Wild, P.S.; Münzel, T.; et al. The association of chronic anxiousness with cardiovascular disease and mortality in the community: Results from the Gutenberg Health Study. Sci. Rep. 2020, 10, 12436. [Google Scholar] [CrossRef]
- Dimsdale, J.E. What does heart disease have to do with anxiety? J. Am. Coll. Cardiol. 2010, 56, 47–48. [Google Scholar] [CrossRef] [Green Version]
- Goddard, A.W.; Ball, S.G.; Martinez, J.; Robinson, M.J.; Yang, C.R.; Russell, J.M.; Shekhar, A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress. Anxiety 2010, 27, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Ralph, A.F.; Grenier, C.; Costello, H.M.; Stewart, K.; Ivy, J.R.; Dhaun, N.; Bailey, M.A. Activation of the Sympathetic Nervous System Promotes Blood Pressure Salt-Sensitivity in C57BL6/J Mice. Hypertension 2021, 77, 158–168. [Google Scholar] [CrossRef]
- Pan, Y.; Cai, W.; Cheng, Q.; Dong, W.; An, T.; Yan, J. Association between anxiety and hypertension: A systematic review and meta-analysis of epidemiological studies. Neuropsychiatr. Dis. Treat. 2015, 11, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peskind, E.R.; Radant, A.; Dobie, D.J.; Hughes, J.; Wilkinson, C.W.; Sikkema, C.; Veith, R.C.; Dorsa, D.M.; Raskind, M.A. Hypertonic saline infusion increases plasma norepinephrine concentrations in normal men. Psychoneuroendocrinology 1993, 18, 103–113. [Google Scholar] [CrossRef]
- Molosh, A.I.; Johnson, P.L.; Fitz, S.D.; Dimicco, J.A.; Herman, J.P.; Shekhar, A. Changes in central sodium and not osmolarity or lactate induce panic-like responses in a model of panic disorder. Neuropsychopharmacology 2010, 35, 1333–1347. [Google Scholar] [CrossRef] [PubMed]
- Arnold, E. Anxiety DSM-5 Diagnostic Criteria and Treatment Overview. Available online: https://pro.psycom.net/assessment-diagnosis-adherence/anxiety (accessed on 30 August 2022).
- Yi, B.; Titze, J.; Rykova, M.; Feuerecker, M.; Vassilieva, G.; Nichiporuk, I.; Schelling, G.; Morukov, B.; Choukèr, A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: A longitudinal study. Transl. Res. 2015, 166, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, B.C.; Kasai, T.; Coelho, F.M.; Zatz, R.; Elias, R.M. Fluid Redistribution in Sleep Apnea: Therapeutic Implications in Edematous States. Front. Med. 2017, 4, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangash, A.; Wajid, F.; Poolacherla, R.; Mim, F.K.; Rutkofsky, I.H. Obstructive Sleep Apnea and Hypertension: A Review of the Relationship and Pathogenic Association. Cureus 2020, 12, e8241. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.; Muppidi, V.; Gupta, S. Hyperaldosteronism. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499983/ (accessed on 1 October 2022).
- Coble, J.P.; Grobe, J.L.; Johnson, A.K.; Sigmund, C.D. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: Importance of the subfornical organ. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R238–R249. [Google Scholar] [CrossRef] [Green Version]
- Tiarks, G. Hypertonic dehydration: What Is It, Causes, Treatment, and More. Available online: https://www.osmosis.org/answers/hypertonic-dehydration (accessed on 1 September 2022).
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, R.; Abhilash, S.; Nayeem, A.; Salam, S.A.; Augustine, P.; Dan, P.; Maureira, P.; Mraiche, F.; Gentile, C.; Hansbro, P.M.; et al. Increased complications of COVID-19 in people with cardiovascular disease: Role of the renin–angiotensin-aldosterone system (RAAS) dysregulation. Chem. Biol. Interact. 2022, 351, 109738. [Google Scholar] [CrossRef]
- Dudenbostel, T.; Calhoun, D.A. Resistant hypertension, obstructive sleep apnoea and aldosterone. J. Hum. Hypertens. 2012, 26, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fereidoun, H.; Pouria, H. Effect of excessive salt consumption on night’s sleep. Pak J. physiol. 2014, 10, 6–9. [Google Scholar]
- Pimenta, E.; Stowasser, M.; Gordon, R.D.; Harding, S.M.; Batlouni, M.; Zhang, B.; Oparil, S.; Calhoun, D.A. Increased dietary sodium is related to severity of obstructive sleep apnea in patients with resistant hypertension and hyperaldosteronism. Chest 2013, 143, 978–983. [Google Scholar] [CrossRef] [Green Version]
- Fiori, C.Z.; Martinez, D.; Montanari, C.C.; Lopez, P.; Camargo, R.; Sezerá, L.; Gonçalves, S.C.; Fuchs, F.D. Diuretic or sodium-restricted diet for obstructive sleep apnea—A randomized trial. Sleep 2018, 41, zsy016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Sodium and Potassium; Oria, M., Harrison, M., Stallings, V.A., Eds.; National Academies Press (US): Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- NHLBI. Implementing Recommendations for Dietary Salt Reduction: Where Are We? Where Are We Going? How Do We Get There?: A Summary of an NHLBI Workshop; National Institutes of Health, National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1996.
- Kasai, T.; Arcand, J.; Allard, J.P.; Mak, S.; Azevedo, E.R.; Newton, G.E.; Bradley, T.D. Relationship between sodium intake and sleep apnea in patients with heart failure. J. Am. Coll. Cardiol. 2011, 58, 1970–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Ko, I.; Kim, D.K. Association of Obstructive Sleep Apnea With the Risk of Affective Disorders. JAMA Otolaryngol. –Head Neck Surg. 2019, 145, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Rezaeitalab, F.; Moharrari, F.; Saberi, S.; Asadpour, H.; Rezaeetalab, F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J. Res. Med. Sci. 2014, 19, 205–210. [Google Scholar] [PubMed]
- Cox, R.C.; Olatunji, B.O. Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Med. Rev. 2020, 51, 101282. [Google Scholar] [CrossRef] [PubMed]
- Akberzie, W.; Hesselbacher, S.; Aiyer, I.; Surani, S.; Surani, Z.S. The Prevalence of Anxiety and Depression Symptoms in Obstructive Sleep Apnea. Cureus 2020, 12, e11203. [Google Scholar] [CrossRef]
- Duan, X.; Zheng, M.; Zhao, W.; Huang, J.; Lao, L.; Li, H.; Lu, J.; Chen, W.; Liu, X.; Deng, H. Associations of Depression, Anxiety, and Life Events With the Risk of Obstructive Sleep Apnea Evaluated by Berlin Questionnaire. Front. Med. 2022, 9, 799792. [Google Scholar] [CrossRef] [PubMed]
- Daabis, R.; Gharraf, H. Predictors of anxiety and depression in patients with obstructive sleep apnea. Egypt. J. Chest Dis. Tuberc. 2012, 61, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behav. Sleep Med. 2020, 18, 35–57. [Google Scholar] [CrossRef]
- Batzikosta, A.; Antoniadou, M.; Tiga, P.; Nena, E.; Xanthoudaki, M.; Voulgaris, A.; Sotiropoulou, R.; Kouratzi, M.; Froudarakis, M.; Steiropoulos, P. AB011. Assessment of anxiety and depressive symptoms in obstructive sleep apnea patients. Ann. Transl. Med. 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.L.; Martinez, F.; Aguila, A.P.; Pal, A.; Aysola, R.S.; Henderson, L.A.; Macey, P.M. Stress in obstructive sleep apnea. Sci. Rep. 2021, 11, 12631. [Google Scholar] [CrossRef] [PubMed]
- Sharafkhaneh, A.; Giray, N.; Richardson, P.; Young, T.; Hirshkowitz, M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep 2005, 28, 1405–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansukhani, M.P.; Kara, T.; Caples, S.M.; Somers, V.K. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr. Hypertens. Rep. 2014, 16, 476. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Seto, S.W.; Golledge, J. Angiotensin II, sympathetic nerve activity and chronic heart failure. Heart Fail. Rev. 2014, 19, 187–198. [Google Scholar] [CrossRef]
- Dendorfer, A.; Thornagel, A.; Raasch, W.; Grisk, O.; Tempel, K.; Dominiak, P. Angiotensin II induces catecholamine release by direct ganglionic excitation. Hypertension 2002, 40, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakim, F.; Gozal, D.; Kheirandish-Gozal, L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front. Neurol. 2012, 3, 7. [Google Scholar] [CrossRef]
- Sica, E.; De Bernardi, F.; Nosetti, L.; Martini, S.; Cosentino, M.; Castelnuovo, P.; Marino, F. Catecholamines and children obstructive sleep apnea: A systematic review. Sleep Med. 2021, 87, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; Colin-Ramirez, E.; Ross, H.; Escobedo, J.; Macdonald, P.; Troughton, R.; Saldarriaga, C.; Alemayehu, W.; McAlister, F.A.; Arcand, J. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): An international, open-label, randomised, controlled trial. The Lancet 2022, 399, 1391–1400. [Google Scholar] [CrossRef]
- Patel, Y.; Joseph, J. Sodium Intake and Heart Failure. Int. J. Mol. Sci. 2020, 21, 9474. [Google Scholar] [CrossRef] [PubMed]
- Harnack, L.J.; Cogswell, M.E.; Shikany, J.M.; Gardner, C.D.; Gillespie, C.; Loria, C.M.; Zhou, X.; Yuan, K.; Steffen, L.M. Sources of Sodium in US Adults From 3 Geographic Regions. Circulation 2017, 135, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B. Low dietary sodium potentially mediates COVID-19 prevention associated with whole-food plant-based diets. Br. J. Nutr. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- cdc.gov. How To Reduce Sodium Intake. Available online: https://www.cdc.gov/salt/reduce_sodium_tips.htm (accessed on 6 October 2022).
- nhlbi.nih.gov. DASH Eating Plan. Available online: https://www.nhlbi.nih.gov/education/dash-eating-plan (accessed on 6 October 2022).
- Hunter, R.W.; Dhaun, N.; Bailey, M.A. The impact of excessive salt intake on human health. Nat. Rev. Nephrol. 2022, 18, 321–335. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.B. Hypertension, Anxiety and Obstructive Sleep Apnea in Cardiovascular Disease and COVID-19: Mediation by Dietary Salt. Diseases 2022, 10, 89. https://doi.org/10.3390/diseases10040089
Brown RB. Hypertension, Anxiety and Obstructive Sleep Apnea in Cardiovascular Disease and COVID-19: Mediation by Dietary Salt. Diseases. 2022; 10(4):89. https://doi.org/10.3390/diseases10040089
Chicago/Turabian StyleBrown, Ronald B. 2022. "Hypertension, Anxiety and Obstructive Sleep Apnea in Cardiovascular Disease and COVID-19: Mediation by Dietary Salt" Diseases 10, no. 4: 89. https://doi.org/10.3390/diseases10040089
APA StyleBrown, R. B. (2022). Hypertension, Anxiety and Obstructive Sleep Apnea in Cardiovascular Disease and COVID-19: Mediation by Dietary Salt. Diseases, 10(4), 89. https://doi.org/10.3390/diseases10040089






