Role of Endoscopy in Management of Upper Gastrointestinal Cancers
Abstract
:1. Introduction
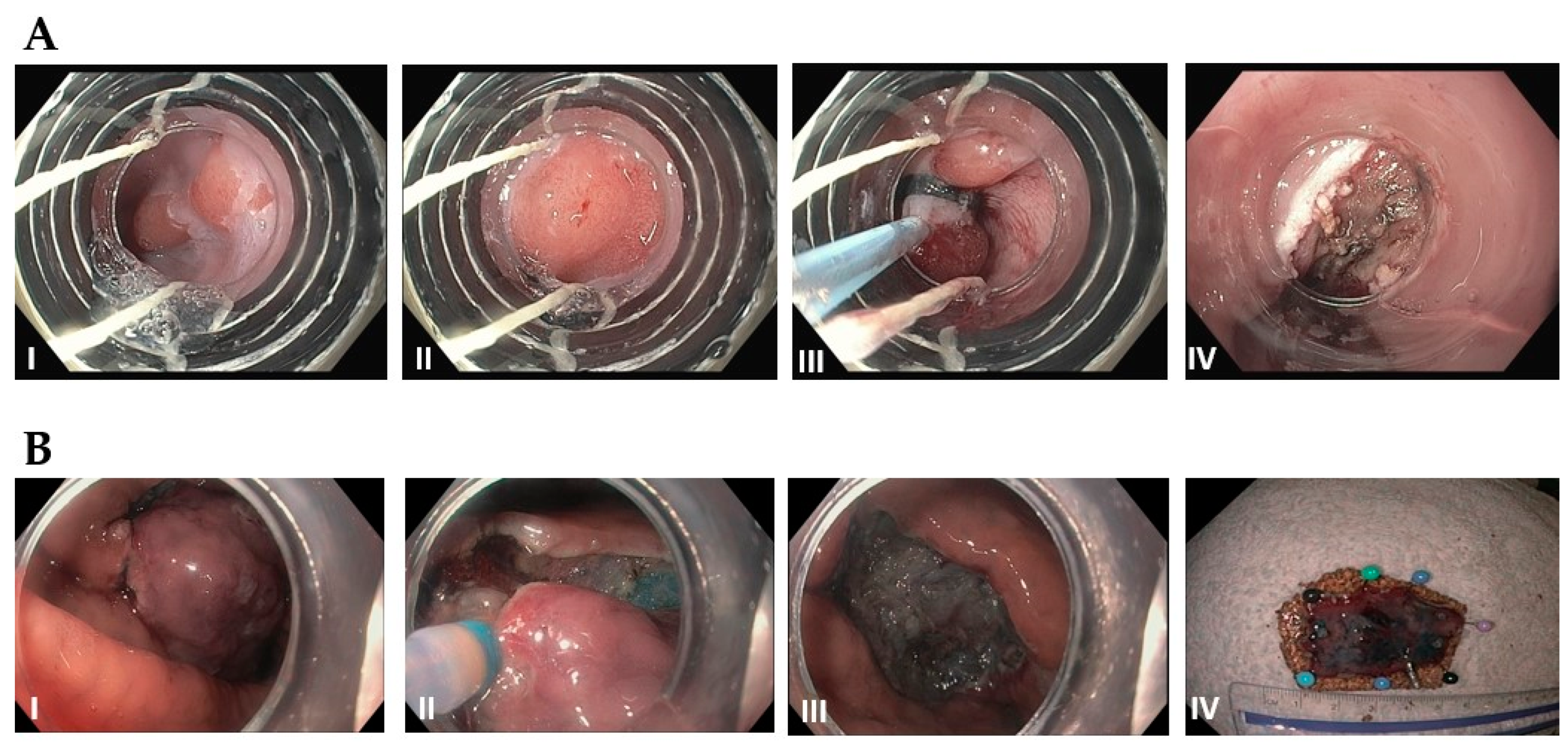
2. Management of Pre-Malignant Lesions
2.1. Barrett’s Esophagus
2.2. Esophageal Epidermoid Metaplasia
2.3. Gastric Intestinal Metaplasia
2.4. Pancreatic Cysts
2.5. Duodenal Adenomas
3. Diagnosis and Staging
3.1. Luminal Upper GI Cancer
3.2. Pancreaticobiliary Cancer
4. Treatment of Cancer
4.1. Esophageal SCC/Adenocarcinoma
4.2. Gastric Cancer
4.3. Gastrointestinal Stromal Tumor
4.4. Pancreatic and Ampullary Cancer
4.5. Extrahepatic Cholangiocarcinoma
5. Palliative Therapy
5.1. Stent Placement
5.2. Enteral Feeding
5.3. Celiac Plexus Block
6. Summary and Future Directions
6.1. AI/Deeping Learning and Endoscopy
6.2. Endoscopic Oncology
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Axon, A.T.R. Fifty years of digestive endoscopy: Successes, setbacks, solutions and the future. Dig. Endosc. 2019, 32, 290–297. [Google Scholar] [CrossRef]
- Teh, J.-L.; Shabbir, A.; Yuen, S.; So, J.B.-Y. Recent advances in diagnostic upper endoscopy. World J. Gastroenterol. 2020, 26, 433–447. [Google Scholar] [CrossRef]
- Subramanian, V.; Ragunath, K. Advanced Endoscopic Imaging: A Review of Commercially Available Technologies. Clin. Gastroenterol. Hepatol. 2014, 12, 368–376.e1. [Google Scholar] [CrossRef]
- Akarsu, M. Evaluation of New Technologies in Gastrointestinal Endoscopy. JSLS J. Soc. Laparoendosc. Surg. 2018, 22, e2017.00053. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Protano, M.-A.; Polydorides, A.D.; Dawsey, S.M.; Pierce, M.C.; Kim, M.K.; Schwarz, R.A.; Quang, T.; Parikh, N.; Bhutani, M.S.; et al. Quantitative Analysis of High-Resolution Microendoscopic Images for Diagnosis of Esophageal Squamous Cell Carcinoma. Clin. Gastroenterol. Hepatol. 2014, 13, 272–279.e2. [Google Scholar] [CrossRef] [Green Version]
- Song, L.-M.W.K.; Banerjee, S.; Desilets, D.; Diehl, D.L.; Farraye, F.A.; Kaul, V.; Kethu, S.R.; Kwon, R.S.; Mamula, P.; Pedrosa, M.C.; et al. Autofluorescence imaging. Gastrointest. Endosc. 2011, 73, 647–650. [Google Scholar] [CrossRef]
- Jang, J.-Y. The Past, Present, and Future of Image-Enhanced Endoscopy. Clin. Endosc. 2015, 48, 466–475. [Google Scholar] [CrossRef]
- Shukla, R.; Abidi, W.M.; Richards-Kortum, R.; Anandasabapathy, S. Endoscopic imaging: How far are we from real-time histology? World J. Gastrointest. Endosc. 2011, 3, 183–194. [Google Scholar] [CrossRef]
- Bhutani, M.S.; Cazacu, I.M.; Chavez, A.A.L.; Saftoiu, A.; Vilmann, P. A quarter century of EUS-FNA: Progress, milestones, and future directions. Endosc. Ultrasound 2018, 7, 141–160. [Google Scholar] [CrossRef] [Green Version]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Hawes, R.; Varadarajulu, S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos). Gastrointest. Endosc. 2018, 88, 9–17. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Ehsan, H.; Wahab, A.; Muneeb, A.; Chaudhary, F.S.; Williams, R.; Haas, C.J. Endoscopic retrograde cholangiopancreatography guided interventions in the management of pancreatic cancer. World J. Gastrointest. Endosc. 2020, 12, 323–340. [Google Scholar] [CrossRef]
- Hanada, K.; Minami, T.; Shimizu, A.; Fukuhara, M.; Yano, S.; Sasaki, K.; Koda, M.; Sugiyama, K.; Yonehara, S.; Yanagisawa, A. Roles of ERCP in the Early Diagnosis of Pancreatic Cancer. Diagnostics 2019, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Friedberg, S.R.; Lachter, J. Endoscopic ultrasound: Current roles and future directions. World J. Gastrointest. Endosc. 2017, 9, 499–505. [Google Scholar] [CrossRef]
- Yeo, S.T.; Bray, N.; Haboubi, H.; Hoare, Z.; Edwards, R.T. Endoscopic ultrasound staging in patients with gastro-oesophageal cancers: A systematic review of economic evidence. BMC Cancer 2019, 19, 900. [Google Scholar] [CrossRef] [Green Version]
- Bhutani, M.; Cazacu, I.; Singh, B.; Saftoiu, A. Recent developments in hepatopancreatobiliary EUS. Endosc. Ultrasound 2019, 8, 146–150. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Souza, R.F.; Yadlapati, R.H.; Sauer, B.G.; Wani, S. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Off. J. Am. Coll. Gastroenterol. ACG 2022, 117, 559–587. [Google Scholar] [CrossRef]
- Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; Gurudu, S.R.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359.e2. [Google Scholar] [CrossRef] [Green Version]
- Kadri, S.R.; Lao-Sirieix, P.; O’Donovan, M.; Debiram, I.; Das, M.; Blazeby, J.; Emery, J.; Boussioutas, A.; Morris, H.; Walter, F.; et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett’s oesophagus in primary care: Cohort study. BMJ 2010, 341, c4372. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, R.C.; di Pietro, M.; O’Donovan, M.; Maroni, R.; Muldrew, B.; Debiram-Beecham, I.; Gehrung, M.; Offman, J.; Tripathi, M.; Smith, S.G.; et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: A multicentre, pragmatic, randomised controlled trial. Lancet 2020, 396, 333–344. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Sharma, P.; Overholt, B.F.; Wolfsen, H.C.; Sampliner, R.E.; Wang, K.K.; Galanko, J.A.; Bronner, M.P.; Goldblum, J.R.; Bennett, A.E.; et al. Radiofrequency Ablation in Barrett’s Esophagus with Dysplasia. N. Engl. J. Med. 2009, 360, 2277–2288. [Google Scholar] [CrossRef]
- Wani, S.; Qumseya, B.; Sultan, S.; Agrawal, D.; Chandrasekhara, V.; Harnke, B.; Kothari, S.; McCarter, M.; Shaukat, A.; Wang, A.; et al. Endoscopic eradication therapy for patients with Barrett’s esophagus–associated dysplasia and intramucosal cancer. Gastrointest. Endosc. 2018, 87, 907–931.e9. [Google Scholar] [CrossRef] [Green Version]
- Chadwick, G.; Groene, O.; Markar, S.R.; Hoare, J.; Cromwell, D.; Hanna, G.B. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: A critical assessment of histologic outcomes and adverse events. Gastrointest. Endosc. 2014, 79, 718–731.e3. [Google Scholar] [CrossRef]
- Singhi, A.D.; Arnold, C.; Crowder, C.D.; Lam-Himlin, D.M.; Voltaggio, L.; A Montgomery, E. Esophageal leukoplakia or epidermoid metaplasia: A clinicopathological study of 18 patients. Mod. Pathol. 2013, 27, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Singhi, A.D.; Arnold, C.A.; Lam-Himlin, D.M.; Nikiforova, M.N.; Voltaggio, L.; Canto, M.I.; McGrath, K.M.; Montgomery, E.A. Targeted next-generation sequencing supports epidermoid metaplasia of the esophagus as a precursor to esophageal squamous neoplasia. Mod. Pathol. 2017, 30, 1613–1621. [Google Scholar] [CrossRef] [Green Version]
- Kamboj, A.K.; Gibbens, Y.Y.; Hagen, C.E.; Wang, K.K.; Iyer, P.G.; Katzka, D.A. Esophageal Epidermoid Metaplasia: Clinical Charac-teristics and Risk of Esophageal Squamous Neoplasia. Off. J. Am. Coll. Gastroenterol. ACG 2021, 116, 1533–1536. [Google Scholar] [CrossRef]
- Shao, L.; Li, P.; Ye, J.; Chen, J.; Han, Y.; Cai, J.; Lu, X. Risk of gastric cancer among patients with gastric intestinal metaplasia. Int. J. Cancer 2018, 143, 1671–1677. [Google Scholar] [CrossRef]
- Huang, R.J.; Choi, A.Y.; Truong, C.D.; Yeh, M.M.; Hwang, J.H. Diagnosis and Management of Gastric Intestinal Metaplasia: Current Status and Future Directions. Gut Liver 2019, 13, 596–603. [Google Scholar] [CrossRef]
- Lee, J.W.J.; Zhu, F.; Srivastava, S.; Tsao, S.K.; Khor, C.; Ho, K.Y.; Fock, K.M.; Lim, W.C.; Ang, T.L.; Chow, W.C.; et al. Severity of gastric intestinal metaplasia predicts the risk of gastric cancer: A prospective multicentre cohort study (GCEP). Gut 2021, 71, 854–863. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. The Participants in the International Workshop on the Histopathology of Gastritis H 1994. Classification and Grading of Gastritis: The Updated Sydney System. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Capelle, L.G.; Haringsma, J.; De Vries, A.C.; Steyerberg, E.; Biermann, K.; Van Dekken, H.; Kuipers, E.J. Narrow Band Imaging for the Detection of Gastric Intestinal Metaplasia and Dysplasia During Surveillance Endoscopy. Dig. Dis. Sci. 2010, 55, 3442–3448. [Google Scholar] [CrossRef]
- Choi, I.J. Endoscopic Gastric Cancer Screening and Surveillance in High-Risk Groups. Clin. Endosc. 2014, 47, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.-H.; Xu, L.-D.; Xing, M.-X.; Li, K.-K.; Xiao, X.-G.; Zhang, Y.; Li, L.; Xiao, Y.-J.; Qu, Y.-L.; Wu, H.-L. Comparison of white-light endoscopy, optical-enhanced and acetic-acid magnifying endoscopy for detecting gastric intestinal metaplasia: A randomized trial. World J. Clin. Cases 2021, 9, 3895–3907. [Google Scholar] [CrossRef] [PubMed]
- Noh, C.-K.; Lee, E.; Lee, G.H.; Lim, S.G.; Park, B.; Shin, S.J.; Cheong, J.Y.; Lee, K.M. Association of Regular Endoscopic Screening with Interval Gastric Cancer Incidence in the National Cancer Screening Program. J. Clin. Med. 2021, 11, 230. [Google Scholar] [CrossRef]
- Choi, K.S.; Jun, J.K.; Park, E.-C.; Park, S.; Jung, K.W.; Han, M.A.; Choi, I.J.; Lee, H.-Y. Performance of Different Gastric Cancer Screening Methods in Korea: A Population-Based Study. PLoS ONE 2012, 7, e50041. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Li, D.; El Serag, H.B.; Davitkov, P.; Altayar, O.; Sultan, S.; Falck-Ytter, Y.; Mustafa, R.A. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 2020, 158, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Demissie, K.; Lu, S.-E.; Rhoads, G.G. Cancer Incidence among Korean-American Immigrants in the United States and Native Koreans in South Korea. Cancer Control 2007, 14, 78–85. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Adams, M.A.; Dorn, S.D.; Dudley-Brown, S.L.; Flamm, S.L.; Gellad, Z.F.; Gruss, C.B.; et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Off. J. Am. Coll. Gastroenterol. ACG 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Muthusamy, V.R.; Chandrasekhara, V.; Acosta, R.D.; Bruining, D.H.; Chathadi, K.V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Gurudu, S.R.; Khashab, M.A.; et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest. Endosc. 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Yang, D.; Samarasena, J.B.; Jamil, L.H.; Chang, K.J.; Lee, D.; Ona, M.A.; Lo, S.K.; Gaddam, S.; Liu, Q.; Draganov, P.V. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: A multicenter study. Endosc. Int. Open 2018, 6, E1423–E1430. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, A.R.; Zhu, H.; Liao, X.; Szporn, A.H.; Kumta, N.A.; Nagula, S.; DiMaio, C.J. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest. Endosc. 2019, 91, 1095–1104. [Google Scholar] [CrossRef]
- Guo, N. Diagnosis and surgical treatment of solid pseudopapillary neoplasm of the pancreas: Analysis of 24 cases. Can. J. Surg. 2011, 54, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Chathadi, K.V.; Khashab, M.A.; Acosta, R.D.; Chandrasekhara, V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Lightdale, J.R.; Saltzman, J.R.; Shaukat, A.; et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest. Endosc. 2015, 82, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Seewald, S.; Omar, S.; Soehendra, N. Endoscopic resection of tumors of the ampulla of Vater: How far up and how deep down can we go? Gastrointest. Endosc. 2006, 63, 789–791. [Google Scholar] [CrossRef]
- Alali, A.; Espino, A.; Moris, M.; Martel, M.; Schwartz, I.; Cirocco, M.; Streutker, C.; Mosko, J.; Kortan, P.; Barkun, A.; et al. Endoscopic Resection of Ampullary Tumours: Long-term Outcomes and Adverse Events. J. Can. Assoc. Gastroenterol. 2019, 3, 17–25. [Google Scholar] [CrossRef]
- Cloyd, J.M.; George, E.; Visser, B.C. Duodenal adenocarcinoma: Advances in diagnosis and surgical management. World J. Gastrointest. Surg. 2016, 8, 212–221. [Google Scholar] [CrossRef]
- Brosens, L.A.A.; Keller, J.J.; Offerhaus, G.J.A.; Goggins, M.; Giardiello, F.M. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut 2005, 54, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Bulow, S.; Björk, J.; Christensen, I.J.; Fausa, O.; Järvinen, H.; Moesgaard, F.; Vasen, H. Duodenal adenomatosis in familial adenomatous polyposis. Gut 2004, 53, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Aihara, H.; Kumar, N.; Thompson, C.C. Diagnosis, surveillance, and treatment strategies for familial adenomatous polyposis. Eur. J. Gastroenterol. Hepatol. 2014, 26, 255–262. [Google Scholar] [CrossRef]
- Campos, F.G.; Sulbaran, M.; Safatle-Ribeiro, A.V.; Martinez, C.A.R. Duodenal adenoma surveillance in patients with familial adenomatous polyposis. World J. Gastrointest. Endosc. 2015, 7, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Mackey, R.; Brown, N.; Church, J.; Burke, C.; Walsh, R.M. Outcome Based on Management for Duodenal Adenomas: Sporadic Versus Familial Disease. J. Gastrointest. Surg. 2009, 14, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cuadrado-Robles, E.; Piessevaux, H.; Moreels, T.G.; Yeung, R.; Aouattah, T.; Komuta, M.; Dano, H.; Jouret-Mourin, A.; Deprez, P.H. Combined excision and ablation of ampullary tumors with biliary or pancreatic intraductal extension is effective even in malignant neoplasms. United Eur. Gastroenterol. J. 2019, 7, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rustagi, T.; Irani, S.; Reddy, D.N.; Abu Dayyeh, B.K.; Baron, T.H.; Gostout, C.J.; Levy, M.J.; Martin, J.; Petersen, B.T.; Ross, A.; et al. Radiofrequency ablation for intraductal extension of ampullary neoplasms. Gastrointest. Endosc. 2016, 86, 170–176. [Google Scholar] [CrossRef]
- Camus, M.; Napoléon, B.; Vienne, A.; Le Rhun, M.; Leblanc, S.; Barret, M.; Chaussade, S.; Robin, F.; Kaddour, N.; Prat, F. Efficacy and safety of endobiliary radiofrequency ablation for the eradication of residual neoplasia after endoscopic papillectomy: A multicenter prospective study. Gastrointest. Endosc. 2018, 88, 511–518. [Google Scholar] [CrossRef]
- Early, D.S.; Ben-Menachem, T.; Decker, G.A.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fukami, N.; Hwang, J.H.; Jain, R.; Jue, T.L.; et al. Appropriate use of GI endoscopy. Gastrointest. Endosc. 2012, 75, 1127–1131. [Google Scholar] [CrossRef]
- Polkowski, M. Endosonographic staging of upper intestinal malignancy. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 649–661. [Google Scholar] [CrossRef]
- Boniface, M.; Wani, S.; Schefter, T.; Koo, P.; Meguid, C.; Leong, S.; Kaplan, J.; Wingrove, L.; McCarter, M. Multidisciplinary management for esophageal and gastric cancer. Cancer Manag. Res. 2016, 8, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Pilonis, N.D.; Januszewicz, W.; di Pietro, M. Confocal laser endomicroscopy in gastro-intestinal endoscopy: Technical aspects and clinical applications. Transl. Gastroenterol. Hepatol. 2022, 7, 7. [Google Scholar] [CrossRef]
- Gaddam, S.; Mathur, S.C.; Singh, M.; Arora, J.; Wani, S.B.; Gupta, N.; Overhiser, A.; Rastogi, A.; Singh, V.; Desai, N.; et al. Novel Probe-Based Confocal Laser Endomicroscopy Criteria and Interobserver Agreement for the Detection of Dysplasia in Barrett’s Esophagus. Am. J. Gastroenterol. 2011, 106, 1961–1969. [Google Scholar] [CrossRef]
- Yoon, H. New approaches to gastric cancer staging: Beyond endoscopic ultrasound, computed tomography and positron emission tomography. World J. Gastroenterol. 2014, 20, 13783–13790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gong, Y.; Xu, H. Clinical and pathological staging of gastric cancer: Current perspectives and implications. Eur. J. Surg. Oncol. (EJSO) 2020, 46, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, I.S.; Triantafyllou, M.; Triantafyllou, K.; Rösch, T. EUS in the management of gastric cancer. Ann. Gastroenterol. 2011, 24, 9–15. [Google Scholar] [PubMed]
- Valero, M.; Robles-Medranda, C. Endoscopic ultrasound in oncology: An update of clinical applications in the gastrointestinal tract. World J. Gastrointest. Endosc. 2017, 9, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, S.; Kaul, V. Endoscopic Ultrasound Stagingof Esophageal Cancer. Gastroenterol. Hepatol. 2020, 16, 14–20. [Google Scholar]
- Thosani, N.; Singh, H.; Kapadia, A.; Ochi, N.; Lee, J.H.; Ajani, J.; Swisher, S.G.; Hofstetter, W.L.; Guha, S.; Bhutani, M.S. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: A systematic review and meta-analysis. Gastrointest. Endosc. 2012, 75, 242–253. [Google Scholar] [CrossRef]
- Krill, T.; Baliss, M.; Roark, R.; Sydor, M.; Samuel, R.; Zaibaq, J.; Guturu, P.; Parupudi, S. Accuracy of endoscopic ultrasound in esophageal cancer staging. J. Thorac. Dis. 2019, 11, S1602–S1609. [Google Scholar] [CrossRef]
- Pouw, R.E.; Heldoorn, N.; Herrero, L.A.; Kate, F.J.T.; Visser, M.; Busch, O.R.; Henegouwen, M.I.V.B.; Krishnadath, K.K.; Weusten, B.L.; Fockens, P.; et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest. Endosc. 2011, 73, 662–668. [Google Scholar] [CrossRef]
- Young, P.E.; Gentry, A.B.; Acosta, R.D.; Greenwald, B.D.; Riddle, M. Endoscopic Ultrasound Does Not Accurately Stage Early Adenocarcinoma or High-Grade Dysplasia of the Esophagus. Clin. Gastroenterol. Hepatol. 2010, 8, 1037–1041. [Google Scholar] [CrossRef]
- He, L.-J. Endoscopic ultrasonography for staging of T1a and T1b esophageal squamous cell carcinoma. World J. Gastroenterol. 2014, 20, 1340–1347. [Google Scholar] [CrossRef]
- Prasad, G.A.; Wu, T.T.; Wigle, D.A.; Buttar, N.S.; Wongkeesong, L.; Dunagan, K.T.; Lutzke, L.S.; Borkenhagen, L.S.; Wang, K.K. Endoscopic and Surgical Treatment of Mucosal (T1a) Esophageal Adenocarcinoma in Barrett’s Esophagus. Gastroenterology 2009, 137, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Ngamruengphong, S.; Ferri, L.; Aihara, H.; Draganov, P.V.; Yang, D.J.; Perbtani, Y.B.; Jue, T.L.; Munroe, C.A.; Boparai, E.S.; Mehta, N.A.; et al. Efficacy of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasia in a Large Cohort in North America. Clin. Gastroenterol. Hepatol. 2020, 19, 1611–1619.e1. [Google Scholar] [CrossRef] [PubMed]
- Saftoiu, A.; Vilmann, P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J. Clin. Ultrasound 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-C.; Wang, K.K.; Paul, N.; Jayaraman, V.; Wang, Q.; Abboud, Y.; Jamil, L.H.; Gaddam, S.; Lo, S.K. Fluoroscopy-guided shaped endobiliary biopsy at endoscopic retrograde cholangiography can accurately diagnose biliary neoplasia: Results from a large cohort. Endosc. Int. Open 2021, 9, E1039–E1048. [Google Scholar] [CrossRef] [PubMed]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Prim. 2021, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.; Capanu, M.; Abou-Alfa, G.; Huitzil, D.; Jarnagin, W.; Fong, Y.; D’Angelica, M.; DeMatteo, R.; Blumgart, L.; O’Reilly, E. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J. Surg. Oncol. 2008, 98, 485–489. [Google Scholar] [CrossRef]
- Gurusamy, K.S.; Abu-Amara, M.; Farouk, M.; Davidson, B.R. Cholecystectomy for gallbladder polyp. Cochrane Database Syst. Rev. 2009, 2009, CD007052. [Google Scholar] [CrossRef]
- Ito, H.; Ito, K.; D’Angelica, M.; Gonen, M.; Klimstra, D.; Allen, P.; DeMatteo, R.P.; Fong, Y.; Blumgart, L.H.; Jarnagin, W.R. Accurate Staging for Gallbladder Cancer: Implications for Surgical Therapy and Pathological Assessment. Ann. Surg. 2011, 254, 320–325. [Google Scholar] [CrossRef]
- Azuma, T.; Yoshikawa, T.; Araida, T.; Takasaki, K. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am. J. Surg. 2001, 181, 65–70. [Google Scholar] [CrossRef]
- Hijioka, S.; Hara, K.; Mizuno, N.; Imaoka, H.; Ogura, T.; Haba, S.; Mekky, M.A.; Bhatia, V.; Hosoda, W.; Yatabe, Y.; et al. Diagnostic yield of endoscopic retrograde cholangiography and of EUS-guided fine needle aspiration sampling in gallbladder carcinomas. J. Hepato-Biliary-Pancreatic Sci. 2011, 19, 650–655. [Google Scholar] [CrossRef]
- Appel, B.L.; Tolat, P.; Evans, D.B.; Tsai, S. Current Staging Systems for Pancreatic Cancer. Cancer J. 2012, 18, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, I.C.; Curvers, W.L.; Schoon, E.J. Endoscopic resection for early esophageal carcinoma. J. Thorac. Dis. 2019, 11, S713–S722. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.M.; Gerdes, H. Endoscopic options for early stage esophageal cancer. J. Gastrointest. Oncol. 2015, 6, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yang, D.-H.; Kim, J.W.; Kim, J.-H.; Min, Y.W.; Lee, S.H.; Bae, J.H.; Chung, H.; Choi, K.D.; Park, J.C.; et al. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Clin. Endosc. 2020, 53, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.Y.; Park, H.J.; Park, Y.S.; Lee, J.H.; Choi, K.-S.; Jeong, K.W.; Kim, D.H.; Choi, K.D.; Song, H.J.; Lee, G.H.; et al. Endoscopic Resection for Undifferentiated-Type Early Gastric Cancer: Immediate Endoscopic Outcomes and Long-Term Survivals. Am. J. Dig. Dis. 2015, 61, 1158–1164. [Google Scholar] [CrossRef]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef]
- Sato, Y.; Hashimoto, S.; Mizuno, K.-I.; Takeuchi, M.; Terai, S. Management of gastric and duodenal neuroendocrine tumors. World J. Gastroenterol. 2016, 22, 6817–6828. [Google Scholar] [CrossRef]
- Scherübl, H.; Cadiot, G.; Jensen, R.; Rösch, T.; Stölzel, U.; Klöppel, G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: Small tumors, small problems? Endoscopy 2010, 42, 664–671. [Google Scholar] [CrossRef]
- Sivandzadeh, G.R.; Ejtehadi, F.; Shoaee, S.; Aminlari, L.; Niknam, R.; Taghavi, A.R.; Geramizadeh, B.; Hormati, A.; Safarpour, A.R.; Lankarani, K.B. Endoscopic mucosal resection: Still a reliable therapeutic option for gastrointestinal neuroendocrine tumors. BMC Gastroenterol. 2021, 21, 1–6. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H. Long-term follow up of endoscopic resection for type 3 gastric NET. World J. Gastroenterol. 2013, 19, 8703–8708. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, X.; Ye, S.; Wang, J.; Liang, J.; Mai, S.; Lai, M.; Feng, H.; Wang, G.; Zhou, Y. A comparison of the efficacy and safety of endoscopic full-thickness resection and laparoscopic-assisted surgery for small gastrointestinal stromal tumors. Surg. Endosc. 2015, 30, 3357–3361. [Google Scholar] [CrossRef]
- Andalib, I.; Yeoun, D.; Reddy, R.; Xie, S.; Iqbal, S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: Methods and feasibility data. Surg. Endosc. 2017, 32, 1787–1792. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.-X.; Niu, Q.; Wang, A.-L.; Shi, N.; Ma, F.-Z.; Hu, Y.-B. OTSC assisted EFTR for the treatment of GIST: 40 cases analysis. Minim. Invasive Ther. Allied Technol. 2020, 31, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Hadjicostas, P.; Malakounides, N.; Varianos, C.; Kitiris, E.; Lerni, F.; Symeonides, P. Radiofrequency ablation in pancreatic cancer. Hpb 2006, 8, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, M.N.; Ehsan, H.; Muneeb, A.; Wahab, A.; Sana, M.K.; Neupane, K.; Chaudhary, F.S. Role of Radiofrequency Ablation in the Management of Unresectable Pancreatic Cancer. Front. Med. 2021, 7, 1070. [Google Scholar] [CrossRef]
- Park, D.H.; Choi, J.-H.; Oh, D.; Lee, S.S.; Seo, D.-W.; Lee, S.K.; Kim, M.-H. Endoscopic Ultrasonography-Guided Ethanol Ablation for Small Pancreatic Neuroendocrine Tumors: Results of a Pilot Study. Clin. Endosc. 2015, 48, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.-C.; Seo, D.W.; Song, T.J.; Moon, S.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.; Kim, J. Endoscopic Ultrasonography-Guided Ethanol Lavage With Paclitaxel Injection Treats Patients With Pancreatic Cysts. Gastroenterology 2011, 140, 172–179. [Google Scholar] [CrossRef]
- Song, T.J.; Seo, D.W.; Lakhtakia, S.; Reddy, N.; Oh, D.W.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.-H. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest. Endosc. 2015, 83, 440–443. [Google Scholar] [CrossRef]
- Sofi, A.A.; Khan, M.A.; Das, A.; Sachdev, M.; Khuder, S.; Nawras, A.; Lee, W. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2018, 87, 944–951.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Zhou, H.; Wang, Y.; Huang, H.; Jin, H.; Lou, Q.; Shah, R.J.; Zhang, X. Endoscopic radiofrequency ablation plus a novel oral 5-fluorouracil compound versus radiofrequency ablation alone for unresectable extrahepatic cholangiocarcinoma. Gastrointest. Endosc. 2020, 92, 1204–1212.e1. [Google Scholar] [CrossRef] [PubMed]
- Halpern, A.L.; McCarter, M.D. Palliative Management of Gastric and Esophageal Cancer. Surg. Clin. North Am. 2019, 99, 555–569. [Google Scholar] [CrossRef]
- Kim, K.Y.; Tsauo, J.; Song, H.-Y.; Kim, P.H.; Park, J.-H. Self-Expandable Metallic Stent Placement for the Palliation of Esophageal Cancer. J. Korean Med Sci. 2017, 32, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, C.; Saluja, S.S.; Pal, S.; Ahuja, V.; Saran, P.; Dash, N.R.; Sahni, P.; Chattopadhyay, T.K. Palliative stenting for relief of dysphagia in patients with inoperable esophageal cancer: Impact on quality of life. Dis. Esophagus 2009, 22, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Xinopoulos, D.; Dimitroulopoulos, D.; Moschandrea, I.; Skordilis, P.; Bazinis, A.; Kontis, M.; Paraskevas, I.; Kouroumalis, E.; Paraskevas, E. Natural course of inoperable esophageal cancer treated with metallic expandable stents: Quality of life and cost-effectiveness analysis. J. Gastroenterol. Hepatol. 2004, 19, 1397–1402. [Google Scholar] [CrossRef]
- Evans, J.A.; Early, D.S.; Chandraskhara, V.; Chathadi, K.V.; Fanelli, R.D.; Fisher, D.A.; Foley, K.Q.; Hwang, J.H.; Jue, T.L.; Pasha, S.F.; et al. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest. Endosc. 2013, 77, 328–334. [Google Scholar] [CrossRef]
- Sawas, T.; Al Halabi, S.; Parsi, M.A.; Vargo, J.J. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: A meta-analysis. Gastrointest. Endosc. 2015, 82, 256–267.e7. [Google Scholar] [CrossRef]
- American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee; Anderson, M.A.; Appalaneni, V.; Ben-Menachem, T.; Decker, G.A.; Early, D.S.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fisher, L.R.; et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest. Endosc. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Qumseya, B.J.; Jamil, L.H.; Elmunzer, B.J.; Riaz, A.; Ceppa, E.P.; Thosani, N.C.; Buxbaum, J.L.; Storm, A.C.; Sawhney, M.S.; Pawa, S.; et al. ASGE guideline on the role of endoscopy in the management of malignant hilar obstruction. Gastrointest. Endosc. 2021, 94, 222–234.e22. [Google Scholar] [CrossRef]
- Jue, T.L.; Storm, A.C.; Naveed, M.; Fishman, D.S.; Qumseya, B.J.; McRee, A.J.; Truty, M.J.; Khashab, M.A.; Agrawal, D.; Al-Haddad, M.; et al. ASGE guideline on the role of endoscopy in the management of benign and malignant gastroduodenal obstruction. Gastrointest. Endosc. 2020, 93, 309–322.e4. [Google Scholar] [CrossRef] [PubMed]
- Tyberg, A.; Perez-Miranda, M.; Sanchez-Ocaña, R.; Peñas, I.; de la Serna, C.; Shah, J.; Binmoeller, K.; Gaidhane, M.; Grimm, I.; Baron, T.; et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: A multicenter, international experience. Endosc. Int. Open 2016, 4, E276–E281. [Google Scholar] [CrossRef] [Green Version]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef] [PubMed]
- Mobily, M.; Patel, J.A. Palliative percutaneous endoscopic gastrostomy placement for gastrointestinal cancer: Roles, goals, and complications. World J. Gastrointest. Endosc. 2015, 7, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sun, H.; Yang, L.; Gao, H.; Cui, Y.; Yu, C.; Xu, H.; Li, L. Nutritional Risk Index as a Prognostic Factor Predicts the Clinical Outcomes in Patients With Stage III Gastric Cancer. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef]
- Han-Geurts, I.; Hop, W.; Tran, T.; Tilanus, H. Nutritional Status as a Risk Factor in Esophageal Surgery. Dig. Surg. 2006, 23, 159–163. [Google Scholar] [CrossRef]
- Daly, J.M.; Weintraub, F.N.; Shou, J.; Rosato, E.F.; Lucia, M. Enteral Nutrition During Multimodality Therapy in Upper Gastroin-testinal Cancer Patients. Ann. Surg. 1995, 221, 327. [Google Scholar] [CrossRef]
- Shastri, Y.M.; Shirodkar, M.; Mallath, M.K. Endoscopic feeding tube placement in patients with cancer: A prospective clinical audit of 2055 procedures in 1866 patients. Aliment. Pharmacol. Ther. 2008, 27, 649–658. [Google Scholar] [CrossRef]
- Strijbos, D.; Keszthelyi, D.; Gilissen, L.P.L.; Lacko, M.; Hoeijmakers, J.G.J.; van der Leij, C.; de Ridder, R.J.J.; de Haan, M.W.; Masclee, A.A.M. Percutaneous endoscopic versus radiologic gastrostomy for enteral feeding: A retrospective analysis on outcomes and complications. Endosc. Int. Open 2019, 7, E1487–E1495. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-K.; Small Intestine and Nutrition Research Group of the Korean Association for the Study of Intestinal Diseases (KASID); Kim, J.Y.; Koh, S.-J.; Lee, Y.J.; Jang, H.J. Complications of percutaneous endoscopic and radiologic gastrostomy tube insertion: A KASID (Korean Association for the Study of Intestinal Diseases) study. Surg. Endosc. 2018, 33, 750–756. [Google Scholar] [CrossRef]
- Kohli, D.R.; Kennedy, K.F.; Desai, M.; Sharma, P. Safety of endoscopic gastrostomy tube placement compared with radiologic or surgical gastrostomy: Nationwide inpatient assessment. Gastrointest. Endosc. 2020, 93, 1077–1085.e1. [Google Scholar] [CrossRef]
- Galaski, A.; Peng, W.W.; Ellis, M.; Darling, P.; Common, A.; Tucker, E. Gastrostomy tube placement by radiological versus endoscopic methods in an acute care setting: A retrospective review of frequency, indications, complications and outcomes. Can. J. Gastroenterol. 2009, 23, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.; Singh, G.; Das, S.; Concha-Parra, R.; Erber, J.; Micames, C.; Gress, F. Efficacy of Endoscopic Ultrasound-guided Celiac Plexus Block and Celiac Plexus Neurolysis for Managing Abdominal Pain Associated With Chronic Pancreatitis and Pancreatic Cancer. J. Clin. Gastroenterol. 2010, 44, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Collins, D.; Penman, I.; Mishra, G.; Draganov, P. EUS-guided celiac block and neurolysis. Endoscopy 2006, 38, 935–939. [Google Scholar] [CrossRef]
- Asif, A.A.; Walayat, S.K.; Bechtold, M.L.; Revanur, V.; Puli, S.R. EUS-guided celiac plexus neurolysis for pain in pancreatic cancer patients—A meta-analysis and systematic review. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Wyse, J.M.; Carone, M.; Paquin, S.C.; Usatii, M.; Sahai, A.V. Randomized, Double-Blind, Controlled Trial of Early Endoscopic Ultrasound–Guided Celiac Plexus Neurolysis to Prevent Pain Progression in Patients With Newly Diagnosed, Painful, Inoperable Pancreatic Cancer. J. Clin. Oncol. 2011, 29, 3541–3546. [Google Scholar] [CrossRef]
- Erdek, M.A.; Halpert, D.E.; Fernández, M.G.; Cohen, S.P. Assessment of Celiac Plexus Block and Neurolysis Outcomes and Technique in the Management of Refractory Visceral Cancer Pain. Pain Med. 2010, 11, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, G.Y.; Schroeder, D.R.; Carns, P.E.; Wilson, J.L.; Martin, D.P.; Kinney, M.O.; Mantilla, C.; Warner, D.O. Effect of Neurolytic Celiac Plexus Block on Pain Relief, Quality of Life, and Survival in Patients With Unresectable Pancreatic Cancer. JAMA 2004, 291, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Kim, E.Y. Endoscopic Management of Pancreatobiliary Malignancies. Am. J. Dig. Dis. 2022, 67, 1635–1648. [Google Scholar] [CrossRef]
- Wang, A.Y.; Yachimski, P.S. Endoscopic Management of Pancreatobiliary Neoplasms. Gastroenterology 2018, 154, 1947–1963. [Google Scholar] [CrossRef]
- Singh, R.; Owen, V.; Shonde, A.; Kaye, P.; Hawkey, C.; Ragunath, K. White light endoscopy, narrow band imaging and chromoendoscopy with magnification in diagnosing colorectal neoplasia. World J. Gastrointest. Endosc. 2009, 1, 45–50. [Google Scholar] [CrossRef] [PubMed]
- El Hajjar, A.; Rey, J.-F. Artificial intelligence in gastrointestinal endoscopy: General overview. Chin. Med. J. 2020, 133, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Berzin, T.M.; Parasa, S.; Wallace, M.B.; Gross, S.A.; Repici, A.; Sharma, P. Position statement on priorities for artificial intelligence in GI endoscopy: A report by the ASGE Task Force. Gastrointest. Endosc. 2020, 92, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Zhou, W.; An, P.; Shen, L.; Liu, J.; Jiang, X.; Huang, X.; Mu, G.; Wan, X.; et al. Randomised controlled trial of WISENSE, a real-time quality improving system for monitoring blind spots during esophagogastroduodenoscopy. Gut 2019, 68, 2161–2169. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Xiao, X.; Wu, C.; Zeng, X.; Zhang, Y.; Du, J.; Bai, S.; Xie, J.; Zhang, Z.; Li, Y.; et al. Real-time automated diagnosis of precancerous lesions and early esophageal squamous cell carcinoma using a deep learning model (with videos). Gastrointest. Endosc. 2019, 91, 41–51. [Google Scholar] [CrossRef]
- Corral, J.E.; Hussein, S.; Kandel, P.; Bolan, C.W.; Bagci, U.; Wallace, M.B. Deep Learning to Classify Intraductal Papillary Mucinous Neoplasms Using Magnetic Resonance Imaging. Pancreas 2019, 48, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.A.; O’Connor, K. A pilot study of endoscopic injection chemo/sclerotherapy of esophageal carcinoma. Gastrointest. Endosc. 1990, 36, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.M.; Van Dam, J. Endoscopic Ultrasound-Guided Intratumoural Therapy for Pancreatic Cancer. Can. J. Gastroenterol. 2008, 22, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Verna, E.C.; Dhar, V. Review: Endoscopic ultrasound-guided fine needle injection for cancer therapy: The evolving role of therapeutic endoscopic ultrasound. Ther. Adv. Gastroenterol. 2008, 1, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Robles-Jara, C.; Robles-Medranda, C. Endoscopic Chemotherapy with 5-Fluorouracil in Advanced Gastric Cancer. J. Gastrointest. Cancer 2009, 41, 75–78. [Google Scholar] [CrossRef]



| Screening/Surveillance Guidelines | High Risk Features | Screening or Surveillance Modalities | |
|---|---|---|---|
| Barrett’s esophagus (BE) | Men w/ chronic GERD (>5 years) occurring > once/week and ≥2 risk factors (ACG 2022) | (1) Age > 50 (2) Caucasian race (3) Tobacco use (4) Obesity (5) First-degree relative with BE or EAC. | Upper endoscopy (EGD) -“gold standard” for diagnosis and treatment Transnasal endoscopy (screening only): -good sensitivity (91%) and specificity (96%) -cheaper than EGD -cannot perform interventions Cytosponge (screening only): -cheaper than EGD -cannot perform interventions -Newer technique; not widely used in the United States |
| Gastric intestinal metaplasia (GIM) | Routine screening NOT recommended. Surveillance every 3–5 years in patients with GIM and high-risk features (AGA 2018) | (1) Incomplete intestinal metaplasia (2) Extensive GIM (3) Family history of gastric cancer (4) Immigration from a high incidence region | Upper endoscopy |
| Pancreatic cystic neoplasms (IPMN and MCN) | Routine screening NOT recommended. Cysts with high-risk features should undergo EUS-FNA to evaluate histology. Cysts without high-risk features should undergo surveillance (ACG 2018). | (1) Cyst size ≥ 2 cm (2) Main pancreatic duct dilation > 5 mm * (3) Solid cystic component (4) Enhancing mural nodule > 5 mm * (5) ≥3 mm growth in 1 year (6) Obstructive jaundice * (7) Symptomatic cyst (8) Family history of pancreatic cancer (9) New onset diabetes | MRCP (preferred) -Generally preferred first-line EUS -Used in high-risk cases and when imaging is non-diagnostic |
| Duodenal adenoma | Patients with familial adenomatous polyposis: based on Spigelman classification (stage 0-IV). No definitive surveillance guidelines for patients without FAP. | Components of Spigelman class: (1) Increased polyp number (>20) (2) Polyp > 10 mm (3) Villous histology (4) High grade dysplasia | Upper endoscopy |
| Cyst Category | Imaging Appearance (MRI and EUS) | Fluid Evaluation | Risk for Malignancy |
|---|---|---|---|
| Pseudocyst | Thick-walled Anechoic | Brown color Elevated amylase/lipase Low CEA | No |
| Serous cystadenoma | Microcystic with “honeycomb” appearance Central calcification | Thin, clear Low amylase/lipase Low CEA | No a |
| Solid pseudopapillary neoplasm | Solid + cystic component | Necrotic debris | Yes |
| Mucinous Cystic Neoplasm (MCN) | Macrocystic +/− septations Peripheral calcifications +/− solid component b | Mucinous Variable amylase (usually low) High CEA | Yes |
| Intraductal Papillary Mucinous Neoplasm (IPMN) | Dilated pancreatic duct c +/− septations +/− solid component | Mucinous High amylase High CEA | Yes d |
| Staging Modalities | Endoscopic Treatment Options | |
|---|---|---|
| Luminal Upper GI cancer a | EUS: -First line for T-staging (sensitivity is 81% for stage T1/T2, >90% for T3/T4) and N-staging -EUS-FNA can help for N-staging via lymph node biopsy, although results can be technique-limited CT: -Used for M-staging -Lower sensitivity and specificity for N-staging, when compared to EUS | Endoscopic techniques (EMR, ESD) generally feasible for T1a tumors ≤2 cm Surgical resection vs systemic therapy for larger and more advanced tumors |
| Extraluminal upper GI cancer b | EUS: -Sensitivity and specificity for T and N staging highest in pancreatic cancer (compared to gallbladder cancer or cholangiocarcinoma) Laparoscopy: -Most accurate diagnostic modality for gallbladder cancer and cholangiocarcinoma Cross-sectional imaging: -Modality of choice for diagnosing intrahepatic cholangiocarcinoma and evaluating resectability in pancreatic cancer -Used for M-staging | Generally, endoscopy has only a palliative role (RFA, stenting) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Jiang, Y.; Abboud, Y.; Gaddam, S. Role of Endoscopy in Management of Upper Gastrointestinal Cancers. Diseases 2023, 11, 3. https://doi.org/10.3390/diseases11010003
Liang J, Jiang Y, Abboud Y, Gaddam S. Role of Endoscopy in Management of Upper Gastrointestinal Cancers. Diseases. 2023; 11(1):3. https://doi.org/10.3390/diseases11010003
Chicago/Turabian StyleLiang, Jeff, Yi Jiang, Yazan Abboud, and Srinivas Gaddam. 2023. "Role of Endoscopy in Management of Upper Gastrointestinal Cancers" Diseases 11, no. 1: 3. https://doi.org/10.3390/diseases11010003
APA StyleLiang, J., Jiang, Y., Abboud, Y., & Gaddam, S. (2023). Role of Endoscopy in Management of Upper Gastrointestinal Cancers. Diseases, 11(1), 3. https://doi.org/10.3390/diseases11010003





