Development of SARS-CoV-2 Vaccine: Challenges and Prospects
Abstract
:1. Introduction
1.1. Genome Structure and Characteristic of COVID-19
1.2. Epidemiology of SARS-CoV-2
1.3. Mutant Variant of SARS-CoV-2
1.4. Clinical Pathology of COVID-19
1.5. Immunopathology of COVID-19
1.6. Management Strategies of COVID-19
| WHO Name | Pango Lineage | GISAID Clade | Next Strain Clade | Country/Date of First Detection | Date of Designation | Pathophysiology | Epidemiology | |
|---|---|---|---|---|---|---|---|---|
| Previously circulating VOCs | Alpha | B.1.1.7 | GRY | 20I (V1) | United Kingdom | VOC: 18 December 2020 |
| |
| September 2020 | ||||||||
| Previous VOC: 9 March 2022 | ||||||||
| Beta | B.1.351 | GH/501Y.V2 | 20H (V2) | South Africa | VOC: 18 December 2020 |
| ||
| May 2020 | ||||||||
| Previous VOC: 9 March 2022 | ||||||||
| Gamma | P.1 | GR/501Y.V3 | 20J (V3) | Brazil, | VOC: 11 January 2021 |
| ||
| November 2020 | ||||||||
| Previous VOC: 9 March 2022 | ||||||||
| Delta | B.1.617.2 | G/478K.V1 | 21A, 21I, 21J | India | VOI: 4 April 2021 |
|
| |
| October 2020 | VOC: 11 May 2021 | |||||||
| Previous VOC: 7 June 2022 | ||||||||
| Currently circulating VOCs | Omicron 1 | B.1.1.529 | GR/484A | 21K, 21L, 21M, 22A, | Multiple countries | VUM: 24 November 2021 |
|
|
| 22B, 22C, 22D | November 21 | VOC: 26 November 2021 |
| Vaccine | Manufacturer/Country | Type | Antigen | Dose/Dosage | Efficacy | Overall Efficacy | Approved Countries | References |
|---|---|---|---|---|---|---|---|---|
| mRNA-1273/SpikeVax | Moderna (US) | mRNA | Full-length spike (S) protein with proline substitutions | 100 μg: 2 doses (28 days apart) | 100% 14 days after second dose | 92.1% 14 days after first dose; 94.1% 14 days after second dose | EUA: the US, EU, Canada, and UK | [57,58,59,60] |
| BNT162b2/Comirnaty | Pfizer.BioNTech (US) | mRNA | Full-length S protein with proline substitutions | 30 μg: 2 doses (21 days apart) | 88.9% after 1 dose | 52% after first dose; 94.6% 7 days after second dose | EUA: the US, EU, Canada, and UK | [61] |
| Ad26.CoV2.S | Janssen/Johnson & Johnson (US) | Viral vector | Recombinant, replication incompetent human adenovirus serotype 26 vector encoding a full-length, stabilized SARS-CoV-2 S protein | 5 × 1010 viral particles: 1 dose | 85% after 28 days; 100% after 49 days | 72% in the US; 66% in Latin America; 57% in South Africa (at 28 days) | EUA: the US, EU, and Canada | [57] |
| ChAdOx1(AZS1222)/ Covishield | AstraZeneca/Oxford (UK) | Viral vector | Replication-deficient chimpanzee adenoviral vector with the SARS-CoV-2 S protein | 5 × 1010 viral particles: 2 doses (28 days apart) | 100% 21 days after first dose | 64.1% after first dose; 70.4% 14 days after second dose | EUA: WHO/Covax, the UK, India, and Mexico | [57] |
| NVX-CoV2373/Nuvaxovid | Novavax, Inc (US) | Protein subunit | Recombinant full-length, prefusion S protein | 5 μg of protein and 50 μg of Matrix-M adjuvant: 2 doses | Unknown | 89.3% in the UK (after 2 doses); 60% in South Africa | EUA application planned | [62] |
| CVnCov | CureVa/GlaxoSmithKline (Germany) | mRNA | Prefusion stabilized full-length S protein of the SARS-CoV-2 virus | 12 μg: 2 doses (28 days apart) | Unknown | Ongoing Phase 3 trial | Not applicable | [62] |
| Gam-COVID-Vac (Sputnik V) | Gamaleya National Research Center for Epidemiology and Microbiology (Russia) | Viral vector | Full-length SARS-CoV-2 virus glycoprotein S carried by adenoviral vectors | 1011 virus particles per dose for each recombinant adenovirus: 2 doses (first rAd26; second rAd5) (21 days apart) | 100% 21 days after first dose | 87.6% 14 days after first dose; 91.1% 7 days after second dose | EUA: Russia, Belarus, Argentina, Serbia, UAE, Algeria, Palestine, and Egypt | [63] |
| CoronaVac | Sinovac Biotech (China) | Inactivated virus | Inactivated CN02 Strain of SARS-CoV-2 created from Vero cells | 3 μg with aluminum hydroxide adjuvant: 2 doses (14 days apart) | Unknown | Phase 3 data not published; reported efficacy 14 days after dose 2: 50.38% (mild) and 78% (mild to severe) in Brazil, 65% in Indonesia, and 91.25% in Turkey | EUA: China, Brazil, Columbia, Bolivia, Brazil, Chile, Uruguay, Turkey, Indonesia and Azerbaijan | [62] |
| BBIBP-CorV | Sinopharm (China) | Inactivated virus | Inactivated HB02 strain of SARS-CoV-2 created from Vero cells | 4 μg with aluminum hydroxide adjuvant: 2 doses (21 days apart) | Unknown | Phase 3 data not published; unpublished reported 79% and 86% efficacy | EUA: China, UAE, Bahrain, Serbia, Peru, and Zimbabwe | [57] |
| BBV152 | Covaxin (Bharat Biotech, India) | Inactivated virus | Whole SARS-CoV-2 Virion (Strain: NIV-2020-770), inactivated Vero Cell | 6 μg of whole-virion with aluminum hydroxide adjuvant: 2 doses (28 days apart) | 78% after second dose | 77.8% (symptomatic); 93.4% (severe); 63.6% (asymptomatic) | Asia, Europe, Africa, South America, North America, Oceania (Australia) | [64] |
| Ad5-nCoV-S recombinant (Ad5-nCoV) | CanSinoBio/Convidecia | Viral vector | Replication-defective adenovirus type 5 vector expressing the SARS-CoV-2 S protein | 0.5 × 1011 virus particles per dose for each recombinant adenovirus: 1 dose | 90.07% (severe) 28 days after single dose and 95.47% (severe) 14 days after single dose. | 65.28% (symptomatic) 28 days after single dose vaccination, and 68.83% (symptomatic) 14 days after single dose. | Asia, Europe, and Latin America | [64] |
2. Current Update on Various Vaccines Targeting SARS-CoV-2
2.1. mRNA Vaccines
2.2. Subunit Vaccines
2.3. Inactivated Virus Vaccines
2.4. Viral Vector Vaccines
2.5. Live Attenuated Vaccines
2.6. Virus-Like Particles Vaccines
2.7. DNA Vaccines
2.8. Mix and Match Vaccine/Boosters
2.9. Vaccines in Clinical Trials Targeting SARS-CoV-2
3. Challenges Associated with SARS-CoV-2 Vaccines
Viral Mutations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T. Novel coronavirus: From discovery to clinical diagnostics. Infec. Genet. Evolut. 2020, 79, 104211. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Crooke, S.N.; Kennedy, R.B. SARS-CoV-2 vaccine development: Current status. Mayo Clin. Proc. 2020, 95, 2172–2188. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Sheikh, F.N.; Jamal, S.; Ezeh, J.K.; Akhtar, A. Coronavirus (COVID-19): A review of clinical features, diagnosis, and treatment. Cureus 2020, 12, e7355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.C.; Wang, C.B.; Bernardini, S. The COVID-19 pandemic. Criti. Rev. Clin. Lab. Sci 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Farooqi, T.; Malik, J.A.; Mulla, A.H.; Al Hagbani, T.; Almansour, K.; Ubaid, M.A.; Alghamdi, S.; Anwar, S. An overview of SARS-CoV-2 epidemiology, mutant variants, vaccines, and management strategies. J. Infect. Public Health 2021, 14, 1299–1312. [Google Scholar] [CrossRef]
- Giovanetti, M.; Benvenuto, D.; Angeletti, S.; Ciccozzi, M. The first two cases of 2019-nCoV in Italy: Where they come from? J. Med. Virol. 2020, 92, 518–521. [Google Scholar] [CrossRef] [Green Version]
- Paraskevis, D.; Kostaki, E.G.; Magiorkinis, G.; Panayiotakopoulos, G.; Sourvinos, G.; Tsiodras, A.S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol 2020, 79, 104212. [Google Scholar] [CrossRef]
- Hampton, T. Bats may be SARS reservoir. JAMA 2005, 294, 2291. [Google Scholar]
- Banerjee, A.; Kulcsar, K.; Misra, V.; Frieman, M.; Mossman, K. Bats and coronaviruses. Viruses 2019, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Shi, Z.; Yu, M.; Ren, W.; Smith, C.; Epstein, J.H.; Wang, H.; Crameri, G.; Hu, Z.; Zhang, H.; et al. Bats are natural reservoirs of SARS-like coronaviruses. Science 2005, 310, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.Y.; Zhang, Y.; Zhou, X.; Huang, K.; Qian, Y.; Leng, Y.; Yan, L.; Huang, B.; He, Y. Molecular evolution of SARS-CoV-2 structural genes: Evidence of positive selection in spike glycoprotein. BioRxiv 2020, 25, 170688. [Google Scholar]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020, 182, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Chiba, S.; Halfmann, P.; Ehre, C.; Kuroda, M.; Dinnon, K.H., III; Leist, S.R.; Schäfer, A.; Nakajima, N.; Takahashi, K.; et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 2020, 370, 1464–1468. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Wang, X.; Pascal, K.E.; Tomkins-Tinch, C.; Nyalile, T.P.; Wang, Y.; Baum, A.; Diehl, W.E.; Dauphin, A.; Carbone, C.; et al. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell 2020, 183, 739–751. [Google Scholar] [CrossRef]
- Volz, E.; Hill, V.; McCrone, J.T.; Price, A.; Jorgensen, D.; O’Toole, Á.; Southgate, J.; Johnson, R.; Jackson, B.; Nascimento, F.F.; et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell 2021, 184, 64–75. [Google Scholar] [CrossRef]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Helfritz, F.A.; et al. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. MedRxiv 2021, 7, 21267432. [Google Scholar]
- Roessler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 B. 1.1. 529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals. MedRxiv 2021, 8, 21267491. [Google Scholar]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.; Greenland, M.; Dinesh, T.; Provstgaard-Morys, S.; Clutterbuck, E.; Ramasamy, M.N.; Aley, P.K.; Farooq, M.Y.; et al. Safety and immunogenicity report from the com-COV study—A single-blind randomised non-inferiority trial comparing heterologous and homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine. EuropePMC 2021. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D.; Laferrière, C.; Ardakani, A. A snapshot of the global race for vaccines targeting SARS-CoV-2 and the COVID-19 pandemic. Front. Pharmacol 2020, 11, 937. [Google Scholar] [CrossRef] [PubMed]
- Geremia, N.; De Vito, A.; Gunnella, S.; Fiore, V.; Princic, E.; Napodano, C.P.; Madeddu, G.; Babudieri, S. A case of vasculitis-like skin eruption associated with COVID-19. Infect. Dis. Clin. Pract 2020, 28, 30–31. [Google Scholar] [CrossRef]
- Viner, R.M.; Whittaker, E. Kawasaki-like disease: Emerging complication during the COVID-19 pandemic. Lancet 2020, 395, 1741-3. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Tirelli, G.; Meloni, P.; Hopkins, C.; Madeddu, G.; De Vito, A.; Gardenal, N.; Valentinotti, R.; Tofanelli, M.; Borsetto, D.; et al. March. Coronavirus disease 2019 (COVID-19)–related smell and taste impairment with widespread diffusion of severe acute respiratory syndrome–coronavirus-2 (SARS-CoV-2) Omicron variant. Int. Forum Allergy Rhinol. 2022, 12, 1273–1281. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.; Yuen, T.T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.; Tsang, J.O.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med 2020, 14, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Bakhiet, M.; Taurin, S. SARS-CoV-2: Targeted managements and vaccine development. Cytokine Growth Fact. Rev 2021, 58, 16–29. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Hill, T.; Li, K.; Peters, C.J.; Tseng, C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. Virol. J. 2009, 83, 3039–3048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ. Res 2020, 127, 1404–1418. [Google Scholar] [CrossRef]
- Fosbøl, E.L.; Butt, J.H.; Østergaard, L.; Andersson, C.; Selmer, C.; Kragholm, K.; Schou, M.; Phelps, M.; Gislason, G.H.; Gerds, T.A.; et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020, 324, 168–177. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Geremia, N.; Princic, E.; Fanelli, C.; Napodano, P.; Muredda, A.A.; Fiore, V.; Maida, I.; Fois, A.G.; Babudieri, S.; et al. Does Angiotensin II receptor blockers increase the risk of SARS-CoV-2 infection? A real-life experience. Eur. Rev. Med. Pharmacol. Sci. 2021, 523–526. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, evaluation, and treatment of coronavirus (COVID-19). Statpearls 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/ (accessed on 20 February 2022).
- Wang, Y.; Wang, Y.; Chen, Y.; Qin, Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020, 92, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Abramowicz, M.; Zucotti, G.; Pflomm, M. Tixagevimab and cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. JAMA 2022, 327, 384–385. [Google Scholar]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.COVID19treatmentguidelines.nih.gov/ (accessed on 28 December 2022).
- Gentile, I.; Scotto, R.; Schiano Moriello, N.; Pinchera, B.; Villari, R.; Trucillo, E.; Ametrano, L.; Fusco, L.; Castaldo, G.; Buonomo, A.R.; et al. Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: Results of a real-life study. Vaccines 2022, 10, 1731. [Google Scholar] [CrossRef]
- Crothers, K.; DeFaccio, R.; Tate, J.; Alba, P.R.; Goetz, M.B.; Jones, B.; King, J.T.; Marconi, V.; Ohl, M.E.; Rentsch, C.T.; et al. Dexamethasone in hospitalised COVID-19 patients not on intensive respiratory support. Eur. Respir. J. 2022, 60, 2102532. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Juneja, K.; Hill, J.A. Outpatient remdesivto prevent progression to severe COVID-19. Reply N. Engl. J. Med. 2022, 386, 1094. [Google Scholar]
- Piccicacco, N.; Zeitler, K.; Ing, A.; Montero, J.; Faughn, J.; Silbert, S.; Kim, K. Real-world effectiveness of early remdesivir and sotrovimab in the highest-risk COVID-19 outpatients during the Omicron surge. J. Antimicrob. Chemother 2022, 77, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Thye, A.Y.; Law, J.W.; Pusparajah, P.; Letchumanan, V.; Chan, K.G.; Lee, L.H. Emerging SARS-CoV-2 variants of concern (VOCs): An impending global crisis. Biomedicines. 2021, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Nainu, F.; Frediansyah, A.; Yatoo, M.I.; Mohapatra, R.K.; Chakraborty, S.; Zhou, H.; Islam, M.R.; Mamada, S.S.; Kusuma, H.I.; et al. Global emerging Omicron variant of SARS-CoV-2: Impacts, challenges and strategies. J. Infect. Public Health 2022, 16, 4–14. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England: Technical Briefing 46 [Internet]. London: Crown Copyright; 2022 [Cited 2022 Oct 7]. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1109820/Technical-Briefing-46.pdf (accessed on 10 January 2023).
- Hisner, R. Proposal for a Sublineage of BA.5.2.1 with S:R346T and N:S33F (Originally Proposed by @FedeGueli) #827 [Internet]. GitHub; 2022 Jul 7 [cited 2022 Oct 6]. Available online: https://github.com/cov-lineages/pango-designation/issues/827 (accessed on 20 February 2022).
- Cao, Y.; Jian, F.; Wang, J.; Yu, Y.; Song, W.; Yisimayi, A.; Wang, J.; An, R.; Chen, X.; Zhang, N.; et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 2022, 614, 1–3. [Google Scholar] [CrossRef]
- Liu, Z.; VanBlargan, L.A.; Bloyet, L.M.; Rothlauf, P.W.; Chen, R.E.; Stumpf, S.; Zhao, H.; Errico, J.M.; Theel, E.S.; Liebeskind, M.J.; et al. Landscape analysis of escape variants identifies SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. BioRxiv 2020, 11, 372037. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news/item/27-10-2022-tag-ve-statement-on-omicron-sublineages-bq.1-and-xbb (accessed on 20 February 2023).
- Sheward, D.J.; Kim, C.; Fischbach, J.; Sato, K.; Muschiol, S.; Ehling, R.A.; Björkström, N.K.; Hedestam, G.B.; Reddy, S.T.; Albert, J.; et al. Omicron sublineage BA. 2.75. 2 exhibits extensive escape from neutralising antibodies. Lancet Infect. Dis. 2022, 22, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- WHO. “Tracking SARS-CoV-2 Variants”. Retrieved 15 July 2022. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 20 February 2023).
- Qu, P.; Faraone, J.N.; Evans, J.P.; Zheng, Y.M.; Carlin, C.; Anghelina, M.; Stevens, P.; Fernandez, S.; Jones, D.; Lozanski, G.; et al. Extraordinary evasion of neutralizing antibody response by Omicron XBB. 1.5, CH. 1.1 and CA. 3.1 Variants. bioRxiv 2023. [Google Scholar] [CrossRef]
- Available online: https://www.fiercehealthcare.com/providers/new-covid-19-omicron-subvariant-ch11-contains-delta-mutation-concerns-health-experts (accessed on 22 February 2023).
- Available online: https://www.sunstar.com.ph/article/1953532/manila/local-news/doh-detects-first-case-of-xbf-subvariant (accessed on 22 February 2023).
- Available online: https://insightplus.mja.com.au/2023/2/omicron-the-circulating-subvariant-facing-new-pressures/ (accessed on 22 February 2023).
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef]
- Boo, S.H.; Kim, Y.K. The emerging role of RNA modifications in the regulation of mRNA stability. Exp. Mol. Med. 2020, 52, 400–408. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Nance, K.D.; Meier, J.L. Modifications in an emergency: The role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent. Sci. 2021, 7, 748–756. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabrouk, M.T.; Huang, W.C.; Martinez-Sobrido, L.; Lovell, J.F. Advanced materials for SARS-CoV-2 vaccines. Adv. Mater. 2022, 34, 2107781. [Google Scholar] [CrossRef] [PubMed]
- Abdul, M.G. Mixing of Sputnik V and AstraZeneca COVID-19 Vaccines. Jpn. J. Gstro Hepato 2021, 6, 1–3. [Google Scholar]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Wlodkowic, D. The 2020 race towards SARS-CoV-2 specific vaccines. Theranostics 2021, 11, 1690. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, H.; Gu, J.; Li, H.; Zheng, L.; Zou, Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines 2020, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Tatsis, N.; Ertl, H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef]
- Zak, D.E.; Andersen-Nissen, E.; Peterson, E.R.; Sato, A.; Hamilton, M.K.; Borgerding, J.; Krishnamurty, A.T.; Chang, J.T.; Adams, D.J.; Hensley, T.R.; et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc. Natl. Acad. Sci. USA 2012, 109, E3503-12. [Google Scholar] [CrossRef] [Green Version]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Bottazzi, M.E.; Du, L.; Lustigman, S.; Tseng, C.T.K.; Curti, E.; Jones, K.; Zhan, B.; Hotez, P.J. Roadmap to developing a recombinant coronavirus S protein receptor-binding domain vaccine for severe acute respiratory syndrome. Expert Rev. Vaccines 2012, 11, 1405–1413. [Google Scholar] [CrossRef] [Green Version]
- Versteeg, L.; Almutairi, M.M.; Hotez, P.J.; Pollet, J. Enlisting the mRNA vaccine platform to combat parasitic infections. Vaccines 2019, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Robbins, G.; Wosen, J. Scientists Are Struggling to Quickly Find a Vaccine That Can Vanquish Coronavirus. San Diego Union-Tribune. 2020. Available online: https://www.sandiegouniontribune.com/news/science/story/2020-06-06/race-for-vaccine (accessed on 20 October 2022).
- Shang, W.; Yang, Y.; Rao, Y.; Rao, X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines npj. Vaccines 2020, 5, 1–3. [Google Scholar]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target. Ther. 2020, 135, 237. [Google Scholar] [CrossRef]
- Rashedi, R.; Samieefar, N.; Masoumi, N.; Mohseni, S.; Rezaei, N. COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts. J. Med. Virol. 2022, 94, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G. Mixing vaccines may boost immune responses. Science 2021, 372, 1138. [Google Scholar] [CrossRef]
- Lewis, D. Mix-and-match COVID vaccines: The case is growing, but questions remain. Nature 2021, 595, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Benning, L.; Töllner, M.; Hidmark, A.; Schaier, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Buylaert, M.; Grenz, J.; Ponath, G.; et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines 2021, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- MJ, E.O.; Juanes, D.T.B. Pfizer-BioNTech, la primera vacuna ARNm contra la COVID-19, parece segura y eficaz. Evid. Pedriatr. 2021, 17, 6. [Google Scholar]
- Edwards, K.M.; Orenstein, W.A. COVID-19: Vaccines; Hirsch, M.S.B., Ed.; UptoDate: Wellesley, MA, USA, 2022. [Google Scholar]
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 3, 1850. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, S.J. Current updates on COVID-19 vaccines and therapeutics: As of June 2022. Biotechnol. Bioprocess Eng. 2022, 27, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- CureVac, A.G. Curevac Final Data from Phase 2b/3 Trial of First-Generation COVID-19 Vaccine Candidate, Cvncov, Demonstrates Protection in Age Group of 18 to 60 2021. Available online: https://www.curevac.com/en/2021/06/30/curevac-final-data-from-phase-2b-3-trial-of-first-generation-COVID-19-vaccine-candidate-cvncov-demonstrates-protection-in-age-group-of-18-to-60/ (accessed on 20 February 2022).
- Röltgen, K.; Nielsen, S.C.; Arunachalam, P.S.; Yang, F.; Hoh, R.A.; Wirz, O.F.; Lee, A.S.; Gao, F.; Mallajosyula, V.; Li, C.; et al. mRNA vaccination compared to infection elicits an IgG-predominant response with greater SARS-CoV-2 specificity and similar decrease in variant spike recognition. MedRxiv 2021, 4, 21254952. [Google Scholar]
- Pollock, K.M.; Cheeseman, H.M.; Szubert, A.J.; Libri, V.; Boffito, M.; Owen, D.; Bern, H.; O'Hara, J.; McFarlane, L.R.; Lemm, N.M.; et al. Safety and immunogenicity of a self-amplifying RNA vaccine against COVID-19: COVAC1, a phase I, dose-ranging trial. EClinicalMedicine 2022, 44, 101262. [Google Scholar] [CrossRef]
- Ochoa-Azze, R.; Chang-Monteagudo, A.; Climent-Ruiz, Y.; Macías-Abraham, C.; Valenzuela-Silva, C.; de los Ángeles García-García, M.; Jerez-Barceló, Y.; Triana-Marrero, Y.; Ruiz-Villegas, L.; Rodríguez-Prieto, L.D.; et al. Safety and immunogenicity of the FINLAY-FR-1A vaccine in COVID-19 convalescent participants: An open-label phase 2a and double-blind, randomised, placebo-controlled, phase 2b, seamless, clinical trial. Lancet Respir. Med. 2022, 10, 785–795. [Google Scholar] [CrossRef]
- Calina, D.; Sarkar, C.; Arsene, A.L.; Salehi, B.; Docea, A.O.; Mondal, M.; Islam, M.T.; Zali, A.; Sharifi-Rad, J. Recent advances, approaches and challenges in targeting pathways for potential COVID-19 vaccines development. Immunol. Res. 2020, 68, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Calina, D.; Falzone, L.; Petrakis, D.; Mitrut, R.; Siokas, V.; Pennisi, M.; Lanza, G.; Libra, M.; Doukas, S.G.; et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020, 146, 111769. [Google Scholar] [CrossRef] [PubMed]
- CDC. Global COVID-19 Vaccinations [Internet]. 2021. Available online: https://covid.cdc.gov/covid-data-tracker/#global-vaccinations (accessed on 3 September 2021).
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm., J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Sesa, G.; Siepmann, I.; Czabanowska, K.; Green, M.; Reid, J.; Middleton, J. The Importance of Health Communication during Emergencies. Mix-and-Match Quest. 2021. Available online: https://www.aspher.org/download/796/aspher_dosemixing-23072021.pdf (accessed on 10 February 2023).
- Soriano, V.; de Mendoza, C.; Gómez-Gallego, F.; Corral, O.; Barreiro, P. Third wave of COVID-19 in Madrid, Spain. Int. J. Infect. Dis. 2021, 107, 212–214. [Google Scholar] [CrossRef]
- El-Shabasy, R.M.; Nayel, M.A.; Taher, M.M.; Abdelmonem, R.; Shoueir, K.R. Three wave changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int. J. Biol. Macromol. 2022, 204, 161–168. [Google Scholar] [CrossRef]
- Chersich, M.F.; Gray, G.; Fairlie, L.; Eichbaum, Q.; Mayhew, S.; Allwood, B.; English, R.; Scorgie, F.; Luchters, S.; Simpson, G.; et al. COVID-19 in Africa: Care and protection for frontline healthcare workers. Glob. Health 2020, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Denison, M.R.; Graham, R.L.; Donaldson, E.F.; Eckerle, L.D.; Baric, R.S. Coronaviruses an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011, 8, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Gong, W. Will mutations in the spike protein of SARS-CoV-2 lead to the failure of COVID-19 vaccines? J. Korean Med. Sci. 2021, 36, e124. [Google Scholar] [CrossRef]
- Gómez, C.E.; Perdiguero, B.; Esteban, M. Emerging SARS-CoV-2 variants and impact in global vaccination programs against SARS-CoV-2/COVID-19. Vaccines 2021, 9, 243. [Google Scholar] [CrossRef]
- Morin, L.; Savale, L.; Pham, T.; Colle, R.; Figueiredo, S.; Harrois, A.; Gasnier, M.; Lecoq, A.L.; Meyrignac, O.; Noel, N.; et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021, 325, 1525–1534. [Google Scholar]
- Lal, A.; Ahmed, N.; Maqsood, A.; Alam, M.K. COVID-19 Omicron-another deadly dilemma. Altamash J. Dent. Med. 2022, 1, 1–5. [Google Scholar]
- Chatterjee, S.; Bhattacharya, M.; Nag, S.; Dhama, K.; Chakraborty, C. A Detailed Overview of SARS-CoV-2 Omicron: Its sub-variants, mutations and pathophysiology, clinical characteristics, immunological landscape, immune escape, and therapies. Viruses 2023, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Vasanthakumaran, T.; Thi Hien, H.N.; Hung, I.C.; Luu, M.N.; Khan, Z.A.; An, N.T.; Tran, V.P.; Lee, W.J.; Abdul Aziz, J.M.; et al. SARS-CoV-2 Omicron and its current known unknowns: A narrative review. Rev. Med. Virol. 2023, 33, e2398. [Google Scholar] [CrossRef] [PubMed]
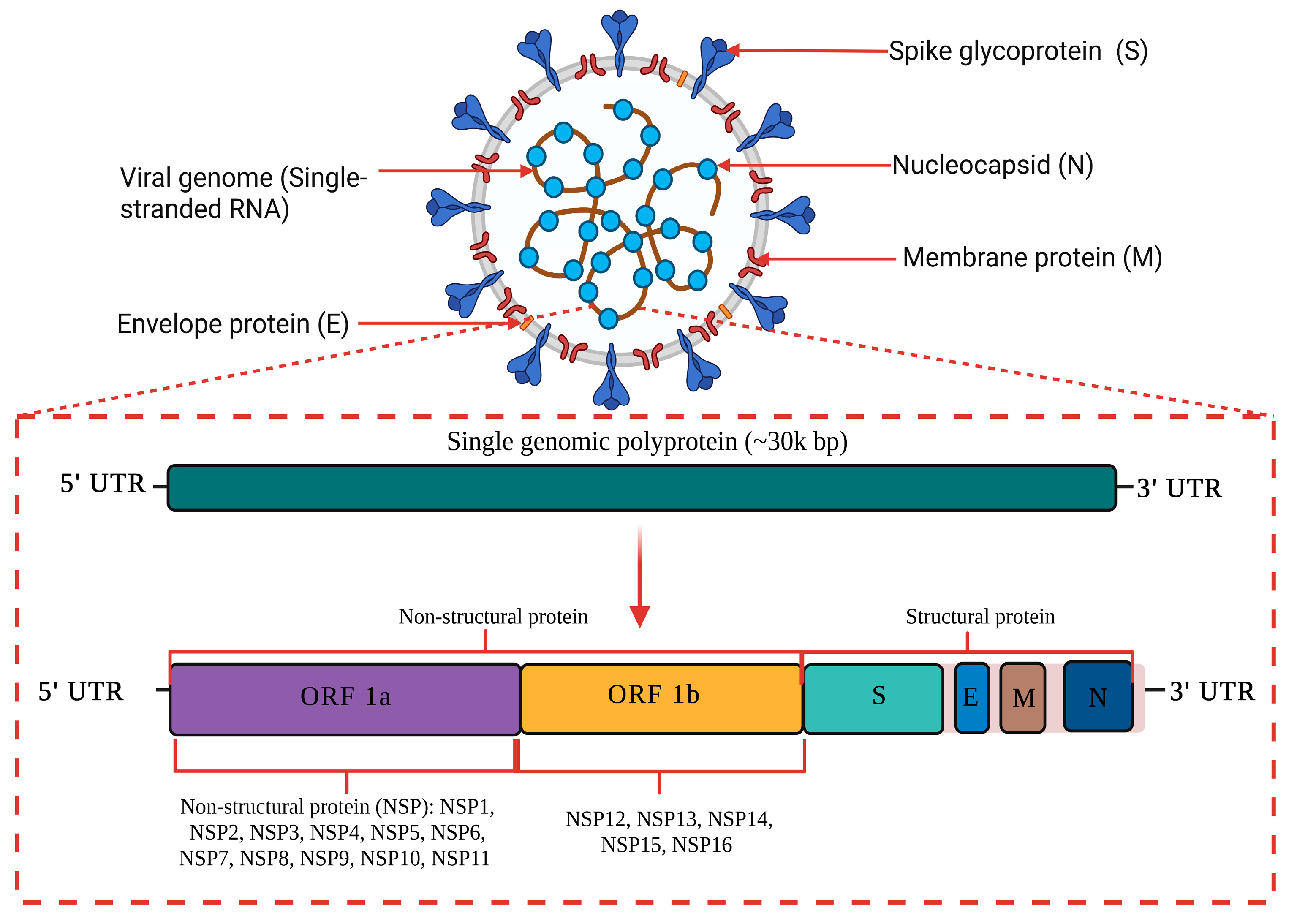
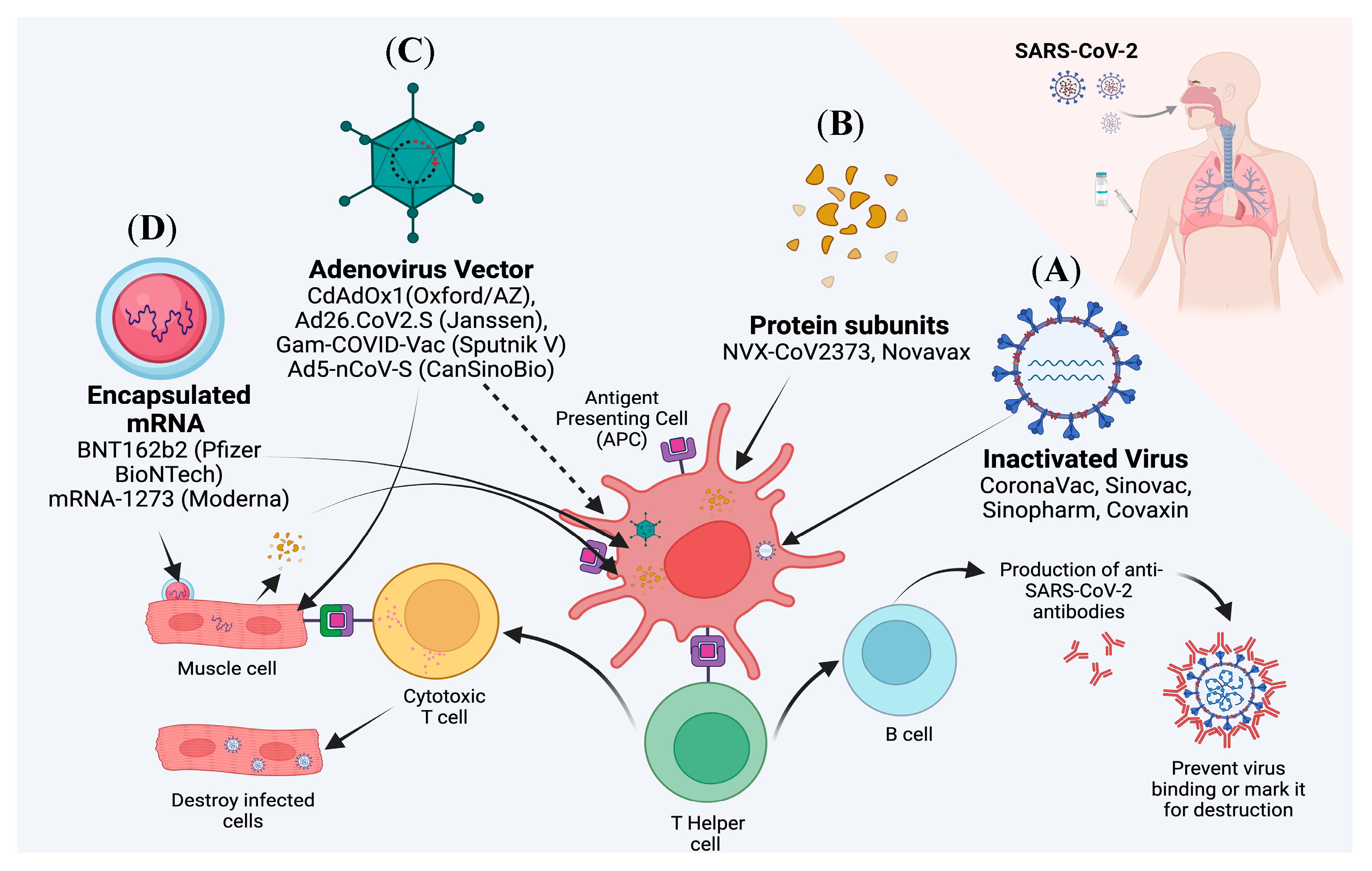
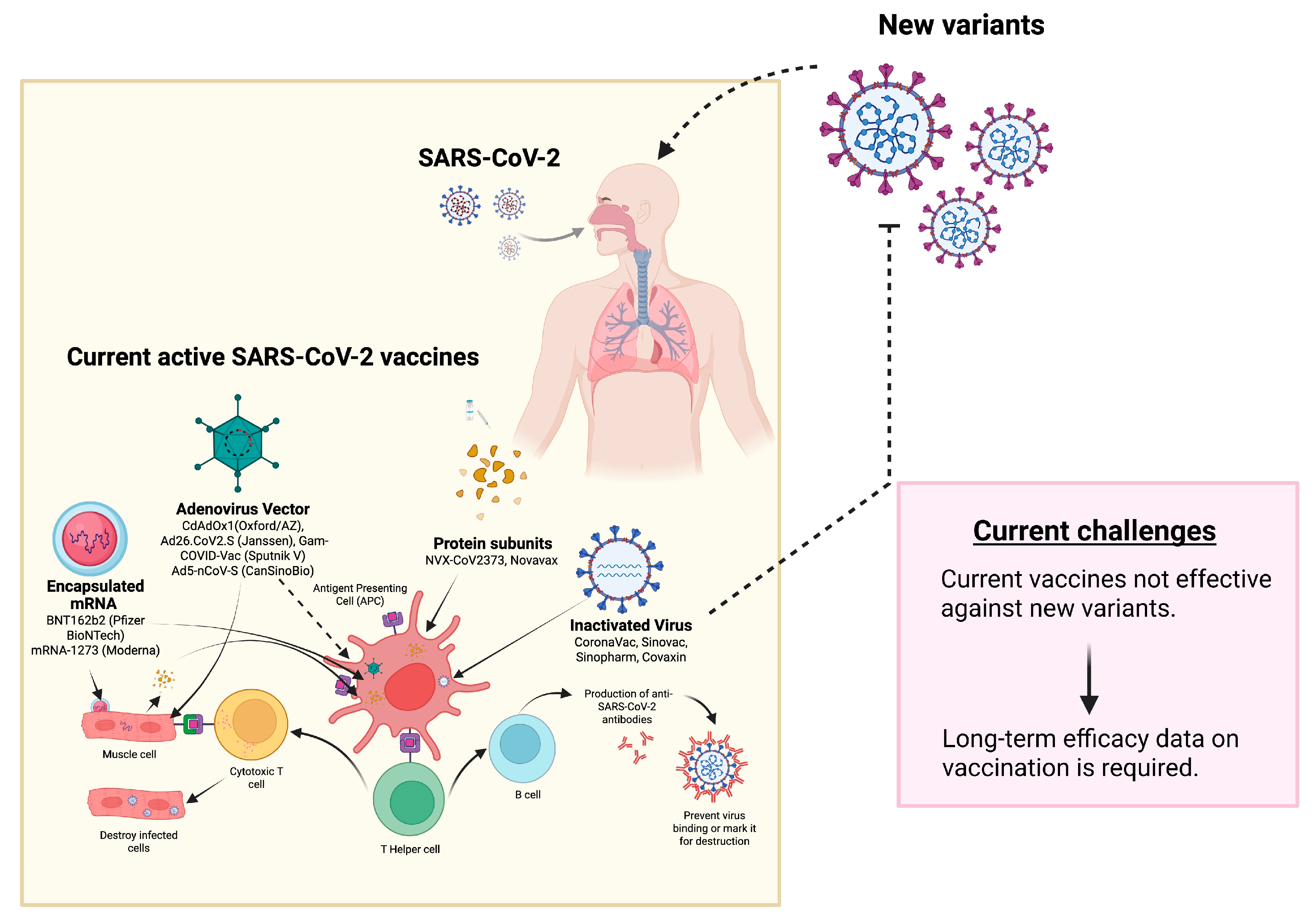
| Pango Lineage #. (+Mutation) | GISAID Clade | Next Strain Clade | Relationship to Circulating VOC Lineages | Spike Genetic Features | Earliest Documented Samples | Pathophysiology | Epidemiology |
|---|---|---|---|---|---|---|---|
| BF.7 * | GRA | 22B | BA.5 sublineages | BA.5 + S:R346T | 24 January 2022 |
|
|
| BQ.1 $ | GRA | 22E | BA.5 sublineages | BQ.1 and BQ.1.1: BA.5 + S:R346T, S:K444T, S:N460K | 7 February 2022 |
|
|
| BA.2.75 § | GRA | 22D | BA.2 sublineage | BA.2.75: BA.2 + S:K147E, S:W152R, S:F157L, S:I210V, S:G257S, S:D339H, S:G446S, S:N460K, S:Q493R reversion | 31 December 2021 |
|
|
| CH.1.1 § | GRA | 22D | BA.2 sublineage | BA.2.75 + S:L452R, S:F486S | 27 July 2022 | ||
| XBB µ | GRAA | 22F | Recombinant of BA.2.10.1 and BA.2.75 sublineages, i.e., BJ1 and BM.1.1.1, with a breakpoint in S1 | BA.2+ S:V83A, S:Y144-, S:H146Q, S:Q183E, S:V213E, S:G252V, S:G339H, S:R346T, S:L368I, S:V445P, S:G446S, S:N460K, S:F486S, S:F490S | 13 August 2022 |
|
|
| XBB.1.5 | GRA | 23A | Recombinant of BA.2.10.1 and BA.2.75 sublineages, i.e., BJ1 and BM.1.1.1, with a breakpoint in S1 | XBB + S:F486P | 15 January 2022 |
|
|
| XBF | GRA | Recombinant of BA.5.2.3 and CJ.1 (BA.2.75.3 sublineage) | BA.5 + S:K147E, S:W152R, S:F157L, S:I210V, S:G257S, S:G339H, S:R346T, S:G446S, S:N460K, S:F486P, S:F490S | 27 July 2022 |
| * very little to no information on XBF, mainly reported on online newspaper only. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahboob, T.; Ismail, A.A.; Shah, M.R.; Rahmatullah, M.; Paul, A.K.; Pereira, M.d.L.; Wiart, C.; Wilairatana, P.; Rajagopal, M.; Dolma, K.G.; et al. Development of SARS-CoV-2 Vaccine: Challenges and Prospects. Diseases 2023, 11, 64. https://doi.org/10.3390/diseases11020064
Mahboob T, Ismail AA, Shah MR, Rahmatullah M, Paul AK, Pereira MdL, Wiart C, Wilairatana P, Rajagopal M, Dolma KG, et al. Development of SARS-CoV-2 Vaccine: Challenges and Prospects. Diseases. 2023; 11(2):64. https://doi.org/10.3390/diseases11020064
Chicago/Turabian StyleMahboob, Tooba, Amni Adilah Ismail, Muhammad Raza Shah, Mohammed Rahmatullah, Alok K. Paul, Maria de Lourdes Pereira, Christophe Wiart, Polrat Wilairatana, Mogana Rajagopal, Karma G. Dolma, and et al. 2023. "Development of SARS-CoV-2 Vaccine: Challenges and Prospects" Diseases 11, no. 2: 64. https://doi.org/10.3390/diseases11020064
APA StyleMahboob, T., Ismail, A. A., Shah, M. R., Rahmatullah, M., Paul, A. K., Pereira, M. d. L., Wiart, C., Wilairatana, P., Rajagopal, M., Dolma, K. G., & Nissapatorn, V. (2023). Development of SARS-CoV-2 Vaccine: Challenges and Prospects. Diseases, 11(2), 64. https://doi.org/10.3390/diseases11020064









