Abstract
In May 2023, the global health emergency status of COVID-19 concluded, marking the onset of an endemic era. This study assessed survival rates among PCR-confirmed adult inpatients during this phase and determined contributing factors. Employing a survival analysis approach, this investigation utilized a nationwide Mexican cohort encompassing 152 adult inpatients. Survival rates were computed using the Kaplan–Meier method, and a proportional Cox model identified mortality risk factors. Survival rates remained above 65% on day 14 after admission. Vaccination status, including the number of doses administered, was not significantly associated with fatal outcomes. Chronic kidney disease or a history of immunosuppression (due to any cause) increased mortality risk. Our findings underscore the persistent severity of COVID-19 beyond the global health emergency, emphasizing the necessity for tailored interventions for vulnerable patients.
1. Introduction
The emergence of the coronavirus disease 2019 (COVID-19) pandemic, resulting from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, necessitated immediate and concerted cooperation among healthcare systems, the scientific community, and governmental bodies to effectively address the repercussions of the infection on public health [1]. Progressing alongside the advancement of vaccines, substantial headway was achieved in curtailing the transmission and mitigating the virulence of the virus [2]. Consequently, the designation of COVID-19 as a global health emergency was lifted, and the disease transitioned into an endemic state [3].
In Mexico, the COVID-19 pandemic became a major social and economic burden. Nonetheless, since the declaration of the end of the global emergency, there have been notable reductions in hospitalization rates, as well as diminished morbidity and mortality [4].
While attention has been largely focused on acute management, transmission control, and vaccine deployment during the pandemic’s peak, the transition from a global health emergency to a post-emergency phase warrants a thorough investigation [5]. This transition not only signifies a pivotal milestone in pandemic management but also presents an opportunity to comprehend the long-term implications of COVID-19 for individuals who have experienced the disease [6]. Understanding the survival outcomes and health trajectories of adult inpatients who tested positive for COVID-19 can provide insights into the lingering effects of the virus and inform healthcare strategies in the post-pandemic era.
This study aims to evaluate the survival experience of adult inpatients who were diagnosed with PCR-confirmed COVID-19 after the conclusion of the global health emergency. Therefore, the investigation aims to contribute to the growing body of knowledge concerning the dynamics of COVID-19 as it transitions from an acute health crisis to a manageable endemic condition. This study intends to provide valuable insights that can guide healthcare policies, clinical decision making, and public health strategies in the aftermath of the global health emergency.
2. Materials and Methods
We conducted a nationwide cohort study in Mexico, starting at the outset of the COVID-19 pandemic, which emerged in late February 2020. A more comprehensive description of the cohort has been previously published [7]. This specific investigation, characterized as a survival analysis study, centered on a subset of adult patients aged 18 years and above, presenting respiratory symptoms indicative of COVID-19 between 5 May and 26 July 2023. The inclusion criteria involved confirming disease through RT-PCR testing. Patients who did not necessitate hospitalization were omitted, as well as those with incomplete clinical and epidemiological data of interest.
Patients were identified using the records of a system for the epidemiological surveillance of viral respiratory diseases, which specifically targets SARS-CoV-2, influenza virus, respiratory syncytial virus, and other pathogens of public health concern. This system is known as SINOLAVE, and it primarily derives data from patients’ medical records and, when applicable, death certificates [8].
Demographic and clinical variables of interest were derived from the audited surveillance system. We employed pneumonia at the time of hospital admission as an indicator of severity. This was defined by the presence of respiratory clinical symptoms (such as cough, fever, dyspnea, and chest pain), as well as radiographic evidence of pneumonia (bilateral ground glass opacities or consolidations visible on CT or X-ray).
Nucleic acids were extracted from 200 μL of clinical specimens (deep nasal swabs) using the MagNa Pure LC Total Nucleic Acid Isolation Kit automated system (catalog: 03038505001; Roche Diagnostics, Mannheim, Germany) [9]. The detection of SARS-CoV-2 was carried out utilizing the primers and probes proposed by Corman et al. [10], employing the SuperScript III Platinum One-step qRT-PCR System (catalog: 12574035; Invitrogen, Carlsbad, CA, USA) in conjunction with the 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
For the analysis of hospitalized patients with molecularly diagnosed COVID-19, we applied the Kaplan–Meier method to compute survival functions and determine 95% confidence intervals (CIs). The primary binary outcome was in-hospital death due to any immediate cause in patients with PCR-confirmed COVID-19, serving as the failure event. Patients who were still hospitalized as of 5 August 2023 (the date of data compilation) were considered censored observations. To assess the factors associated with patient survival, a multiple proportional Cox hazard regression model was employed.
This study underwent review and approval by the Local Committee of Ethics in Health Research (601) of the IMSS (approval R-2022-601-022). None of the participants were physically located or interviewed during any stage of this study, and all researchers adhered to strict ethical guidelines.
3. Results
Data from 152 inpatients with laboratory-confirmed COVID-19 were analyzed, resulting in a total follow-up period of 1143 person-days. They represented 6.2% (n = 152/2441) of the total laboratory-positive patients registered during the study period. The remaining cases (93%, n = 2289/2441) were patients with mild symptoms who did not require hospital admission and were therefore excluded.
We documented a total of 25 deaths, as indicated by the discharge information present in medical records and subsequently validated by death certificates. Thus, the overall mortality rate was calculated as 2.2 per 100 person-days (25 deaths over 1143 person-days). The median survival time (and the interquartile range) was 6 (4–9) days among cases resulting in a fatal outcome. For patients who successfully recovered, the median duration of in-hospital stay was 6 (3–11) days. No statistically significant differences (p = 0.615) were observed between these latter two groups.
As presented in Table 1, the participants who experienced a fatal in-hospital outcome were more likely to exhibit pneumonia at the time of hospital admission (40.0% vs. 14.2%, p = 0.002). They also had a higher prevalence of a personal history of type 2 diabetes mellitus (52.0% vs. 20.5%, p = 0.001), chronic kidney disease (CKD; 28.0% vs. 6.3%, p = 0.001), and immunosuppression due to any cause (20.0% vs. 1.6%, p < 0.001). No significant differences were observed in terms of disease outcomes in relation to COVID-19 vaccination status, gender, age, or other clinical characteristics.

Table 1.
Characteristics of the study sample (n = 152) for selected variables, Mexico 2023.
The average time span between the date of the final vaccine administration and the onset of symptoms was 504.4 ± 221.1 days for individuals who had received two doses and 445.9 ± 196.9 days for those who had received a vaccine booster (three doses). The lone patient within the study cohort who had received only one vaccine shot encountered symptom onset 463 days after being vaccinated.
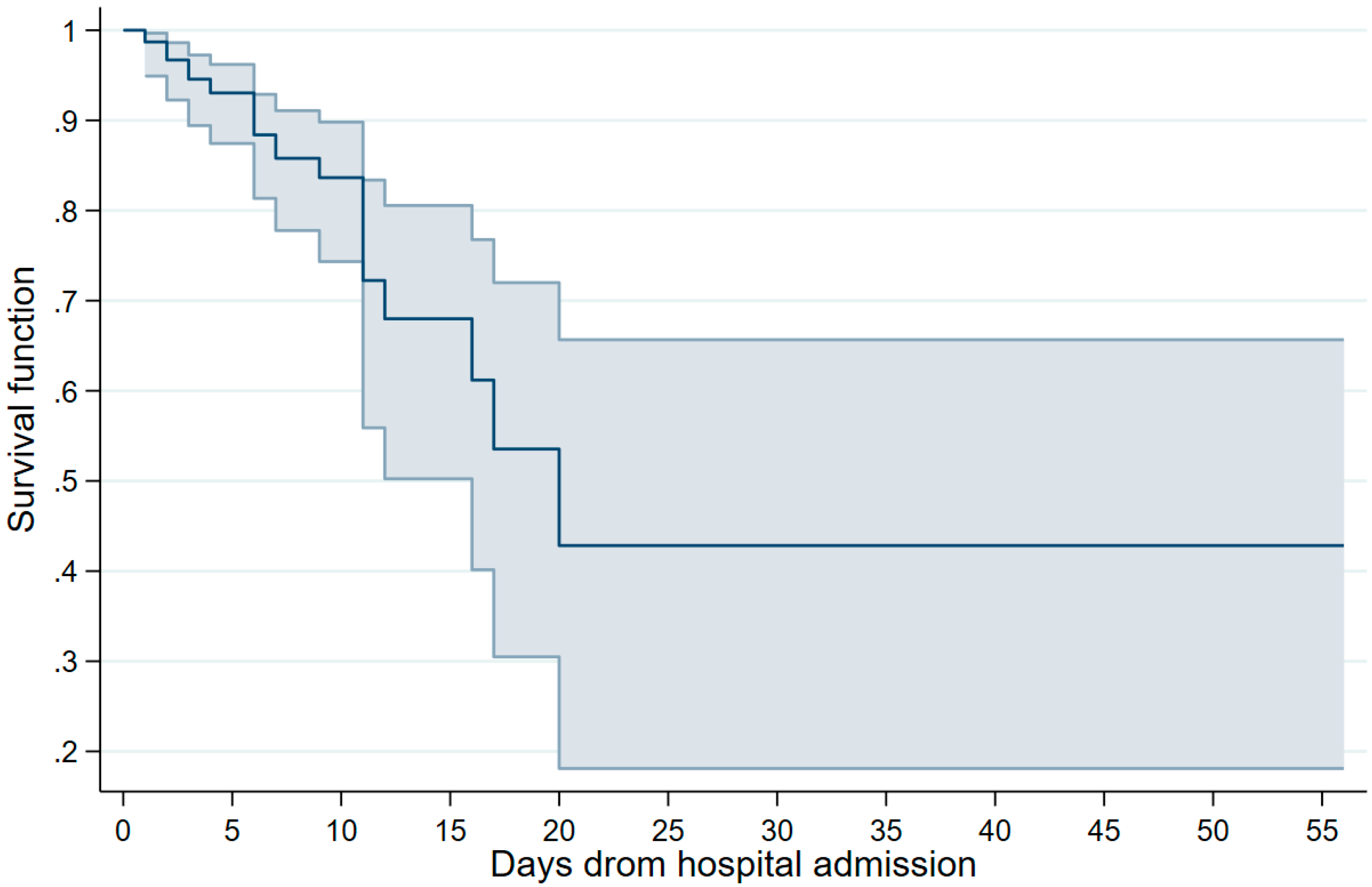
The survival rates are summarized in Figure 1. The estimates, along with their corresponding 95% CIs, were as follows, based on the days elapsed since hospital admission: 1 day, 0.98 (0.96–0.99); 3 days, 0.94 (0.91–0.96); 7 days, 0.84 (0.80–0.88); 14 days, 0.66 (0.56–0.73); 21 days, 0.56 (0.43–0.66); and 28 days, 0.47 (0.31–0.61).
Figure 1.
Survival curve of analyzed patients, Mexico 2023. Note: the point estimates and 95% confidence intervals are presented.
In the multiple proportional hazards model (Table 2), the patients with a personal history of CKD had a 3.4-fold increased risk of death due to COVID-19 (HR = 3.43, 95% CI 1.07–11.0). The highest risk was observed in immunosuppressed patients (HR = 6.44, 95% CI 1.49–17.8). No other significant associations were observed in the study sample.

Table 2.
Factors associated with survival rates, Mexico 2023.
4. Discussion
The present study analyzed the survival experience among adult inpatients who were diagnosed with PCR-confirmed COVID-19 following the cessation of the global health emergency and during the SARS-CoV-2 endemic era. This research provides valuable insights into the complex interplay between patient characteristics, disease course, and outcomes in this post-emergency phase.
The survival rates, in general, exceeded those documented in the same cohort throughout the wild-type emergency and subsequent pandemic waves [11]. Moreover, even with a hospitalization period extending to 14 days, the computed rates remained higher than 65% (0.66, 95% CI 0.56–0.73).
Interestingly, there were no significant differences in disease outcomes concerning COVID-19 vaccination status, gender, age, or other clinical characteristics that consistently exhibited associations with adverse disease outcomes throughout the pandemic, such as a personal history of type 2 diabetes mellitus [12,13]. The absence of a protective effect from vaccines might be attributed to the extended interval (over 6 months) between the last vaccine administration and the onset of illness, possibly resulting in decreased antibody titers [14]. As for type 2 diabetes mellitus, the absence of an association within the study sample might stem from a potential deficiency in statistical power within the multiple regression model, indicated by the computed p-value slightly exceeding the significance threshold ( = 0.056).
The linkage between certain comorbidities and an increased mortality risk was also starkly evident. Participants with a personal history of CKD and immunosuppression exhibited a significantly higher prevalence in the group with fatal outcomes. Similar findings have been previously published [15,16,17,18,19]. These findings underscore the persisting vulnerability of patients with pre-existing health conditions in the post-emergency phase. Collectively, these findings suggest that the post-emergency phase is characterized by a more even distribution of risk factors and that vaccination might not be the sole determinant of survival.
The inclusion of exclusively PCR-confirmed cases represents a significant strength of this study. However, it is essential to acknowledge the potential limitations inherent to the research. Firstly, our analysis was constrained by a limited sample size, a consequence of the low hospitalization rates observed during the post-emergency phase under examination. We focused exclusively on non-ambulatory cases, which exhibited more severe clinical symptoms and, consequently, a higher risk of adverse outcomes. Therefore, conducting larger-scale investigations that also encompass patients with mild symptoms not necessitating hospital admission could potentially yield more comprehensive insights into this area.
Secondly, a notable limitation is the absence of genomic data to ascertain the prevalent SARS-CoV-2 variants. Thirdly, we were unable to measure antibody levels subsequent to SARS-CoV-2 infection or vaccination. Incorporating such measurements could have held clinical and epidemiological value, particularly in assessing the impact of COVID-19 vaccination status on survival rates. Finally, our study did not encompass an analysis of age-stratified data, a methodological omission that curtailed our ability to investigate how various age groups responded to the exposures under scrutiny.
5. Conclusions
This study’s findings underscore the persistent gravity of COVID-19, even beyond the global health emergency phase, highlighting the need for targeted interventions for patients with specific comorbidities and vulnerabilities. The complex interplay of patient characteristics, disease severity, and underlying health conditions continues to shape survival outcomes. As the world navigates the post-pandemic landscape, these insights contribute to the informed formulation of healthcare policies and individualized patient management strategies.
Author Contributions
This study’s conception and methodological framework were driven by V.B.-G., O.M.-C. and E.M.-Z. Data analysis was conducted by X.T., M.R.-S., J.A.B.-B., G.M.B.-R. and A.D.O.-R. The interpretation of the research findings was a collaborative effort involving A.L.-R., J.A.B.-B., H.B.C.-A., E.F.R.-B., W.S.-M., Y.C. and G.M.B.-R. The initial manuscript draft was led by V.B.-G., O.M.-C. and E.M.-Z. Subsequently, comprehensive review and editing were undertaken by X.T., M.R.-S., A.L.-R., H.B.C.-A., E.F.R.-B., W.S.-M., Y.C. and A.D.O.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was reviewed and approved by the Local Committee of Ethics in Health Research (601) of the IMSS (approval R-2020-601-022).
Informed Consent Statement
Since the data set analyzed was derived from the normative surveillance of COVID-19 and was fully deidentified prior to delivery to the research group, the requirement for consent to participate was waived.
Data Availability Statement
The data and materials analyzed in this study are available from the corresponding author upon request.
Acknowledgments
The group of researchers expresses gratitude to the Health Research Coordination of the Mexican Social Security Institute for their invaluable support in conducting the research and disseminating the findings.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Balqis-Ali, N.Z.; Fun, W.H.; Ismail, M.; Ng, R.J.; Jaaffar, F.S.A.; Low, L.L. Addressing Gaps for Health Systems Strengthening: A Public Perspective on Health Systems’ Response towards COVID-19. Int. J. Environ. Res. Public. Health 2021, 18, 9047. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Pant, A.B. Mitigating COVID-19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. J. Autoimmun. 2022, 127, 102792. [Google Scholar] [CrossRef] [PubMed]
- United Nations. UN News: WHO Chief Declares End to COVID-19 as a Global Health Emergency. Available online: https://news.un.org/en/story/2023/05/1136367#:~:text=WHO%20chief%20declares%20end%20to%20COVID%2D19%20as%20a%20global%20health%20emergency,-5%20May%202023&text=The%20head%20of%20the%20UN,no%20longer%20a%20global%20threat (accessed on 12 August 2023).
- National Autonomous University of Mexico. UNAM’s Geographic Information Platform on COVID-19 in Mexico. Available online: https://covid19.ciga.unam.mx/ (accessed on 10 August 2023). (In Spanish).
- Leach, M.; MacGregor, H.; Scoones, I.; Wilkinson, A. Post-pandemic transformations: How and why COVID-19 requires us to rethink development. World Dev. 2021, 138, 105233. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Zamora, E.; Hernandez-Suarez, C.M. Survival in adult inpatients with COVID-19. Public Health 2021, 190, 1–3. [Google Scholar] [CrossRef] [PubMed]
- General Directorate of Epidemiology of Mexico. Standardized Guideline for Epidemiological and Laboratory Surveillance of Viral Respiratory Disease. 2022. Available online: https://www.gob.mx/salud/documentos/lineamiento-estandarizado-para-la-vigilancia-epidemiologica-y-por-laboratorio-de-la-enfermedad-respiratoria-viral (accessed on 11 August 2023). (In Spanish).
- Fernandes-Matano, L.; Monroy-Munoz, I.E.; Bermudez de Leon, M.; Leal-Herrera, Y.A.; Palomec-Nava, I.D.; Ruiz-Pacheco, J.A.; Escobedo-Guajardo, B.L.; Marin-Budip, C.; Santacruz-Tinoco, C.E.; Gonzalez-Ibarra, J.; et al. Analysis of influenza data generated by four epidemiological surveillance laboratories in Mexico, 2010–2016. Epidemiol. Infect. 2019, 147, e183. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brunink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Zamora, E.; Guzman-Esquivel, J.; Bricio-Barrios, J.A.; Mendoza-Cano, O. Comparing the survival of adult inpatients with COVID-19 during the wild-type, Delta, and Omicron emergence. Public Health 2022, 213, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Fiolet, T.; Rebeaud, M.E.; Mulot, M.; Guihur, A.; El Fatouhi, D.; Laouali, N.; Peiffer-Smadja, N.; Aune, D.; Severi, G. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: A systematic review and meta-analysis of observational studies. BMJ Open 2021, 11, e052777. [Google Scholar] [CrossRef] [PubMed]
- Varikasuvu, S.R.; Dutt, N.; Thangappazham, B.; Varshney, S. Diabetes and COVID-19: A pooled analysis related to disease severity and mortality. Prim. Care Diabetes 2021, 15, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Suarez, C.; Murillo-Zamora, E. Waning immunity to SARS-CoV-2 following vaccination or infection. Front. Med. 2022, 9, 972083. [Google Scholar] [CrossRef] [PubMed]
- Pecly, I.M.D.; Azevedo, R.B.; Muxfeldt, E.S.; Botelho, B.G.; Albuquerque, G.G.; Diniz, P.H.P.; Silva, R.; Rodrigues, C.I.S. COVID-19 and chronic kidney disease: A comprehensive review. J. Bras. Nefrol. 2021, 43, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Mahalingasivam, V.; Su, G.; Iwagami, M.; Davids, M.R.; Wetmore, J.B.; Nitsch, D. COVID-19 and kidney disease: Insights from epidemiology to inform clinical practice. Nat. Rev. Nephrol. 2022, 18, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, S.; Luong Nguyen, L.B.; Tartour, E.; de Lamballerie, X.; Wittkop, L.; Loubet, P.; Launay, O. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: A systematic review. Clin. Microbiol. Infect. 2022, 28, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Robinson, P.C.; Uldrick, T.S.; Ljungman, P. COVID-19 in immunocompromised populations: Implications for prognosis and repurposing of immunotherapies. J. Immunother. Cancer 2021, 9, e002630. [Google Scholar] [CrossRef] [PubMed]
- Culqui, D.R.; Ortega Segura, J.; Da Costa-Venancio, E.; Renom-Guiteras, A.; Roquer, E.; Muñoz Tejada, S.M.; Rodriguez, P.; Alba Travieso, A.L.; Medrano, I.; Canchucaja-Gutarra, L. Risk factors associated with the mortality of COVID-19 patients aged ≥60 years neither Intubated nor treated with mechanical ventilation: A multicentre retrospective cohort study during the first wave in Spain. BioMed 2022, 2, 341–358. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).