Propofol Affords No Protection against Delayed Cerebral Ischemia in a Mouse Model of Subarachnoid Hemorrhage
Abstract
:1. Introduction
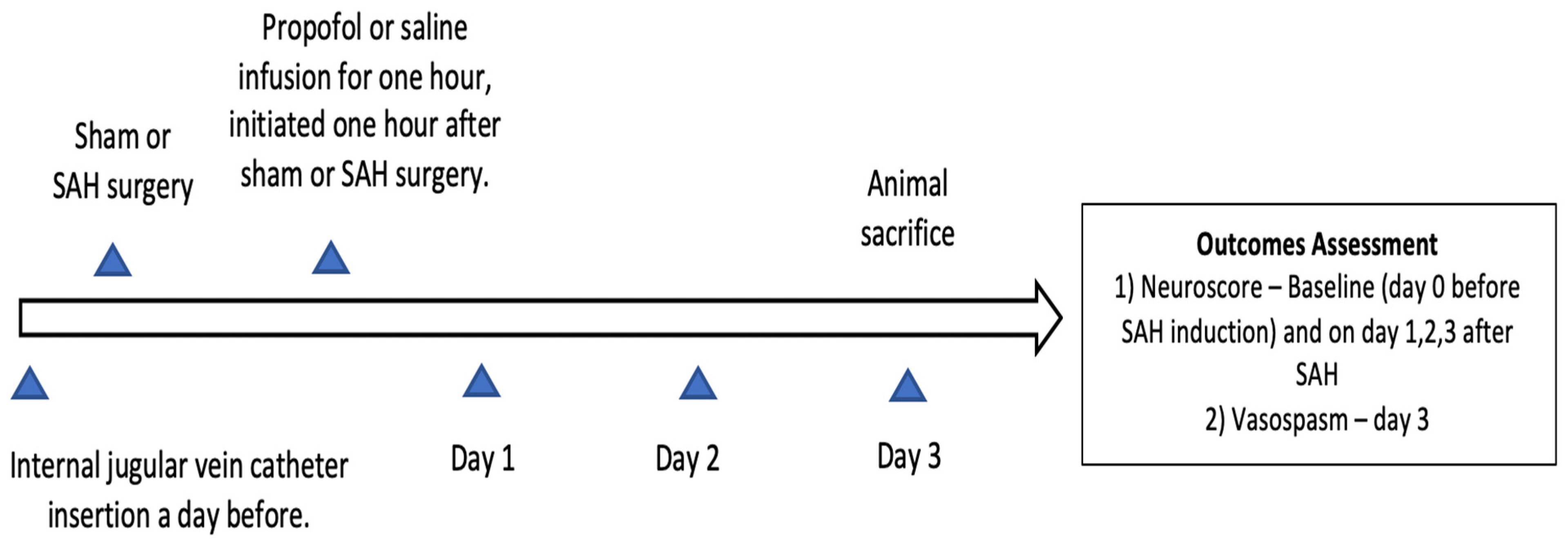
2. Materials and Methods
2.1. Mouse Endovascular Perforation SAH Model
2.2. Propofol Conditioning
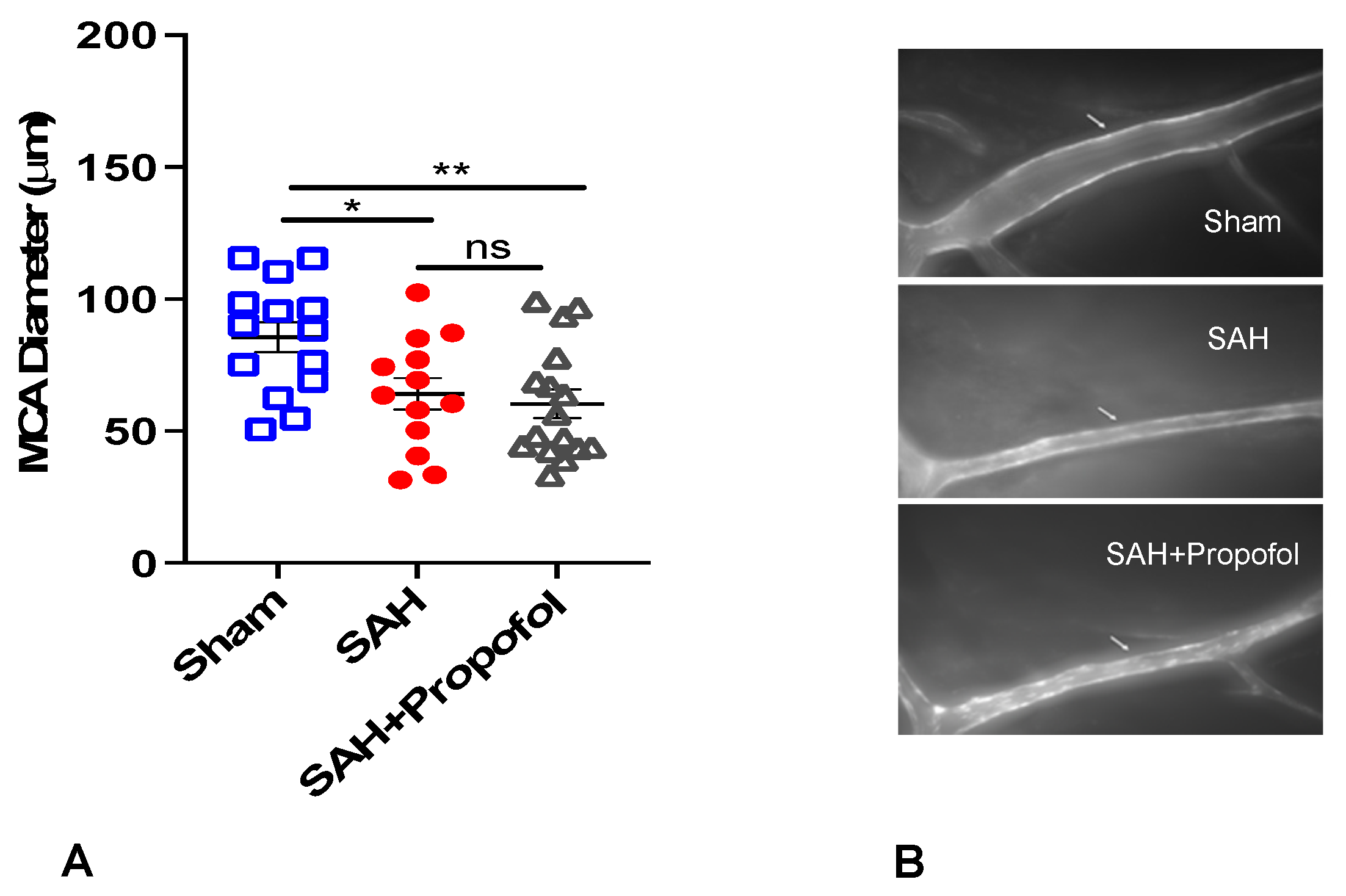
2.3. Cerebral Vasospasm Measurement
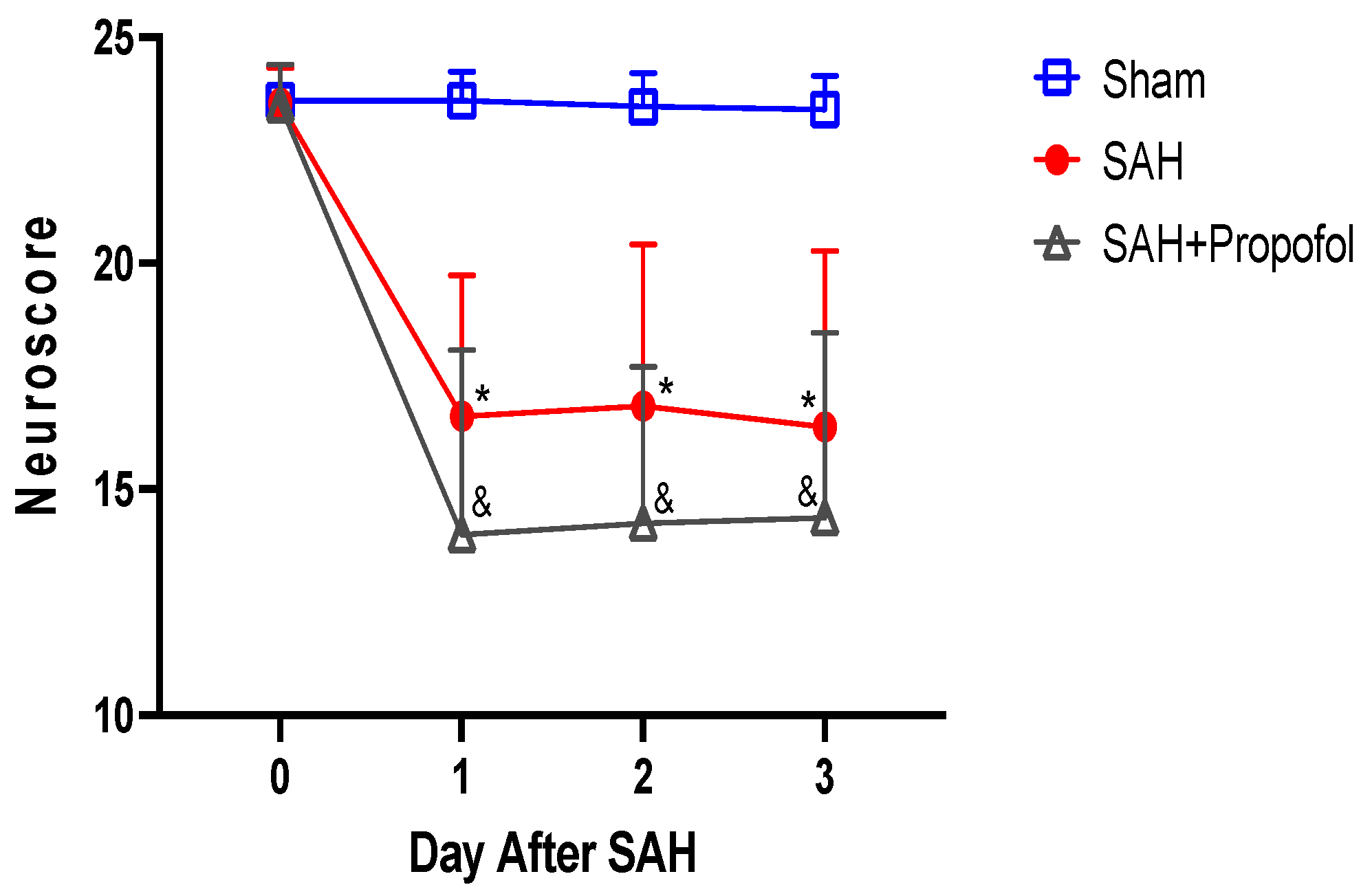
2.4. Neurobehavioral Assessment
2.5. Statistical Analysis
3. Results
3.1. Propofol Conditioning Did Not Afford Protection against SAH-Induced Large Artery Vasospasm in Wild-Type Mice
3.2. Propofol Conditioning Did Not Afford Protection against SAH-Induced Neurological Deficits in Wild-Type Mice
4. Discussion
4.1. Propofol against SAH-Induced DCI
4.2. Volatile vs. Intravenous Anesthetics for SAH-Induced DCI
4.3. Clinical Benefits of Volatile Anesthetics in SAH Patients
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brathwaite, S.; Macdonald, R.L. Current management of delayed cerebral ischemia: Update from results of recent clinical trials. Transl. Stroke Res. 2014, 5, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jayaraman, K.; Norris, A.J.; Hussein, A.; Nelson, J.W.; Mehla, J.; Diwan, D.; Vellimana, A.; Abu-Amer, Y.; Zipfel, G.J.; et al. Isoflurane Conditioning-Induced Delayed Cerebral Ischemia Protection in Subarachnoid Hemorrhage-Role of Inducible Nitric Oxide Synthase. J. Am. Heart Assoc. 2023, 12, e029975. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jayaraman, K.; Giri, T.; Zipfel, G.J.; Athiraman, U. Role of SIRT1 in Isoflurane conditioning-induced neurovascular protection against delayed cerebral ischemia secondary to subarachnoid hemorrhage. Int. J. Mol. Sci. 2021, 22, 4291. [Google Scholar] [CrossRef] [PubMed]
- Gidday, J.M. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006, 7, 437–448. [Google Scholar] [CrossRef]
- Diwan, D.; Vellimana, A.K.; Aum, D.J.; Clarke, J.; Nelson, J.W.; Lawrence, M.; Han, B.H.; Gidday, J.M.; Zipfel, G.J. Sirtuin 1 Mediates Protection Against Delayed Cerebral Ischemia in Subarachnoid Hemorrhage in Response to Hypoxic Postconditioning. J. Am. Heart Assoc. 2021, 10, e021113. [Google Scholar] [CrossRef]
- Jayaraman, K.; Liu, M.; Zipfel, G.J.; Athiraman, U. Sevoflurane and Desflurane Exposures Following Aneurysmal Subarachnoid Hemorrhage confer multifaceted protection against Delayed Cerebral Ischemia. Biomedicines 2021, 9, 820. [Google Scholar] [CrossRef]
- Athiraman, U.; Aum, D.; Vellimana, A.K.; Osbun, J.W.; Dhar, R.; Tempelhoff, R.; Zipfel, G.J. Evidence for a conditioning effect of inhalational anesthetics on angiographic vasospasm after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2019, 133, 152–158. [Google Scholar] [CrossRef]
- Athiraman, U.; Dhar, R.; Jayaraman, K.; Karanikolas, M.; Helsten, D.; Yuan, J.; Lele, A.; Rath, G.P.; Tempelhoff, R.; Roth, S.; et al. Conditioning Effect of Inhalational Anesthetics on Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2021, 88, 394–401. [Google Scholar] [CrossRef]
- Athiraman, U.; Lele, A.V.; Karanikolas, M.; Dhulipala, V.B.; Jayaraman, K.; Fong, C.; Kentner, R.; Sheolal, R.; Vellimana, A.; Gidday, J.M.; et al. Inhalational Versus Intravenous Anesthetic Conditioning for Subarachnoid Hemorrhage-Induced Delayed Cerebral Ischemia. Stroke 2022, 53, 904–912. [Google Scholar] [CrossRef]
- Shortal, B.P.; Reitz, S.L.; Aggarwal, A.; Meng, Q.C.; McKinstry-Wu, A.R.; Kelz, M.B.; Proekt, A. Development and validation of brain target controlled infusion of propofol in mice. PLoS ONE 2018, 13, e0194949. [Google Scholar] [CrossRef]
- Luo, F.; Ji, N.; Zhang, S.; Zhao, J.; Wang, T. Changes of endothelin and calcitonin gene-related peptide concentrations in plasma during propofol anesthesia. J. Neurosurg. Anesthesiol. 2009, 21, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Woo, J.H.; Baik, H.J.; Kim, D.Y.; Chae, J.S.; Yang, N.R.; Seo, E.K. The effect of anesthetic agents on cerebral vasospasms after subarachnoid hemorrhage: A retrospective study. Medicine 2018, 97, e11666. [Google Scholar] [CrossRef] [PubMed]
- Slupe, A.M.; Kirsch, J.R. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J. Cereb. Blood Flow Metab. 2018, 38, 2192–2208. [Google Scholar] [CrossRef] [PubMed]
- Schlünzen, L.; Juul, N.; Hansen, K.V.; Cold, G.E. Regional cerebral blood flow and glucose metabolism during propofol anaesthesia in healthy subjects studied with positron emission tomography. Acta Anaesthesiol. Scand. 2012, 56, 248–255. [Google Scholar] [CrossRef]
- Oshima, T.; Karasawa, F.; Okazaki, Y.; Wada, H.; Satoh, T. Effects of sevoflurane on cerebral blood flow and cerebral metabolic rate of oxygen in human beings: A comparison with isoflurane. Eur. J. Anaesthesiol. 2003, 20, 543–547. [Google Scholar] [CrossRef]
- Milner, E.; Johnson, A.W.; Nelson, J.W.; Harries, M.D.; Gidday, J.M.; Han, B.H.; Zipfel, G.J. HIF-1α Mediates Isoflurane-Induced Vascular Protection in Subarachnoid Hemorrhage. Ann. Clin. Transl. Neurol. 2015, 2, 325–337. [Google Scholar] [CrossRef]
- Hieber, S.; Huhn, R.; Hollmann, M.W.; Weber, N.C.; Preckel, B. Hypoxia-inducible factor 1 and related gene products in anaesthetic-induced preconditioning. Eur. J. Anaesthesiol. 2009, 26, 201–206. [Google Scholar] [CrossRef]
- Nagel, S.; Papadakis, M.; Chen, R.; Hoyte, L.C.; Brooks, K.J.; Gallichan, D.; Sibson, N.R.; Pugh, C.; Buchan, A.M. Neuroprotection by dimethyloxalylglycine following permanent and transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2011, 31, 132–143. [Google Scholar] [CrossRef]
- Tanaka, T.; Takabuchi, S.; Nishi, K.; Oda, S.; Wakamatsu, T.; Daijo, H.; Fukuda, K.; Hirota, K. The intravenous anesthetic propofol inhibits lipopolysaccharide-induced hypoxia-inducible factor 1 activation and suppresses the glucose metabolism in macrophages. J. Anesth. 2010, 24, 54–60. [Google Scholar] [CrossRef]
- Park, K.W.; Dai, H.B.; Metais, C.; Comunale, M.E.; Sellke, F.W. Isoflurane does not further impair microvascular vasomotion in a rat model of subarachnoid hemorrhage. Can. J. Anaesth. 2002, 49, 427–433. [Google Scholar] [CrossRef]
- Wang, T.; Luo, F.; Shan, R.; Zhen, Y.; Zhao, J.; Zhang, S. Changes of endothelin and calcitonin gene-related peptide during desflurane anesthesia in patients undergoing intracranial aneurysm clipping. J. Neurosurg. Anesthesiol. 2004, 16, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, Y.; Liu, X.; Yang, J.; Munoz, D.; Zhang, C. Unexpected pro-injury effect of propofol on vascular smooth muscle cells with increased oxidative stress. Crit. Care Med. 2011, 39, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Lin, C.F.; Li, C.F.; Sun, D.P.; Wang, L.Y.; Hsing, C.H. Anesthetic propofol overdose causes vascular hyperpermeability by reducing endothelial glycocalyx and ATP production. Int. J. Mol. Sci. 2015, 16, 12092–12107. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Chen, C.L.; Yang, T.T.; Choi, P.C.; Hsing, C.H.; Lin, C.F. Anesthetic propofol overdose causes endothelial cytotoxicity in vitro and endothelial barrier dysfunction in vivo. Toxicol. Appl. Pharmacol. 2012, 265, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ham, A.; Kim, K.Y.; Brown, K.M.; Lee, H.T. The volatile anesthetic isoflurane increases endothelial adenosine generation via microparticle ecto-5′-nucleotidase (CD73) release. PLoS ONE 2014, 9, e99950. [Google Scholar] [CrossRef] [PubMed]
- Bakar, A.M.; Park, S.W.; Kim, M.; Lee, H.T. Isoflurane protects against human endothelial cell apoptosis by inducing sphingosine kinase-1 via ERK MAPK. Int. J. Mol. Sci. 2012, 13, 977–993. [Google Scholar] [CrossRef]
- de Klaver, M.J.; Manning, L.; Palmer, L.A.; Rich, G.F. Isoflurane pretreatment inhibits cytokine-induced cell death in cultured rat smooth muscle cells and human endothelial cells. Anesthesiology 2002, 97, 24–32. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, J.E.; Kim, H.Y.; Kim, J. Volatile sedation in the intensive care unit: A systematic review and meta-analysis. Medicine 2017, 96, e8976. [Google Scholar] [CrossRef]
- Bösel, J.; Purrucker, J.C.; Nowak, F.; Renzland, J.; Schiller, P.; Pérez, E.B.; Poli, S.; Brunn, B.; Hacke, W.; Steiner, T. Volatile isoflurane sedation in cerebrovascular intensive care patients using AnaConDa(®): Effects on cerebral oxygenation, circulation, and pressure. Intensive Care Med. 2012, 38, 1955–1964. [Google Scholar] [CrossRef]
- Ditz, C.; Baars, H.; Schacht, H.; Leppert, J.; Smith, E.; Tronnier, V.M.; Küchler, J. Volatile Sedation with Isoflurane in Neurocritical Care Patients After Poor-grade Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2023, 173, e194–e206. [Google Scholar] [CrossRef]
- Villa, F.; Iacca, C.; Molinari, A.F.; Giussani, C.; Aletti, G.; Pesenti, A.; Citerio, G. Inhalation versus endovenous sedation in subarach-noid hemorrhage patients: Effects on regional cerebral blood flow. Crit. Care Med. 2012, 40, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Athiraman, U.; Liu, M.; Jayaraman, K.; Yuan, J.; Mehla, J.; Zipfel, G.J. Anesthetic and subanesthetic doses of isoflurane conditioning provides strong protection against delayed cerebral ischemia in a mouse model of subarachnoid hemorrhage. Brain Res. 2021, 1750, 147169. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Jayaraman, K.; Nelson, J.W.; Mehla, J.; Diwan, D.; Vellimana, A.K.; Zipfel, G.J.; Athiraman, U. Propofol Affords No Protection against Delayed Cerebral Ischemia in a Mouse Model of Subarachnoid Hemorrhage. Diseases 2023, 11, 130. https://doi.org/10.3390/diseases11040130
Liu M, Jayaraman K, Nelson JW, Mehla J, Diwan D, Vellimana AK, Zipfel GJ, Athiraman U. Propofol Affords No Protection against Delayed Cerebral Ischemia in a Mouse Model of Subarachnoid Hemorrhage. Diseases. 2023; 11(4):130. https://doi.org/10.3390/diseases11040130
Chicago/Turabian StyleLiu, Meizi, Keshav Jayaraman, James W. Nelson, Jogender Mehla, Deepti Diwan, Ananth K. Vellimana, Gregory J. Zipfel, and Umeshkumar Athiraman. 2023. "Propofol Affords No Protection against Delayed Cerebral Ischemia in a Mouse Model of Subarachnoid Hemorrhage" Diseases 11, no. 4: 130. https://doi.org/10.3390/diseases11040130
APA StyleLiu, M., Jayaraman, K., Nelson, J. W., Mehla, J., Diwan, D., Vellimana, A. K., Zipfel, G. J., & Athiraman, U. (2023). Propofol Affords No Protection against Delayed Cerebral Ischemia in a Mouse Model of Subarachnoid Hemorrhage. Diseases, 11(4), 130. https://doi.org/10.3390/diseases11040130





