Comparative Efficacy and Safety of Statin Monotherapy and Statin plus Ezetimibe Combination in a Real-World Setting
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Setting
2.2. Participants
2.3. Study Design
2.4. Ethical Approval
2.5. Study Procedures
2.6. Endpoints
2.7. Statistical Methods
3. Results
3.1. Study Population
3.2. Efficacy of Treatment, FAS (Full Analysis Set) Population
3.2.1. Achievement of the LDL-C Target
3.2.2. LDL-C
3.2.3. Total Cholesterol
3.2.4. HDL Cholesterol
3.2.5. Triglycerides
3.3. Target Achievement in CV Risk Differentiated Subgroups
3.4. Safety
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, K.K.; Ference, B.A.; Séverin, T.; Blom, D.; Nicholls, S.J.; Shiba, M.H.; Almahmeed, W.; Alonso, R.; Daccord, M.; Ezhov, M.; et al. World Heart Federation Cholesterol Roadmap 2022. Glob. Heart 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Théroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: The DA VINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef]
- Ray, K.K.; Haq, I.; Bilitou, A.; Manu, M.C.; Burden, A.; Aguiar, C.; Arca, M.; Connolly, D.L.; Eriksson, M.; Ferrières, J.; et al. Treatment gaps in the implementation of LDL cholesterol control among high- and very high-risk patients in Europe between 2020 and 2021: The multinational observational SANTORINI study. Lancet Reg. Health Eur. 2023, 29, 100624. [Google Scholar] [CrossRef]
- Ray, K.K.; Reeskamp, L.F.; Laufs, U.; Banach, M.; Mach, F.; Tokgözoğlu, L.S.; Connolly, D.L.; Gerrits, A.J.; Stroes, E.S.G.; Masana, L.; et al. Combination lipid-lowering therapy as first-line strategy in very high-risk patients. Eur. Heart J. 2022, 43, 830–833. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Metelskaya, V.A.; Shalnova, S.A.; Deev, A.D.; Perova, N.V.; Gomyranova, N.V.; Litinskaya, O.A.; Evstifeeva, S.E.; Artamonova, G.V.; Gatagonova, T.M.; Grinshtein, Y.I.; et al. Analysis of atherogenic dyslipidemias prevalence among population of Russian Federation (results of the ESSE-RF Study). Profil. Meditsina 2016, 19, 15–23. [Google Scholar] [CrossRef]
- Shalnova, S.A.; Deev, A.; Metelskaya, V.A.; Evstifeeva, S.E.; Rotar, O.P.; Zhernakova, Y.V.; Boytsov, S.; Balanova, Y.A.; Gomyranova, N.V.; Imaeva, A.E.; et al. Awareness and Treatment Specifics of Statin Therapy in Persons with Various Cardiovasular Risk: The Study Esse-Rf. Cardiovasc. Ther. Prev. 2016, 15, 29–37. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Weiss, R.; Moccetti, T.; Vogt, A.; Eber, B.; Sosef, F.; Duffield, E. Efficacy and Safety of Rosuvastatin 40 mg Alone or in Combination with Ezetimibe in Patients at High Risk of Cardiovascular Disease (Results from the EXPLORER Study). Am. J. Cardiol. 2007, 99, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Farnier, M.; Santos, R.D.; Cosin-Sales, J.; Ezhov, M.V.; Liu, J.; Granados, D.; Santoni, S.; Khan, I.; Catapano, A.L. Projected impact of treatment intensification with statin, ezetimibe, and statin plus ezetimibe fixed-dose combination on MACE across six countries. Eur. J. Prev. Cardiol. 2022, 29, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, S.; Shen, P.; Sun, Y.; Lin, H.; Zhai, S. Achievement of low-density lipoprotein cholesterol targets in Chinese patients with atherosclerotic cardiovascular disease after receiving statins and ezetimibe. Front. Cardiovasc. Med. 2022, 9, 988576. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Pintó, X.; Escobar, C.; Varona, J.F.; Gámez, J.M. Real-World Attainment of Low-Density Lipoprotein Cholesterol Goals in Patients at High Risk of Cardiovascular Disease Treated with High-Intensity Statins: The TERESA Study. J. Clin. Med. 2023, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.H.; Kim, H.; Lee, S.; Cho, J.H.; Lee, H.; Yim, H.W.; Yoon, K.; Kim, H.; Kim, J.H. Low-density lipoprotein cholesterol reduction and target achievement after switching from statin monotherapy to statin/ezetimibe combination therapy: Real-world evidence. J. Clin. Pharm. Ther. 2020, 46, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, J.L.; Sorio-Vilela, F.; Dornstauder, E.; Fraas, U.; Smieszek, T.; Zappacosta, S.; Laufs, U. Non-statin lipid-lowering therapy over time in very-high-risk patients: Effectiveness of fixed-dose statin/ezetimibe compared to separate pill combination on LDL-C. Clin. Res. Cardiol. 2022, 111, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Houri, J.; Notarbartolo, A.; Melani, L.; Lipka, L.J.; Suresh, R.; Sun, S.; LeBeaut, A.P.; Sager, P.T.; Veltri, E.P. Effect of Ezetimibe Coadministered with Atorvastatin in 628 Patients with Primary Hypercholesterolemia: A Prospective, Randomized, Double-Blind Trial. Circulation 2003, 107, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Gagné, C.; Bays, E.H.; Weiss, S.R.; Mata, P.; Quinto, K.; Melino, M.; Cho, M.; Musliner, A.T.; Gumbiner, B. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am. J. Cardiol. 2002, 90, 1084–1091. [Google Scholar] [CrossRef]
- Hong, S.J.; Jeong, H.S.; Ahn, J.C.; Cha, D.-H.; Won, K.H.; Kim, W.; Cho, S.K.; Kim, S.-Y.; Yoo, B.-S.; Sung, K.C.; et al. A Phase III, Multicenter, Randomized, Double-blind, Active Comparator Clinical Trial to Compare the Efficacy and Safety of Combination Therapy with Ezetimibe and Rosuvastatin Versus Rosuvastatin Monotherapy in Patients with Hypercholesterolemia: I-ROSETTE (Ildong Rosuvastatin & Ezetimibe for Hypercholesterolemia) Randomized Controlled Trial. Clin. Ther. 2018, 40, 226–241.e4. [Google Scholar] [CrossRef] [PubMed]
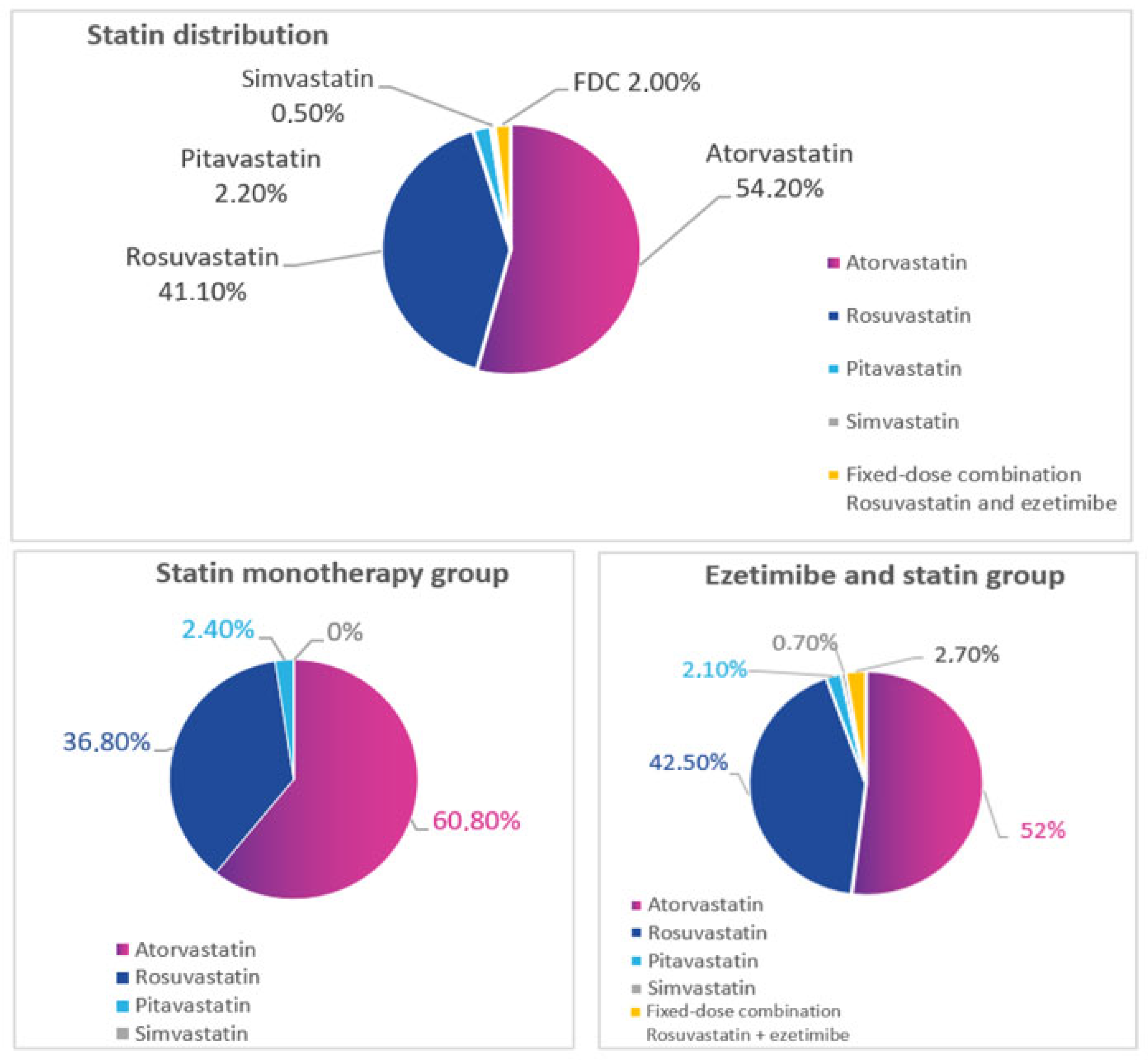



| Parameters | Statin Monotherapy (n = 250) | Statin Plus Ezetimibe (n = 750) | p |
|---|---|---|---|
| Males, n (%) | 155 (62.0) | 429 (52.7) | 0.18 |
| Age, years | 59.9 ± 10.9 | 60.2 ± 10.5 | 0.55 |
| Body weight, kg | 84.4 ± 15.2 | 84.2 ± 15.5 | 0.75 |
| Smoking status | |||
| Former smoker, n (%) | 46 (18.4) | 143 (19.1) | 0.49 |
| Current smoker, n (%) | 51 (20.4) | 128 (17.1) | |
| Never smoked, n (%) | 153 (61.2) | 479 (63.9) | |
| Concomitant diseases | |||
| Coronary heart disease | 140 (56.0) | 424 (56.5) | 0.88 |
| Acute coronary syndrome | 7 (2.8) | 38 (5.1) | 0.19 |
| Myocardial infarction | 35 (14.0) | 138 (18.4) | 0.11 |
| Hypertension | 202 (80.8) | 559 (74.5) | 0.044 |
| Type 2 diabetes mellitus | 40 (16.0) | 108 (14.4) | 0.54 |
| Ischemic stroke | 7 (2.8) | 28 (3.7) | 0.62 |
| Chronic kidney disease | 40 (16.0) | 152 (20.3) | 0.14 |
| Lipid parameters | |||
| Total cholesterol | 4.20 (3.53, 5.10) | 3.90 (3.40, 4.59) | <0.001 |
| Low-density lipoprotein cholesterol | 2.20 (1.67, 3.00) | 1.90 (1.50, 2.57) | <0.001 |
| High-density lipoprotein cholesterol | 1.20 (1.00, 1.41) | 1.21 (1.02, 1.45) | 0.094 |
| Triglycerides | 1.41 (1.03, 1.90) | 1.30 (0.96, 1.83) | 0.049 |
| Revascularization surgery | |||
| Coronary stent placement | 80 (32.0) | 270 (36.1) | 0.25 |
| Percutaneous coronary intervention | 16 (6.4) | 48 (6.4) | 1.00 |
| Coronary artery bypass grafting | 10 (4.0) | 41 (5.5) | 0.36 |
| Peripheral artery surgery | 2 (0.8) | 14 (1.9) | 0.38 |
| Concomitant therapy | |||
| Beta-blockers | 152 (60.8) | 468 (62.4) | 0.65 |
| Angiotensin-converting enzyme inhibitors | 93 (37.2) | 295 (39.3) | 0.55 |
| Angiotensin II receptor antagonists | 74 (29.6) | 217 (28.9) | 0.84 |
| Calcium channel blockers | 65 (26.0) | 211 (28.1) | 0.51 |
| Diuretics | 79 (31.6) | 250 (33.3) | 0.61 |
| Cardiovascular Risk Category | Statin Therapy Intensity | Statin Monotherapy | Ezetimibe and Statin | p | Δ (Difference between the Groups) | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| High risk | High | 24 | 25 | 93 | 32 | 0.492 | 7% |
| Moderate | 42 | 33 | 110 | 26 | 0.394 | −7% | |
| Very high risk | High | 103 | 16 | 353 | 24 | 0.059 | 8% |
| Moderate | 40 | 15 | 98 | 33 | 0.035 | 18% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezhov, M.V.; Sergienko, I.V.; Kryzhanovskiy, S.M.; Manko, K.S.; Timoshina, E.V. Comparative Efficacy and Safety of Statin Monotherapy and Statin plus Ezetimibe Combination in a Real-World Setting. Diseases 2023, 11, 168. https://doi.org/10.3390/diseases11040168
Ezhov MV, Sergienko IV, Kryzhanovskiy SM, Manko KS, Timoshina EV. Comparative Efficacy and Safety of Statin Monotherapy and Statin plus Ezetimibe Combination in a Real-World Setting. Diseases. 2023; 11(4):168. https://doi.org/10.3390/diseases11040168
Chicago/Turabian StyleEzhov, Marat V., Igor V. Sergienko, Sergey M. Kryzhanovskiy, Kirill S. Manko, and Elena V. Timoshina. 2023. "Comparative Efficacy and Safety of Statin Monotherapy and Statin plus Ezetimibe Combination in a Real-World Setting" Diseases 11, no. 4: 168. https://doi.org/10.3390/diseases11040168
APA StyleEzhov, M. V., Sergienko, I. V., Kryzhanovskiy, S. M., Manko, K. S., & Timoshina, E. V. (2023). Comparative Efficacy and Safety of Statin Monotherapy and Statin plus Ezetimibe Combination in a Real-World Setting. Diseases, 11(4), 168. https://doi.org/10.3390/diseases11040168








