iCan, Empowering Recovery: Evaluating a Patient-Centred Cancer Rehabilitation Programme across the Cancer Care Continuum
Abstract
:1. Introduction
2. Materials and Methods
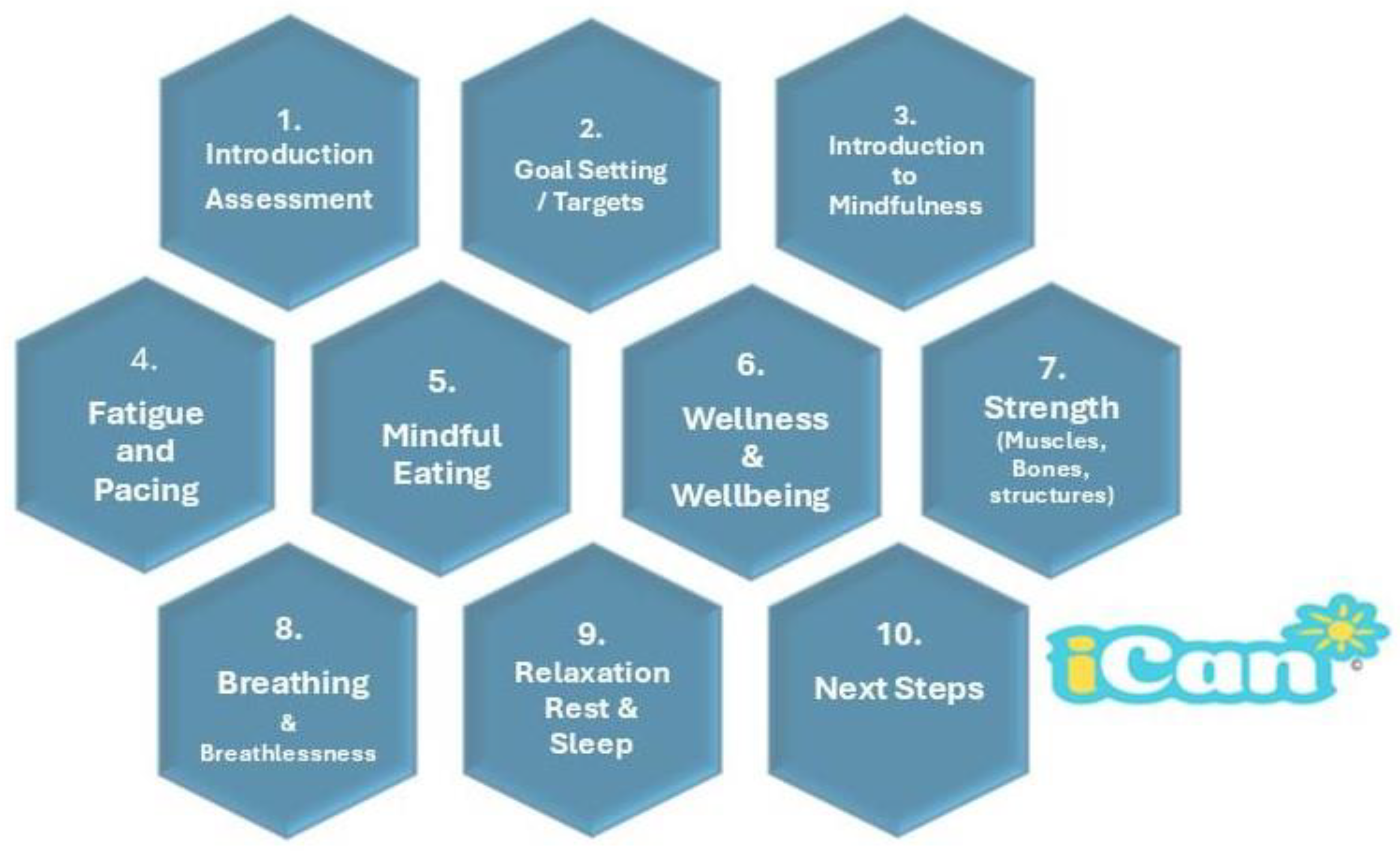
2.1. iCan Cancer Rehabilitation Service
2.2. Data Collection
2.3. Outcome Measures
2.3.1. Functional Capacity
2.3.2. Mental Wellbeing and Psychological Functioning
2.3.3. Physical Activity
2.3.4. Behaviour Change
2.3.5. Fatigue
2.3.6. Data Analysis
3. Results
3.1. Physical Outcome Measures
Functional Capacity, Physical Activity, and Fatigue
3.2. Psychological Outcome Measures
Mental Wellbeing, Psychological Functioning, and Behaviour Change
4. Discussion
4.1. Limitations
4.2. Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rezende, G.; Gomes-Ferraz, C.A.; Bacon, I.G.F.I.; De Carlo, M.M.R.P. The importance of a continuum of rehabilitation from diagnosis of advanced cancer to palliative care. Disabil. Rehabil. 2023, 45, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- U.K. Cancer Research. Cancer Incidence for All Cancers Combined. 2024, no. 27/06/. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/all-cancers-combined#:~:text=It%20is%20estimated%20that%20there,and%20around%209%2C800%20in%20males (accessed on 31 July 2024).
- Smittenaar, C.R.; Petersen, K.A.; Stewart, K.; Moitt, N. Cancer incidence and mortality projections in the UK until 2035. Br. J. Cancer 2016, 115, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- The Shrewsbury and Telford Hospital NHS Trust. Integrated Cancer Strategy for Shropshire, Telford and Wrekin, 2022/2027. Available online: https://www.shropshiretelfordandwrekin.nhs.uk/wp-content/uploads/Integrated-Cancer-Strategy-STW-2022-2027.pdf (accessed on 31 July 2024).
- Powys Regional Partnership Board. Improving the Cancer Journey in Powys, 2024, no. 27/06/. Available online: https://www.powysrpb.org/icjpowys (accessed on 31 July 2024).
- Kokkonen, K.; Saarto, T.; Mäkinen, T.; Pohjola, L.; Kautio, H.; Järvenpää, S.; Puustjärvi-Sunabacka, K. The functional capacity and quality of life of women with advanced breast cancer. Breast Cancer 2016, 24, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Kessels, E.; Husson, O.; Van der Feltz-Cornelis, C.M. The effect of exercise on cancer-related fatigue in cancer survivors: A systematic review and meta-analysis. Neuropsychiatr. Dis. Treat. 2018, volume 14, 479–494. [Google Scholar] [CrossRef]
- Hendifar, A.E.; Petzel, M.Q.; Zimmers, T.A.; Denlinger, C.S.; Matrisian, L.M.; Picozzi, V.J.; Rahib, L.; Consortium, P.P.; Hendifar, A.; Tuli, R. Pancreas cancer-associated weight loss. Oncologist 2018, 24, 691–701. [Google Scholar] [CrossRef]
- Thorsen, L.; Gjerset, G.M.; Loge, J.H.; Kiserud, C.E.; Skovlund, E.; Fløtten, T.; Fosså, S.D. Cancer patients’ needs for rehabilitation services. Acta Oncol. 2011, 50, 212–222. [Google Scholar] [CrossRef]
- World Health Organization. Rehabilitation Competency Framework; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Lehmann, J.; Rothmund, M.; Riedl, D.; Rumpold, G.; Grote, V.; Fischer, M.J.; Holzner, B. Clinical outcome assessment in cancer rehabilitation and the central role of patient-reported outcomes. Cancers 2021, 14, 84. [Google Scholar] [CrossRef]
- Stout, N.L.; Mina, D.S.; Lyons, K.D.; Robb, K.; Silver, J.K. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J. Clin. 2021, 71, 149–175. [Google Scholar] [CrossRef]
- Chan, R.J.; Milch, V.E.; Crawford-Williams, F.; Agbejule, O.A.; Joseph, R.; Johal, J.; Dick, N.; Wallen, M.P.; Ratcliffe, J.; Agarwal, A. Patient navigation across the cancer care continuum: An overview of systematic reviews and emerging literature. CA Cancer J. Clin. 2023, 73, 565–589. [Google Scholar] [CrossRef]
- Alfano, C.M.; Cheville, A.L.; Mustian, K. Developing High-Quality Cancer Rehabilitation Programs: A Timely Need. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 241–249. [Google Scholar] [CrossRef]
- Hauken, M.A.; Holsen, I.; Fismen, E.; Larsen, T.M.B. Working toward a good life as a cancer survivor: A longitudinal study on positive health outcomes of a rehabilitation program for young adult cancer survivors. Cancer Nurs. 2015, 38, 3–15. [Google Scholar] [CrossRef]
- Dalzell, M.; Smirnow, N.; Sateren, W.; Sintharaphone, A.; Ibrahim, M.; Mastroianni, L.; Zambrano, L.D.; O’Brien, S. Rehabilitation and exercise oncology program: Translating research into a model of care. Curr. Oncol. 2017, 24, 191–198. [Google Scholar] [CrossRef]
- Sleight, A.; Gerber, L.H.; Marshall, T.F.; Livinski, A.; Alfano, C.M.; Harrington, S.; Flores, A.M.; Virani, A.; Hu, X.; Mitchell, S.A. Systematic review of functional outcomes in cancer rehabilitation. Arch. Phys. Med. Rehabil. 2022, 103, 1807–1826. [Google Scholar] [CrossRef]
- Spence, J.C.; Rhodes, R.E.; McCurdy, A.; Mangan, A.; Hopkins, D.; Mummery, W.K. Determinants of physical activity among adults in the United Kingdom during the COVID-19 pandemic: The DUK-COVID study. Br. J. Health Psychol. 2020, 26, 588–605. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2010, 28, 753–765. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Shaw, E.; Neilson, H.K.; Brenner, D.R. Epidemiology and biology of physical activity and cancer recurrence. J. Mol. Med. 2017, 95, 1029–1041. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Spence, R.R.; Heesch, K.C.; Brown, W.J. Exercise and cancer rehabilitation: A systematic review. Cancer Treat. Rev. 2010, 36, 185–194. [Google Scholar] [CrossRef]
- Canário, A.C.G.; Cabral, P.U.L.; Paiva, L.C.D.; Florencio, G.L.D.; Spyrides, M.H.; Gonçalves, A.K.D.S. Physical activity, fatigue and quality of life in breast cancer patients. Rev. Assoc. Médica Bras. 2016, 62, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Piraux, E.; Caty, G.; Reychler, G. Effects of preoperative combined aerobic and resistance exercise training in cancer patients undergoing tumour resection surgery: A systematic review of randomised trials. Surg. Oncol. 2018, 27, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Lingens, S.P.; Schulz, H.; Bleich, C. Evaluations of psychosocial cancer support services: A scoping review. PLoS ONE 2021, 16, e0251126. [Google Scholar] [CrossRef]
- Ye, Z.J.; Peng, C.H.; Zhang, H.W.; Liang, M.Z.; Zhao, J.J.; Sun, Z.; Hu, G.Y.; Yu, Y.L. A biopsychosocial model of resilience for breast cancer: A preliminary study in mainland China. Eur. J. Oncol. Nurs. 2018, 36, 95–102. [Google Scholar] [CrossRef]
- Kudre, D.; Chen, Z.; Richard, A.; Cabaset, S.; Dehler, A.; Schmid, M.; Rohrmann, S. Multidisciplinary outpatient cancer rehabilitation can improve cancer patients’ physical and psychosocial status—A systematic review. Curr. Oncol. Rep. 2020, 22, 122. [Google Scholar] [CrossRef]
- Mikkelsen, M.K.; Nielsen, D.L.; Vinther, A.; Lund, C.M.; Jarden, M. Attitudes towards physical activity and exercise in older patients with advanced cancer during oncological treatment–a qualitative interview study. Eur. J. Oncol. Nurs. 2019, 41, 16–23. [Google Scholar] [CrossRef]
- Lin, L.; Lin, L.; Tzeng, G.; Huang, Y.; Tai, J.; Chen, Y.; Wu, C.; Chen, P.; Lin, P.; Hung, P. Effects of mindfulness-based therapy for cancer patients: A systematic review and meta-analysis. J. Clin. Psychol. Med. Settings 2022, 29, 432–445. [Google Scholar] [CrossRef]
- Schuman-Olivier, Z.; Trombka, M.; Lovas, D.A.; Brewer, J.A.; Vago, D.R.; Gawande, R.; Dunne, J.P.; Lazar, S.W.; Loucks, E.B.; Fulwiler, C. Mindfulness and behavior change. Harv. Rev. Psychiatry 2020, 28, 371–394. [Google Scholar] [CrossRef]
- Levit, L.A.; Byatt, L.; Lyss, A.P.; Paskett, E.D.; Levit, K.; Kirkwood, K.; Schenkel, C.; Schilsky, R.L. Closing the rural cancer care gap: Three institutional approaches. JCO Oncol. Pract. 2020, 16, 422–430. [Google Scholar] [CrossRef]
- Feuerstein, M. Defining cancer survivorship. J. Cancer Surviv. Res. Pract. 2007, 1, 5–7. [Google Scholar] [CrossRef]
- Surbone, A.; Annunziata, M.A.; Santoro, A.; Tirelli, U.; Tralongo, P. Cancer patients and survivors: Changing words or changing culture? Ann. Oncol. 2013, 24, 2468–2471. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.R.; Steed, L.; Quirk, H.; Greasley, R.U.; Saxton, J.M.; Taylor, S.J.; Rosario, D.J.; Thaha, M.A.; Bourke, L. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst. Rev. 2018, 9, CD010192. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.C.; Wheeler, S.B. A systematic review of motivational interviewing interventions in cancer patients and survivors. Patient Educ. Couns. 2016, 99, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Shennan, C.; Payne, S.; Fenlon, D. What is the evidence for the use of mindfulness-based interventions in cancer care? A review. Psychoncology 2020, 20, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, J.; Kamper, R.S.; Aagaard, P.; Haddock, B.; Prescott, E.; Ara, I.; Suetta, C. Relation between leg extension power and 30-s sit-to-stand muscle power in older adults: Validation and translation to functional performance. Sci. Rep. 2020, 10, 16337. [Google Scholar] [CrossRef] [PubMed]
- Eden, M.M.; Tompkins, J.; Verheijde, J.L. Reliability and a correlational analysis of the 6MWT, ten-meter walk test, thirty second sit to stand, and the linear analog scale of function in patients with head and neck cancer. Physiother. Theory Pract. 2020, 34, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Tennant, R.; Hiller, L.; Fishwick, R.; Platt, S.; Joseph, S.; Weich, S.; Parkinson, J.; Secker, J.; Stewart-Brown, S. The Warwick-Edinburgh mental well-being scale (WEMWBS): Development and UK validation. Health Qual. Life Outcomes 2007, 5, 63. [Google Scholar] [PubMed]
- Hagströmer, M.; Oja, P.; Sjöström, M. Physical activity and inactivity in an adult population assessed by accelerometry. Med. Sci. Sports Exerc. 2007, 39, 1502–1508. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Michie, S.; Van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Behaviour Change: Digital and Mobile Health Interventions. Available online: https://www.nice.org.uk/guidance/ng183 (accessed on 31 July 2024).
- Keyworth, C.; Epton, T.; Goldthorpe, J.; Calam, R.; Armitage, C.J. Acceptability, reliability, and validity of a brief measure of capabilities, opportunities, and motivations (“COM-B”). Br. J. Health Psychol. 2020, 25, 474–501. [Google Scholar] [CrossRef]
- Tennant, K. Assessment of fatigue in older adults: The FACIT Fatigue Scale (version 4). Support. Care Cancer 2015, 23, 1355–1364. [Google Scholar]
- Dittner, A.J.; Wessely, S.C.; Brown, R.G. The assessment of fatigue: A practical guide for clinicians and researchers. J. Psychosom. Res. 2004, 56, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Bhella, S.; Schentag, C.; Gladman, D.D. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann. Rheum. Dis. 2007, 66, 936–939. [Google Scholar] [CrossRef]
- Vincent, C.; Burnett, S.; Carthey, J. Safety measurement and monitoring in healthcare: A framework to guide clinical teams and healthcare organisations in maintaining safety. BMJ Qual. Saf. 2014, 23, 670–677. [Google Scholar] [CrossRef]
- Madhushri, P.; Dzhagaryan, A.; Jovanov, E.; Milenkovic, A. An mHealth tool suite for mobility assessment. Information 2016, 7, 47. [Google Scholar] [CrossRef]
- Zanini, A.; Crisafulli, E.; D’Andria, M.; Gregorini, C.; Cherubino, F.; Zampogna, E.; Azzola, A.; Spanevello, A.; Schiavone, N.; Chetta, A. Minimum clinically important difference in 30-s sit-to-stand test after pulmonary rehabilitation in subjects with COPD. Respir. Care 2019, 64, 1261–1269. [Google Scholar] [CrossRef]
- O’Grady, H.K.; Edbrooke, L.; Farley, C.; Berney, S.; Denehy, L.; Puthucheary, Z.; Kho, M.E. The sit-to-stand test as a patient-centered functional outcome for critical care research: A pooled analysis of five international rehabilitation studies. Crit. Care 2022, 26, 175. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Crumley, D.; McTiernan, A.; Bernstein, L.; Baumgartner, R.; Gilliland, F.D.; Kriska, A.; Ballard-Barbash, R. Physical activity levels before and after a diagnosis of breast carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 1746–1757. [Google Scholar] [CrossRef]
- Lahart, I.; Metsios, G.; Kite, C. Physical activity and health. In Physical Activity: The Evidence Explained; Draper, N., Stratton, G., Eds.; Routledge: Oxfordshire, UK, 2018; pp. 66–94. Available online: https://www.taylorfrancis.com/chapters/edit/10.4324/9781315523859-5/physical-activity-health-ian-lahart-george-metsios-chris-kite?context=ubx&refId=3e2855ca-4845-4f27-a321-f8c031aef7ad (accessed on 31 July 2024).
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; de Gonzalez, A.B.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure Time Physical Activity and Mortality: A Detailed Pooled Analysis of the Dose-Response Relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef]
- Moore, S.C.; Patel, A.V.; Matthews, C.E.; de Gonzalez, A.B.; Park, Y.; Katki, H.A.; Linet, M.S.; Weiderpass, E.; Visvanathan, K.; Helzlsouer, K.J. Leisure time physical activity of moderate to vigorous intensity and mortality: A large pooled cohort analysis. PLoS Med. 2012, 9, e1001335. [Google Scholar] [CrossRef]
- Sport England. Active People Interactive. 2024. Available online: https://activepeople.sportengland.org/ (accessed on 31 July 2024).
- Howlett, N.; Schulz, J.; Trivedi, D.; Troop, N.; Chater, A. A prospective study exploring the construct and predictive validity of the COM-B model for physical activity. J. Health Psychol. 2019, 24, 1378–1391. [Google Scholar] [CrossRef] [PubMed]
- Kite, C.; Atkinson, L.; McGregor, G.; Clark, C.C.; Randeva, H.S.; Kyrou, I. Capability, Opportunity, and Motivation—Identifying Constructs for Increasing Physical Activity Behaviours in Women with Polycystic Ovary Syndrome (PCOS). Int. J. Environ. Res. Public Health 2023, 20, 2309. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Wang, Q.; Ding, Y.; Zhao, W.; Jia, Z.; Wang, B. Barriers and facilitators to physical activity participation in patients with head and neck cancer: A scoping review. Support. Care Cancer 2022, 30, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.L.; Connolly, B.; Denehy, L.; Hart, N.; Antippa, P.; Lin, K.; Parry, S.M. Understanding factors influencing physical activity and exercise in lung cancer: A systematic review. Support. Care Cancer 2016, 25, 983–999. [Google Scholar] [CrossRef]
- Cao, C.; Friedenreich, C.M.; Yang, L. Association of daily sitting time and leisure-time physical activity with survival among US cancer survivors. JAMA Oncol. 2022, 8, 395–403. [Google Scholar] [CrossRef]
- Fishman, E.I.; Steeves, J.A.; Zipunnikov, V.; Koster, A.; Berrigan, D.; Harris, T.A.; Murphy, R. Association between objectively measured physical activity and mortality in NHANES. Med. Sci. Sports Exerc. 2016, 48, 1303. [Google Scholar] [CrossRef]
- Montan, I.; Löwe, B.; Cella, D.; Mehnert, A.; Hinz, A. General population norms for the functional assessment of chronic illness therapy (FACIT)-fatigue scale. Value Health 2018, 21, 1313–1321. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.; Chang, C.; Peterman, A.; Slavin, M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002, 94, 528–538. [Google Scholar] [CrossRef]
- Butt, Z.; Rao, A.V.; Lai, J.; Abernethy, A.P.; Rosenbloom, S.K.; Cella, D. Age-associated differences in fatigue among patients with cancer. J. Pain Symptom Manag. 2010, 40, 217–223. [Google Scholar] [CrossRef]
- Leung, Y.W.; Brown, C.; Cosio, A.P.; Dobriyal, A.; Malik, N.; Pat, V.; Irwin, M.; Tomasini, P.; Liu, G.; Howell, D. Feasibility and diagnostic accuracy of the Patient-Reported Outcomes Measurement Information System (PROMIS) item banks for routine surveillance of sleep and fatigue problems in ambulatory cancer care. Cancer 2016, 122, 2906–2917. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, Y.; Yun, B.; Wang, Q.; Huang, C.; Han, L. Exercise for fatigue in breast cancer patients: An umbrella review of systematic reviews. Int. J. Nurs. Sci. 2020, 7, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Belloni, S.; Arrigoni, C.; Caruso, R. Effects from physical exercise on reduced cancer-related fatigue: A systematic review of systematic reviews and meta-analysis. Acta Oncol. 2021, 60, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Eton, D.T.; Lai, J.; Peterman, A.H.; Merkel, D.E. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J. Pain Symptom Manag. 2002, 24, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Fat, L.N.; Scholes, S.; Boniface, S.; Mindell, J.; Stewart-Brown, S. Evaluating and establishing national norms for mental wellbeing using the short Warwick–Edinburgh Mental Well-being Scale (SWEMWBS): Findings from the Health Survey for England. Qual. Life Res. 2016, 26, 1129–1144. [Google Scholar] [CrossRef] [PubMed]
- Simm, R.; England, S.; Patel, F. Delivering NHS Community Pain Service Pain Management Programmes during Covid 19 via video conferencing: Patient and clinician experiences and outcomes. Pain Rehabil.-J. Physiother. Pain Assoc. 2022, 52, 42–52. [Google Scholar]
- Hariton, E.; Locascio, J.J. Randomised controlled trials—The gold standard for effectiveness research. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1716. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Gorber, S.C.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef]
- Prince, S.A.; Cardilli, L.; Reed, J.L.; Saunders, T.J.; Kite, C.; Douillette, K.; Fournier, K.; Buckley, J.P. A comparison of self-reported and device measured sedentary behaviour in adults: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 31. [Google Scholar] [CrossRef]
- Ruiz-Casado, A.; Alejo, L.B.; Santos-Lozano, A.; Soria, A.; Ortega, M.J.; Pagola, I.; Fiuza-Luces, C.; Palomo, I.; Garatachea, N.; Cebolla, H. Validity of the physical activity questionnaires IPAQ-SF and GPAQ for cancer survivors: Insights from a Spanish cohort. Int. J. Sports Med. 2016, 37, 979–985. [Google Scholar] [CrossRef]
- Latham, N.K.; Mehta, V.; Nguyen, A.M.; Jette, A.M.; Olarsch, S.; Papanicolaou, D.; Chandler, J. Performance-based or self-report measures of physical function: Which should be used in clinical trials of hip fracture patients? Arch. Phys. Med. Rehabil. 2008, 89, 2146–2155. [Google Scholar] [CrossRef]
- Dishman, R.K.; Washburn, R.A.; Schoeller, D.A. Measurement of physical activity. Quest 2001, 53, 295–309. [Google Scholar] [CrossRef]
| Outcome | Total | Female | Male |
|---|---|---|---|
| Age (years) | 65.0 ± 9.7 | 62.6 ± 9.2 | 71.4 ± 7.8 |
| Treatment stage (n) | |||
| Pre | 3 (2.3%) | 3 (3.2%) | - |
| During | 54 (42.2%) | 35 (37.2%) | 19 (55.9%) |
| Post | 71 (55.5%) | 56 (59.6%) | 15 (44.1%) |
| Cancer Type | Frequency | Percentage |
|---|---|---|
| Breast | 76 | 59.4% |
| Prostate | 22 | 17.2% |
| Head and Neck | 6 | 4.7% |
| Bowel and Colorectal | 6 | 4.6% |
| Other | 18 | 14.1% |
| Outcome | Pairs (n) | Pre iCan | Post iCan | Cohen’s d | p Value |
|---|---|---|---|---|---|
| 30 s Sit-to-Stand | 59 | 8.0 (6.0–9.0) | 14.0 (11.5–17.0) | −1.00 | <0.001 |
| FACIT | 93 | 29.3 (21.0–39.0) | 37.0 (28.0–42.0) | −0.77 | <0.001 |
| SWEMWBS | 91 | 25.0 (22.5–27.0) | 26.0 (23.0–29.0) | −0.69 | <0.001 |
| Importance | 80 | 9.0 (8.0–10.0) | 10.0 (8.75–10.0) | −0.44 | 0.016 |
| Confidence | 81 | 8.0 (6.0–9.0) | 9.0 (8.0–10.0) | −0.76 | <0.001 |
| Physical Activity (days/wk) | |||||
| Vigorous Activity | 95 | 0.0 (0.0–0.0) | 0.0 (0.0–2.0) | −0.78 | <0.001 |
| Moderate Activity | 95 | 0.0 (0.0–3.0) | 3.0 (2.0–5.0) | −0.81 | <0.001 |
| Walking | 95 | 4.0 (2.0–7.0) | 5.0 (3.5–7.0) | −0.57 | <0.001 |
| Physical Activity (mins/day) | |||||
| Vigorous Activity | 95 | 0.0 (0.0–0.0) | 0.0 (0.0–45.0) | −0.73 | <0.001 |
| Moderate Activity | 95 | 0.0 (0.0–60.0) | 45.0 (30.0–82.5) | −0.65 | <0.001 |
| Walking | 95 | 60.0 (30.0–60.0) | 60.0 (30.0–60.0) | −0.10 | 0.506 |
| Sitting | 95 | 360.0 (300.0–480.0) | 300.0 (240.0–360.0) | 0.85 | <0.001 |
| Physical Activity (MET-mins/wk) | |||||
| Total Activity | 95 | 933.0 (479.0–2318.0) | 2106.0 (1263.0–3593.0) | −0.76 | <0.001 |
| Vigorous Activity | 95 | 0.0 (0.0–0.0) | 0.0 (0.0–720.0) | −0.81 | <0.001 |
| Moderate Activity | 95 | 0.0 (0.0–680.0) | 720.0 (360.0–1350.0) | −0.69 | <0.001 |
| Walking | 95 | 693.0 (256.0–1172.0) | 693.0 (363.0–1386.0) | −0.37 | 0.008 |
| COM-B domain scores | |||||
| Physical Opportunity | 89 | 8.0 (6.0–9.0) | 8.0 (7.0–10.0) | −0.46 | 0.002 |
| Social Opportunity | 89 | 7.0 (6.0–9.0) | 8.0 (6.0–9.0) | −0.32 | 0.028 |
| Reflective Motivation | 89 | 7.0 (5.0–8.0) | 8.0 (6.0–9.0) | −0.56 | <0.001 |
| Automatic Motivation | 89 | 5.0 (4.0–7.0) | 6.0 (5.0–8.0) | −0.56 | <0.001 |
| Physical Capability | 89 | 6.0 (5.0–8.0) | 8.0 (7.0–9.0) | −0.73 | <0.001 |
| Psychological Capability | 89 | 7.0 (6.0–8.0) | 8.0 (7.0–9.0) | −0.59 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loweth, T.A.; Taylor, S.R.; Mapp, G.; Bebbington, K.; Atkin, N.; Kite, C. iCan, Empowering Recovery: Evaluating a Patient-Centred Cancer Rehabilitation Programme across the Cancer Care Continuum. Diseases 2024, 12, 236. https://doi.org/10.3390/diseases12100236
Loweth TA, Taylor SR, Mapp G, Bebbington K, Atkin N, Kite C. iCan, Empowering Recovery: Evaluating a Patient-Centred Cancer Rehabilitation Programme across the Cancer Care Continuum. Diseases. 2024; 12(10):236. https://doi.org/10.3390/diseases12100236
Chicago/Turabian StyleLoweth, Thomas A., Suzan R. Taylor, Gareth Mapp, Kim Bebbington, Naomi Atkin, and Chris Kite. 2024. "iCan, Empowering Recovery: Evaluating a Patient-Centred Cancer Rehabilitation Programme across the Cancer Care Continuum" Diseases 12, no. 10: 236. https://doi.org/10.3390/diseases12100236






