Unraveling Pneumomediastinum in COVID-19 Patients: Insights from a High-Volume-Center Case–Control Study
Abstract
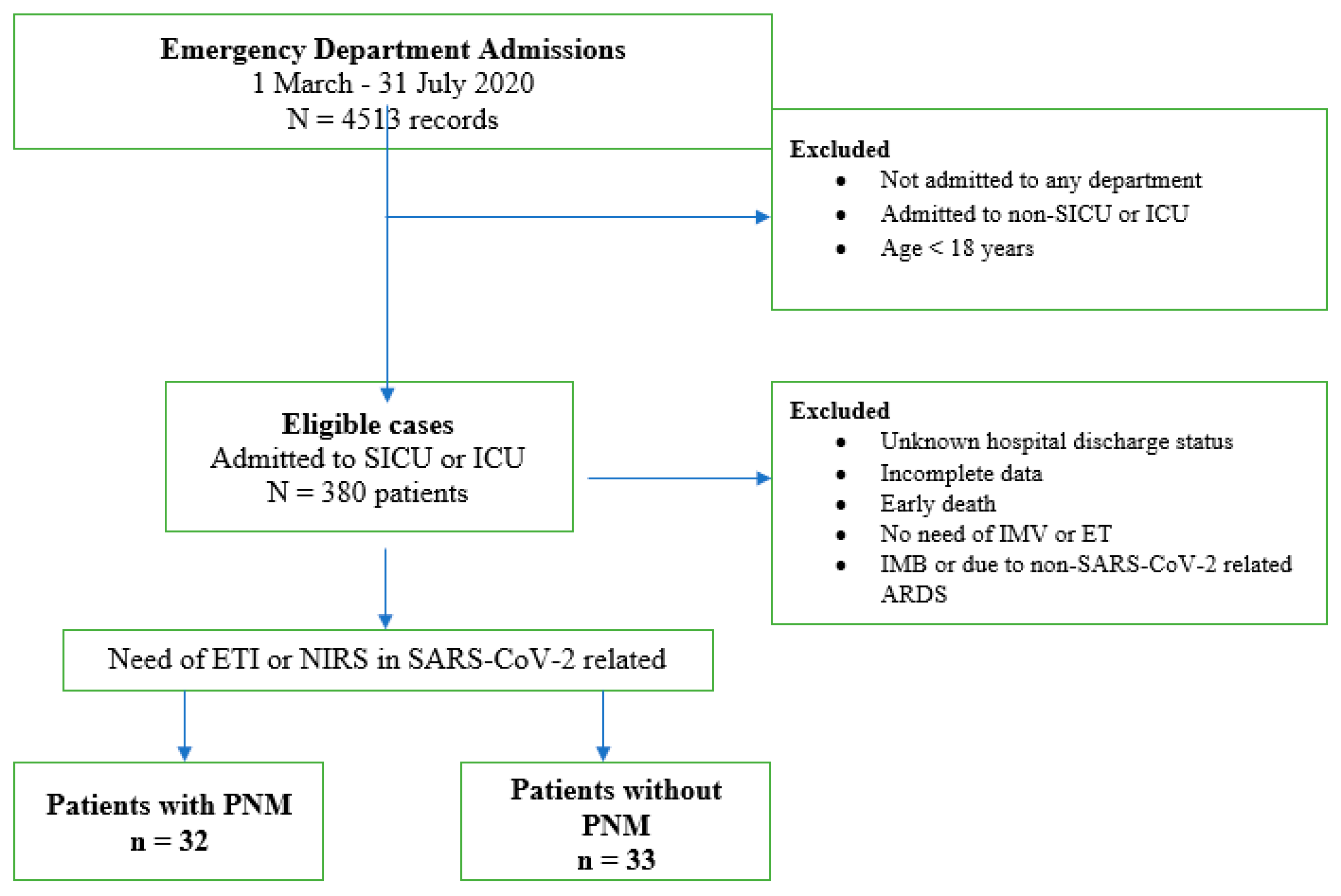
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tetaj, N.; Garotto, G.; Albarello, F.; Mastrobattista, A.; Maritti, M.; Stazi, G.V.; Marini, M.C.; Caravella, I.; Macchione, M.; De Angelis, G.; et al. Incidence of Pneumothorax and Pneumomediastinum in 497 COVID-19 Patients with Moderate–Severe ARDS over a Year of the Pandemic: An Observational Study in an Italian Third Level COVID-19 Hospital. J. Clin. Med. 2021, 10, 5608. [Google Scholar] [CrossRef] [PubMed]
- Belletti, A.; Palumbo, D.; Zangrillo, A.; Fominskiy, E.V.; Franchini, S.; Dell’Acqua, A.; Marinosci, A.; Monti, G.; Vitali, G.; Colombo, S.; et al. Predictors of Pneumothorax/Pneumomediastinum in Mechanically Ventilated COVID-19 Patients. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3642–3651. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, J.; Lee, K.H.; Lee, J.A.; Kim, C.H.; Lee, S.H.; Park, B.J.; Kim, J.H.; Ahn, J.Y.; Jeong, S.J.; et al. Risk factors of pneumothorax and pneumomediastinum in COVID-19: A matched case–control study. BMC Infect. Dis. 2023, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chong, W.H.; Saha, B.K.; Hu, K.; Chopra, A. The incidence, clinical characteristics, and outcomes of pneumothorax in hospitalized COVID-19 patients: A systematic review. Hear. Lung 2021, 50, 599–608. [Google Scholar] [CrossRef]
- Piroth, L.; Cottenet, J.; Mariet, A.-S.; Bonniaud, P.; Blot, M.; Tubert-Bitter, P.; Quantin, C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021, 9, 251–259. [Google Scholar] [CrossRef]
- Rafiee, M.J.; Fard, F.B.; Samimi, K.; Rasti, H.; Pressacco, J. Spontaneous pneumothorax and pneumomediastinum as a rare complication of COVID-19 pneumonia: Report of 6 cases. Radiol. Case Rep. 2021, 16, 687–692. [Google Scholar] [CrossRef]
- Reddy, R.K.; Charles, W.N.; Sklavounos, A.; Dutt, A.; Seed, P.T.; Khajuria, A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J. Med. Virol. 2020, 93, 1045–1056. [Google Scholar] [CrossRef]
- Sanduzzi, A.; Ciasullo, E.; Capitelli, L.; Zamparelli, S.S.; Bocchino, M. Alpha-1-Antitrypsin Deficiency and Bronchiectasis: A Concomitance or a Real Association? Int. J. Environ. Res. Public Health 2020, 17, 2294. [Google Scholar] [CrossRef]
- Prescott, H.C.; Sussman, J.B.; Wiersinga, W.J. Postcritical illness vulnerability. Curr. Opin. Crit. Care 2020, 26, 500–507. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Juárez-Lloclla, J.P.; León-Jiménez, F.; Urquiaga-Calderón, J.; Temoche-Nizama, H.; Bryce-Alberti, M.; Portmann-Baracco, A.; Bryce-Moncloa, A. Spontaneous Pneumopericardium and Pneumomediastinum in Twelve COVID-19 Patients. Arch. De Bronconeumol. 2020, 57, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.F.S.; Seposo, X.T.; Moi, M.L.; Tajudin, M.A.B.A.; Madaniyazi, L.; Sahani, M. Characteristics of COVID-19 epidemic and control measures to curb transmission in Malaysia. Int. J. Infect. Dis. 2020, 101, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Kojima, S.; Kawamoto, A.; Fukushima, M. COVID-19 pathogenesis, prognostic factors, and treatment strategy: Urgent recommendations. J. Med. Virol. 2020, 93, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Quartier, P. Juvenile Idiopathic Arthritis-Associated Chronic Uveitis: Recent Therapeutic Approaches. J. Clin. Med. 2021, 10, 2934. [Google Scholar] [CrossRef]
- Veldhoen, S.; Heidenreich, J.F.; Metz, C.; Petritsch, B.; Benkert, T.; Hebestreit, H.U.; Bley, T.A.; Köstler, H.; Weng, A.M. Three-dimensional Ultrashort Echotime Magnetic Resonance Imaging for Combined Morphologic and Ventilation Imaging in Pediatric Patients With Pulmonary Disease. J. Thorac. Imaging 2020, 36, 43–51. [Google Scholar] [CrossRef]
- Khaire, N.; Deshmukh, S.; Agarwal, E.; Mahale, N.; Khaladkar, S.; Desai, S.; Kulkarni, A. Pneumomediastinum: A marker of severity in COVID-19 disease. Heliyon 2023, 9, e12981. [Google Scholar] [CrossRef]
- Hu, F.; Yin, G.; Chen, Y.; Song, J.; Ye, M.; Liu, J.; Chen, C.; Song, Y.; Tang, X.; Zhang, Y. Corticosteroid, oseltamivir and delayed admission are independent risk factors for prolonged viral shedding in patients with Coronavirus Disease 2019. Clin. Respir. J. 2020, 14, 1067–1075. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Caruso, D.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Polidori, T.; Rucci, C.; Guido, G.; Bracci, B.; De Dominicis, C.; Laghi, A. Chest CT Features of COVID-19 in Rome, Italy. Radiology 2020, 296, E79–E85. [Google Scholar] [CrossRef] [PubMed]
- Tamaskani, N.; Khandashpoor, M.; Livani, S. Pneumothorax, Pneumomediastinum, and Subcutaneous Emphysema as Complications of COVID-19 Infection: A Case Series. Tanaffos 2021, 20, 368–372. [Google Scholar]
- Eperjesiova, B.; Hart, E.; Shokr, M.; Sinha, P.; Ferguson, G.T. Spontaneous Pneumomediastinum/Pneumothorax in Patients with COVID-19. Cureus 2020, 12, e8996. [Google Scholar] [CrossRef] [PubMed]
- Tacconi, F.; Rogliani, P.; Leonardis, F.; Sarmati, L.; Fabbi, E.; De Carolis, G.; La Rocca, E.; Vanni, G.; Ambrogi, V. Incidence of pneumomediastinum in COVID-19: A single-center comparison between 1st and 2nd wave. Respir. Investig. 2021, 59, 661–665. [Google Scholar] [CrossRef]
- McGuinness, G.; Zhan, C.; Rosenberg, N.; Azour, L.; Wickstrom, M.; Mason, D.M.; Thomas, K.M.; Moore, W.H. Increased Incidence of Barotrauma in Patients with COVID-19 on Invasive Mechanical Ventilation. Radiology 2020, 297, E252–E262. [Google Scholar] [CrossRef]
- Anzueto, A.; Frutos–Vivar, F.; Esteban, A.; Alía, I.; Brochard, L.; Stewart, T.; Benito, S.; Tobin, M.J.; Elizalde, J.; Palizas, F.; et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004, 30, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Aquila, I.; Sacco, M.A.; Scarlata, G.G.M.; Procopio, A.C.; Boccuto, L.; Scarpellini, E.; Greco, M.; Foti, D.P.; Ricci, P.; et al. Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series. Diseases 2023, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Negri, S.; Mazzuca, E.; Lococo, F.; Mondoni, M.; Covino, M.; Kuzmych, K.; Agati, S.; Amata, M.; Giuseppe, A.; Gabbrielli, L.; et al. Pneumomediastinum in COVID-19: Risk Factors and Outcomes from a Multicentre Case-control Study. Respir. Med. 2024, 230, 107684. [Google Scholar] [CrossRef]
- Chua, N.G.; Loo, L.; Hee, D.K.H.; Lim, T.P.; Ng, T.M.; Hoo, G.S.R.; Soong, J.L.; Ong, J.C.L.; Tang, S.S.L.; Zhou, Y.P.; et al. Therapeutic drug monitoring of meropenem and piperacillin-tazobactam in the Singapore critically ill population—A prospective, multi-center, observational study (BLAST 1). J. Crit. Care 2022, 68, 107–113. [Google Scholar] [CrossRef]
- Teo, Y.X.; Geetha, H.S.; Mishra, A.K.; Lal, A. Pneumomediastinum in COVID-19: Incidence, Pathogenesis, and Management. Respiratory Care. Mediastinum 2023, 8, 3. [Google Scholar] [CrossRef]
| No PNM (n = 33) | PNM (n = 32) | p-Value | |
|---|---|---|---|
| Men | 21 (63%) | 23 (72%) | 0.5977 |
| Age (years) * | 65.4 ± 14.3 | 54.9 ± 18.5 | 0.0214 |
| Smoking exposure | |||
| Never a smoker | 11 (33.3%) | 9 (28.1%) | 0.7893 |
| Former smoker | 14 (42.4%) | 10 (31.3%) | 0.4431 |
| Current smoker | 8 (24.3%) | 13 (40.6%) | 0.1912 |
| Nutritional status | |||
| BMI * | 27.7 ± 6.5 | 26.5 ± 4.9 | 0.5727 |
| Underweight (BMI < 18.5) | 0 | 2 (6.3%) | 0.2385 |
| Overweight (BMI 25–29.9) | 17 (51.5%) | 18 (56.3%) | 0.8050 |
| Obese (BMI > 30) | 10 (30.3%) | 4 (12.5%) | 0.0131 |
| Blood parameters | |||
| CRP at admission (mg/L) * | 73.6 ± 108.7 | 108.1 ± 87.6 | 0.0187 |
| Maximum CRP (mg/L) * | 126.3 ± 123.6 | 139.4 ± 85.9 | 0.3584 |
| D-dimer at admission (ng/mL) * | 7467 ± 12,243 | 5497 ± 10,060 | 0.7195 |
| Maximum D-dimer (ng/mL) * | 10,412 ± 13,075 | 8354 ± 10,150 | 0.9958 |
| WBC at admission | 6.5 ± 4.1 | 11.4 ± 5.4 | <0.0001 |
| Maximum WBC | 8.9 ± 5.1 | 16.4 ± 6.1 | <0.0001 |
| Comorbidities | |||
| Hypertension | 20 (60.6%) | 16 (50%) | 0.4586 |
| COPD | 5 (15.1%) | 15 (46.9%) | 0.0148 |
| Diabetes mellitus type II | 7 (21.2%) | 3 (9.4%) | 0.3020 |
| CAD | 7 (21.2%) | 6 (18.6%) | >0.0999 |
| Other respiratory diseases (fibrosis and bronchiectasis) | 7 (21.2%) | 5 (15.6%) | 0.7505 |
| No PNM (n = 33) | PNM (n = 32) | p-Value | |
|---|---|---|---|
| Extent of lung involvement * | 52.7 ± 27.4 (1–100) | 61.3 ± 20 (1–100) | 0.3278 |
| Number of lobes involved * | 2.6 ± 1.1 (1–5) | 2.6 ± 0.9 (1–5) | 0.8294 |
| Bilateral distribution | 29 (87.9%) | 28 (87.5%) | >0.9999 |
| Ground-glass opacities | 17 (51.5%) | 30 (93.7%) | 0.0002 |
| Consolidations | 14 (42.2%) | 25 (78.1%) | 0.0051 |
| Crazy paving | 6 (18.2%) | 3 (9.4%) | 0.4752 |
| Pleural effusion | 3 (9%) | 9 (28.1%) | 0.0606 |
| Pneumothorax | 1 (3%) | 20 (62.5%) | <0.0001 |
| Emphysema | 0 | 6 (18.6%) | 0.0110 |
| No PNM (n = 33) | PNM (n = 32) | p-Value | |
|---|---|---|---|
| Conventional OT | 13 (39.4%) | 9 (28.1%) | 0.4339 |
| CPAP | 4 (12.1%) | 8 (25%) | 0.3389 |
| BiPAP | 10 (30.3%) | 8 (25%) | 0.1548 |
| HFNC | 6 (18.2%) | 7 (21.9%) | 0.7642 |
| ETI | 10 (30.3%) | 17 (53.1%) | 0.0619 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzmych, K.; Covino, M.; Paratore, M.; Campanella, A.; Abenavoli, L.; Calabrese, G.; Napolitano, A.G.; Sassorossi, C.; Margaritora, S.; Lococo, F. Unraveling Pneumomediastinum in COVID-19 Patients: Insights from a High-Volume-Center Case–Control Study. Diseases 2024, 12, 242. https://doi.org/10.3390/diseases12100242
Kuzmych K, Covino M, Paratore M, Campanella A, Abenavoli L, Calabrese G, Napolitano AG, Sassorossi C, Margaritora S, Lococo F. Unraveling Pneumomediastinum in COVID-19 Patients: Insights from a High-Volume-Center Case–Control Study. Diseases. 2024; 12(10):242. https://doi.org/10.3390/diseases12100242
Chicago/Turabian StyleKuzmych, Khrystyna, Marcello Covino, Mattia Paratore, Annalisa Campanella, Ludovico Abenavoli, Giuseppe Calabrese, Antonio Giulio Napolitano, Carolina Sassorossi, Stefano Margaritora, and Filippo Lococo. 2024. "Unraveling Pneumomediastinum in COVID-19 Patients: Insights from a High-Volume-Center Case–Control Study" Diseases 12, no. 10: 242. https://doi.org/10.3390/diseases12100242









