Characteristics of Hepatocellular Carcinoma by Sex in Mexico: A Multi-Institutional Collaboration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mexican Interdisciplinary Network for Hepatocellular Cancer Research (RIMICH)
2.2. Medical Record Review and Data Extraction
2.3. Statistical Analyses
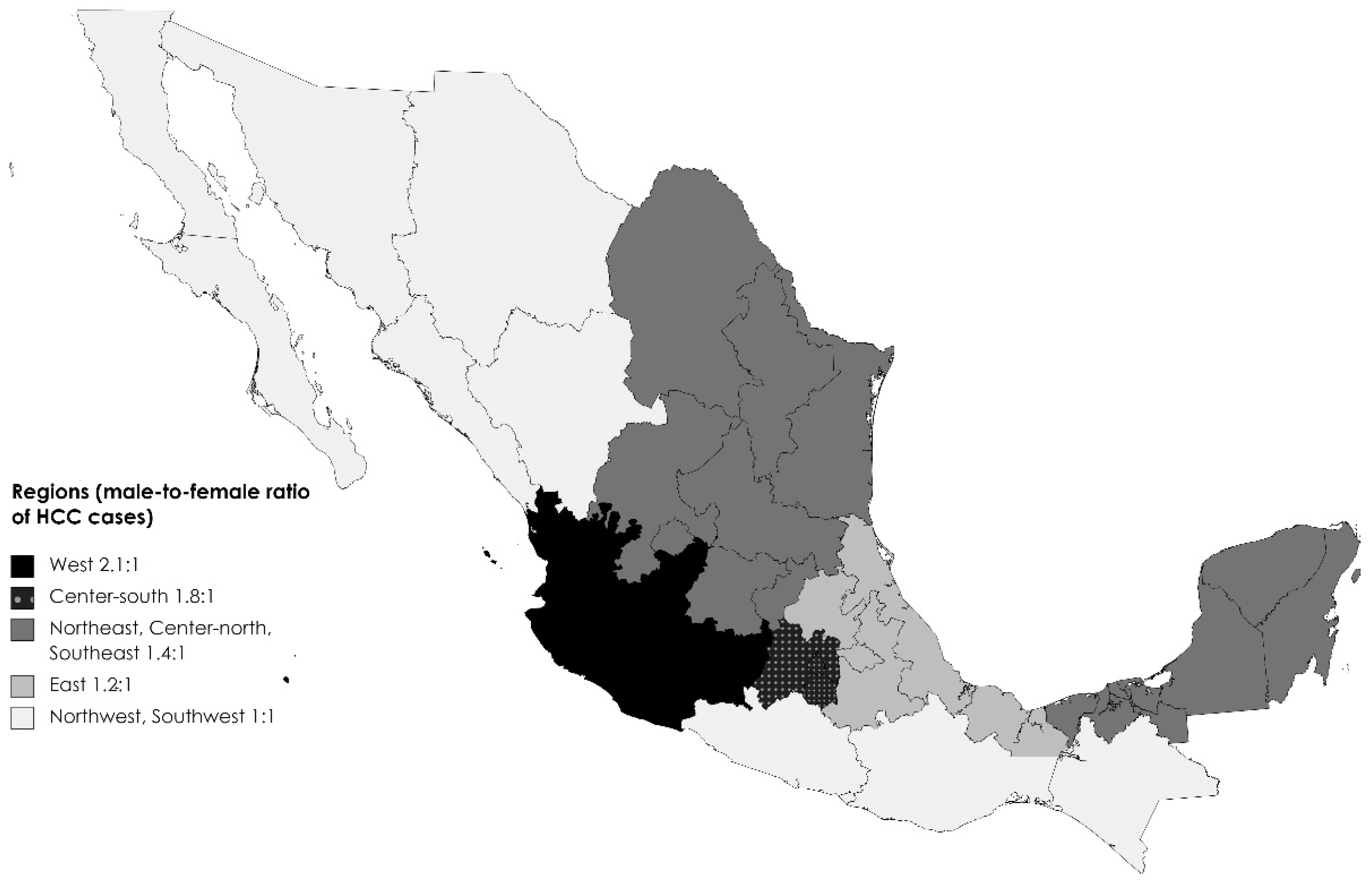
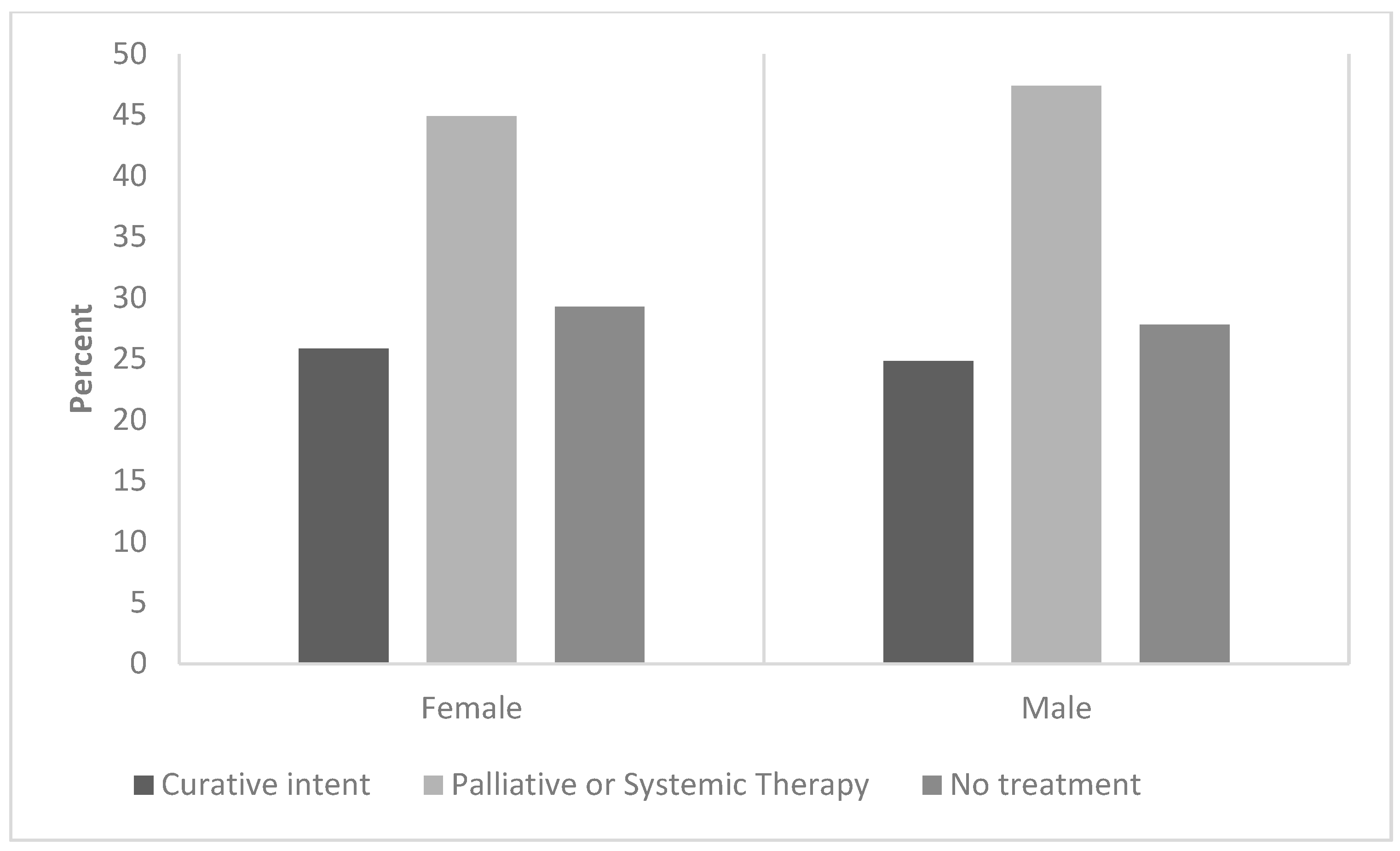
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349.e315. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef]
- Carnalla, M.; Vidaña-Pérez, D.; Alpuche-Aranda, C.; Chávez-Tapia, N.C.; Romero-Martínez, M.; Shamah-Levy, T.; Barrientos-Gutiérrez, T. Hepatitis B infection in Mexican adults: Results of a nationally representative survey. Ann. Hepatol. 2022, 27, 100583. [Google Scholar] [CrossRef]
- Carnalla, M.; Barrientos-Gutiérrez, T.; Vidaña-Perez, D.; Romero-Martínez, M.; Martínez-Bohorquez, M.C.; González-Pier, E.; Fagundo-Sierra, R.; Kershenobich, D.; Alpuche-Aranda, C.; Lazcano-Ponce, E.; et al. Prevalence of hepatitis C in the adult Mexican population: National Survey of Health and Nutrition 2018. Lancet Reg. Health-Am. 2022, 8, 100165. [Google Scholar] [CrossRef]
- World Health Organization. The Global Health Observatory. Alcohol, Heavy Episodic Drinking (15+) Past 30 Days (%), Age-Standardized with 95%CI. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/alcohol-heavy-episodic-drinking-(15-)-past-30-days-(-)-age-standardized-with-95-ci (accessed on 11 September 2023).
- Gonzalez-Chagolla, A.; Olivas-Martinez, A.; Ruiz-Manriquez, J.; Servín-Rojas, M.; Kauffman-Ortega, E.; Chávez-García, L.C.; Juárez-León, O.; Cordova-Gallardo, J.; Díaz-García, J.D.; Gonzalez-Huezo, M.S. Cirrhosis etiology trends in developing countries: Transition from infectious to metabolic conditions. Report from a multicentric cohort in central Mexico. Lancet Reg. Health-Am. 2022, 7, 100151. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; World Health Organization. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; World Health Organization: Geneva, Switzerland, 2002.
- Monge, A.; Romero-Martínez, M.; Groopman, J.D.; McGlynn, K.A.; Santiago, L.; Villalpando-Hernández, S.; Mannan, R.; Burke, S.M.; Remes-Troche, J.M.; Lajous, M. Aflatoxin Exposure in Adults in Southern and Eastern Mexico in 2018: A Descriptive Study. Int. J. Hyg. Environ. Health 2023, 253, 114249. [Google Scholar] [CrossRef]
- Farah, M.; Anugwom, C.; Ferrer, J.D.; Baca, E.L.; Mattos, A.Z.; Possebon, J.P.P.; Arrese, M.; Prieto, J.; Balderramo, D.; Carrera, E.; et al. Changing epidemiology of hepatocellular carcinoma in South America: A report from the South American liver research network. Ann. Hepatol. 2023, 28, 100876. [Google Scholar] [CrossRef]
- Álvarez, C.S.; Espinosa-Tamez, P.; López-Ridaura, R.; Lamadrid-Figueroa, H.; Melchor-Ruan, J.; McGlynn, K.A.; Lajous, M. Liver cancer mortality in Mexico: Trend analysis from 1998 to 2018. Salud Pública México 2022, 64, 14–25. [Google Scholar] [CrossRef]
- Huezo, M.S.G.; Ávila, J.F.S.; de Gastroenterología, A.M.; de Radiología, S.M.; de Oncología, S.M.; de Carcinoma, G.M.d.C. Consenso mexicano de diagnóstico y manejo del carcinoma hepatocelular. Rev. Gastroenterol. México 2014, 79, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Leal, Y.A.; Torres, J.; Gamboa, R.; Mantilla-Morales, A.; Piña-Sanchez, P.; Arrieta, O.; Bonifaz, L.; Meneses, A.; Duque, C.; Piñeros, M. Cancer Incidence in Merida, Mexico 2015-2018: First Report from the Population-based Cancer Registry. Arch. Med. Res. 2022, 53, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Leal, Y.A.; Reynoso-Noverón, N.; Aguilar-Castillejos, L.F.; Meneses-García, A.; Mohar, A.; Piñeros, M. Implementation of the population-based cancer registry in the city of Mérida, Mexico: Process and early results. Salud Pública México 2020, 62, 96–104. [Google Scholar] [CrossRef]
- Bray, F.; Znaor, A.; Cueva, P. Planning and Developing Population-Based Cancer Registration in Low- and Middle-Income Settings; IARC (International Agency for Research on Cancer): Lyon, France, 2014. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Sanyal, A.J.; Harrison, S.A.; Ratziu, V.; Abdelmalek, M.F.; Diehl, A.M.; Caldwell, S.; Shiffman, M.L.; Schall, R.A. The conundrum of cryptogenic cirrhosis: Adverse outcomes without treatment options. J. Hepatol. 2018, 69, 1365–1370. [Google Scholar] [CrossRef]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 1999; pp. 329–338. [Google Scholar]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Tangkijvanich, P.; Anukulkarnkusol, N.; Suwangool, P.; Lertmaharit, S.; Hanvivatvong, O.; Kullavanijaya, P.; Poovorawan, Y. Clinical characteristics and prognosis of hepatocellular carcinoma: Analysis based on serum alpha-fetoprotein levels. J. Clin. Gastroenterol. 2000, 31, 302–308. [Google Scholar] [CrossRef]
- Hayes-Larson, E.; Kezios, K.L.; Mooney, S.J.; Lovasi, G. Who is in this study, anyway? Guidelines for a useful Table 1. J. Clin. Epidemiol. 2019, 114, 125–132. [Google Scholar] [CrossRef]
- INEGI. Esperanza de Vida al Nacimiento por Entidad Federativa Según Sexo, Serie Anual de 2010 a 2022. Available online: https://www.inegi.org.mx/app/tabulados/interactivos/?pxq=Mortalidad_Mortalidad_09_61312f04-e039-4659-8095-0ce2cd284415 (accessed on 11 September 2023).
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Cisneros-Garza, L.; González-Huezo, M.; López-Cossio, J.; Kuljacha-Gastelum, A. Caracterización del carcinoma hepatocelular en México. Rev. Gastroenterol. México 2018, 83, 223–227. [Google Scholar] [CrossRef]
- Carrilho, F.J.; Kikuchi, L.; Branco, F.; Goncalves, C.S.; de Mattos, A.A.; Brazilian HCC Study Group. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics 2010, 65, 1285–1290. [Google Scholar] [CrossRef]
- Fassio, E.; Díaz, S.; Santa, C.; Reig, M.E.; Artola, Y.M.; de Mattos, A.A.; Míguez, C.; Galizzi, J.; Zapata, R.; Ridruejo, E. Etiology of hepatocellular carcinoma in Latin America: A prospective, multicenter, international study. Ann. Hepatol. 2010, 9, 63–69. [Google Scholar] [CrossRef] [PubMed]
- DGE. Dirección de Vigilancia Epidemiológica de Enfermedades No Transmisibles. Informe Anual de Vigilancia Epidemiológica de Hepatitis Virales, México 2020. Available online: https://www.gob.mx/cms/uploads/attachment/file/615926/HepatitisViralesInformeAnual2020.pdf (accessed on 11 September 2023).
- World Health Organization. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017.
- World Health Organization. World Health Organization Global Health Observatory; World Health Organization: Geneva, Switzerland, 2023.
- Shamah-Levy, T.; Romero-Martínez, M.; Barrientos-Gutiérrez, T.; Cuevas-Nasu, L.; Bautista-Arredondo, S.; Colchero, M.; Gaona-Pineda, E.; Lazcano-Ponce, E.; Martínez-Barnetche, J.; Alpuche-Arana, C. Encuesta Nacional de Salud y Nutrición 2020 Sobre COVID-19; Resultados Nacionales; Instituto Nacional de Salud Pública: Cuernavaca, México, 2021. [Google Scholar]
- Nagral, A.; Bangar, M.; Menezes, S.; Bhatia, S.; Butt, N.; Ghosh, J.; Manchanayake, J.H.; Al Mahtab, M.; Singh, S.P. Gender Differences in Nonalcoholic Fatty Liver Disease. Euroasian J. Hepato-Gastroenterol. 2022, 12, S19. [Google Scholar]
- Wu, E.M.; Wong, L.L.; Hernandez, B.Y.; Ji, J.-F.; Jia, W.; Kwee, S.A.; Kalathil, S. Gender differences in hepatocellular cancer: Disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018, 4, 66. [Google Scholar] [CrossRef] [PubMed]
| Female | Male | |||
|---|---|---|---|---|
| (n = 294) | Missing, % | (n = 403) | Missing, % | |
| Mean age at diagnosis, years | 66.3(12.1) | 0 | 64.8(11.7) | 0 |
| <50 | 7.1 | 10.7 | ||
| 50–59, % | 14.3 | 15.9 | ||
| 60–69, % | 34.0 | 37.2 | ||
| 70+, % | 44.6 | 36.2 | ||
| Mean body mass index, kg/m2 | 26.9(5.2) | 0 | 26.1(4.3) | 0 |
| Overweight/Obese, % | 61.2 | 0 | 59.1 | 0 |
| Cirrhosis, % | 65.5 | 0 | 68.5 | 0 |
| Cirrhosis etiology | 36.7 | 33.0 | ||
| Unknown, % | 51.1 | 20.7 | ||
| Alcohol, % | 10.8 | 59.6 | ||
| Hepatitis C virus, % | 20.4 | 12.6 | ||
| MAFLD, % | 8.6 | 3.0 | ||
| Hepatitis B virus, % | 3.8 | 3.3 | ||
| Autoimmune Hepatitis, % | 2.7 | 0.7 | ||
| Other, % | 2.7 | 0.0 | ||
| Child Score | 35.4 | 32.8 | ||
| A, % | 58.4 | 54.2 | ||
| B, % | 31.6 | 32.1 | ||
| C, % | 7.9 | 11.1 | ||
| Biopsy, % | 59.4 | 0 | 56.2 | 0 |
| Treated, % | 70.4 | 0 | 72.2 | 0 |
| Female (n = 294) | Male (n = 403) | |||
|---|---|---|---|---|
| Missing, % | Missing, % | |||
| Differentiation | 41.5 | 44.4 | ||
| Well, % | 25.6 | 30.8 | ||
| Moderate, % | 36.0 | 35.7 | ||
| Poor to Undifferentiated, % | 12.2 | 16.5 | ||
| Not specified, % | 26.2 | 17.0 | ||
| Number of nodules | 0 | 0 | ||
| 1, % | 51 | 47.9 | ||
| 2–3, % | 23.1 | 19.6 | ||
| 4+, % | 7.5 | 14.9 | ||
| Not determined, % | 18.4 | 17.6 | ||
| Size of largest nodule, cm | 7.5(4.3) | 19.4 | 7.9(4.5) | 17.9 |
| Nodule > 5 cm, % | 77.6 | 0 | 77.9 | 0 |
| Satellite lesions, % | 17.0 | 2.4 | 19.1 | 3.2 |
| Extrahepatic disease, % | 24.5 | 2.7 | 24.3 | 4.2 |
| Metastasis | 0 | 0 | ||
| Yes, % | 19.4 | 19.9 | ||
| Not determined, % | 78.2 | 75.9 | ||
| BCLC | 11.2 | 12.2 | ||
| Early (0 or A) | 26.2 | 22.6 | ||
| Intermediate (B) | 24.8 | 20.8 | ||
| Advanced (C or D) | 37.8 | 44.4 | ||
| AFP at diagnosis, ng/mL | ||||
| <20, % | 29.3 | 29.0 | ||
| 20–399, % | 33.3 | 34.0 | ||
| 400+, % | 37.4 | 37.0 | ||
| Tumor type | 41.8 | 44.2 | ||
| Classic, % | 26.3 | 27.6 | ||
| Fibrolamellar, % | 4.7 | 1.8 | ||
| Clear cell, % | 1.8 | 3.1 | ||
| Other, % | 4.7 | 3.4 | ||
| Not determined, % | 62.6 | 64.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchor-Ruan, J.; Santiago-Ruiz, L.; Murillo-Ortiz, B.O.; Rivera-Rivera, S.; Leal-Herrera, Y.A.; Suárez-García, D.; Remes-Troche, J.M.; Grube, P.; Martínez-Mier, G.; Ruiz-García, E.; et al. Characteristics of Hepatocellular Carcinoma by Sex in Mexico: A Multi-Institutional Collaboration. Diseases 2024, 12, 262. https://doi.org/10.3390/diseases12100262
Melchor-Ruan J, Santiago-Ruiz L, Murillo-Ortiz BO, Rivera-Rivera S, Leal-Herrera YA, Suárez-García D, Remes-Troche JM, Grube P, Martínez-Mier G, Ruiz-García E, et al. Characteristics of Hepatocellular Carcinoma by Sex in Mexico: A Multi-Institutional Collaboration. Diseases. 2024; 12(10):262. https://doi.org/10.3390/diseases12100262
Chicago/Turabian StyleMelchor-Ruan, Javier, Luis Santiago-Ruiz, Blanca Olivia Murillo-Ortiz, Samuel Rivera-Rivera, Yelda A. Leal-Herrera, David Suárez-García, José María Remes-Troche, Peter Grube, Gustavo Martínez-Mier, Erika Ruiz-García, and et al. 2024. "Characteristics of Hepatocellular Carcinoma by Sex in Mexico: A Multi-Institutional Collaboration" Diseases 12, no. 10: 262. https://doi.org/10.3390/diseases12100262
APA StyleMelchor-Ruan, J., Santiago-Ruiz, L., Murillo-Ortiz, B. O., Rivera-Rivera, S., Leal-Herrera, Y. A., Suárez-García, D., Remes-Troche, J. M., Grube, P., Martínez-Mier, G., Ruiz-García, E., Ramos-Mayo, A., Velarde-Ruiz-Velasco, J. A., Gamboa-Gutierrez, R., Ordoñez-Escalante, K. G., Cisneros-Garza, L. E., Leal-Leyte, P., Sepúlveda-Delgado, J., González-Huezo, M. S., Arvizu-Castillo, R., ... Zamora-Valdés, D. (2024). Characteristics of Hepatocellular Carcinoma by Sex in Mexico: A Multi-Institutional Collaboration. Diseases, 12(10), 262. https://doi.org/10.3390/diseases12100262






