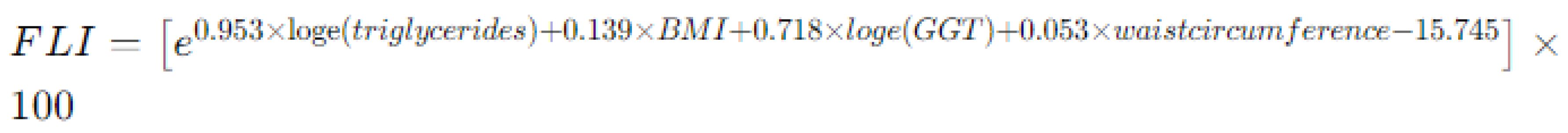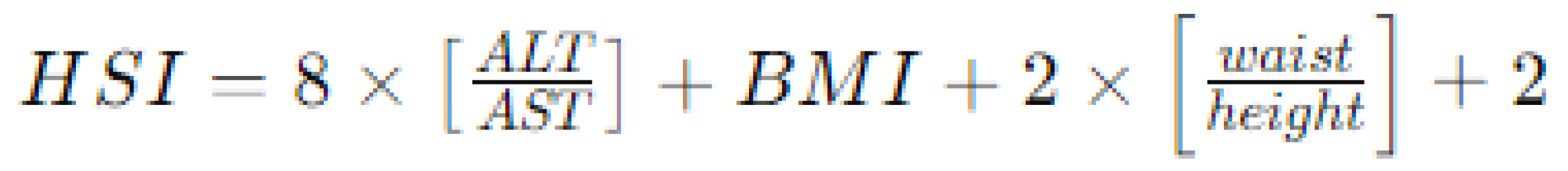Abstract
Background: Individuals with hyperinsulinemia may initially not meet any diagnostic criteria for metabolic syndrome, though displaying a higher risk of cardiovascular complications combined with obesity, diabetes, and hypertension. Aim: The main objective of our study was to assess the diagnostic accuracy of various cardiovascular risk indices in hyperinsulinemic children and adolescents; a secondary objective was to estimate the optimal cut-offs of these indices. Patients and methods: This retrospective single-center study was conducted on 139 patients aged 12.1 ± 2.9 years, managed for hyperinsulinism. Results: We found statistically significant differences in homeostasis model assessment of insulin resistance index (HOMA-IR), triglyceride glucose index (TyG), TyG-body mass index, visceral adiposity index, lipid accumulation product index, fatty liver index, and hepatic steatosis index. At the linear logistic regression assessment, we found that insulin growth factor-1 (IGF-1), HOMA-IR, and ALT/AST ratio were independently associated with confirmed hyperinsulinism. At the multivariate analysis, IGF-1 levels over 203 ng/mL and HOMA-IR higher than 6.2 were respectively associated with a 9- and 18-times higher odds ratio for hyperinsulinism. The other investigated parameters were not significantly related to hyperinsulinism, and could not predict either the presence of hyperinsulinemia or a subsequent cardiovascular risk in our patients. Conclusion: Commonly used indices of cardiovascular risk in adults cannot be considered accurate in confirming hyperinsulinism in children, with the exception of HOMA-IR. Further studies are needed to verify the usefulness of specific cardiovascular risk indices in hyperinsulinemic children and adolescents.
1. Introduction
Metabolic syndrome is defined by a cluster of cardiovascular risk factors associated with insulin resistance, visceral obesity, and unbridled systemic inflammation [1]. Although more common in adulthood, the metabolic syndrome has become a relevant cause of morbidity in the pediatric age, due to an increasing incidence of non-genetic obesity determined by both unbalanced lifestyles and incorrect dietary habits [2].
The etiology of obesity differs between adults and pediatric populations: in fact, childhood obesity is influenced by a complex interplay of genetic, environmental, and behavioral factors, including parental obesity, early feeding practices, and socio-economic status [3]. However, long-term adverse effects of obesity are shared between the two categories, and it has been demonstrated that the cardiovascular risk in the adult population is also associated with different metabolic features in early life [2].
According to the International Diabetes Federation [4], children aged 10–15 years meet the criteria for metabolic syndrome if they exhibit obesity with waist circumference (WC) > 90th percentile and two risk factors among blood pressure ≥ 130/85 mmHg, density lipoprotein (HDL) cholesterol ≤ 40 mg/dL, triglycerides ≥ 150 mg/dL, or fasting glucose ≥ 100 mg/dL. Notwithstanding, it is not possible to diagnose this syndrome in children under 10 years due to the absence of age-specific reference values. Even if individuals with hyperinsulinemia may not initially meet the diagnostic criteria, it is known that they are at higher risk of developing metabolic syndrome, metabolic dysfunction-associated steatotic fatty liver disease, diabetes, hypertension, and further cardiovascular complications [1,4]. Cardiovascular diseases are currently the leading cause of mortality in the industrialized countries [1], and the rate of cardiovascular events may even start during the pediatric age [5], warranting the need to mitigate this risk with the aim of improving morbidities in adulthood and overall life expectancy.
It is also known that certain anthropometric parameters, alone or in combination with laboratory data, might identify groups of patients at higher risk of developing the metabolic syndrome [6]. Over time, cardiovascular risk indices have therefore been developed based on the combination of multiple auxological parameters, laboratory markers, or a combination of both. An alteration of these indirect indices has been statistically associated with both hyperinsulinemia and risk of developing metabolic syndrome [7]. However, the majority of studies pertains to adult patients, while there are limited data referred to the pediatric population. We performed this retrospective study to evaluate the applicability of various cardiovascular risk indices to the pediatric population diagnosed with hyperinsulinism.
2. Patients and Methods
This is a cross-sectional, retrospective, single-center study conducted at the Pediatric Endocrinology Day Hospital of the Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, in children evaluated for hyperinsulinism. The main objective of our study was to assess the diagnostic accuracy of various cardiovascular risk indices—commonly used in adulthood—for studying hyperinsulinemic pediatric patients; a secondary objective was to estimate the optimal cut-offs of these indices in our sample.
2.1. Inclusion and Exclusion Criteria
The inclusion criteria of our study were as follows:
- Age < 18 years.
- Suspected hyperinsulinism (defined as basal insulin > 25 OR family history of hyperinsulinism and/or diabetes OR presence of acanthosis nigricans and/or other signs of hyperinsulinism on the physical examination OR body mass index (BMI), waist circumference (WC), and/or hip circumference (HC) > 95th percentile according to age and sex).
- Complete endocrinological follow-up, including blood pressure measurement, abdominal ultrasound with assessment of hepatic parenchyma features, auxological parameters (weight, height, BMI, WC, HC; for each variable we calculated the respective standard deviation [SD] for age and sex), laboratory test (transaminases, complete thyroid profile, including thyroid-stimulating hormone (TSH), triiodothyronine (fT3), thyroxine (fT4), insulin growth factor-1 (IGF-1), insulin, glucose, glycated hemoglobin, uric acid, complete lipid profile including total cholesterol, triglycerides, low density lipoprotein (LDL), HDL, very low density lipoprotein (VLDL)), and oral glucose tolerance test (OGTT) results, including insulin and glucose peak.
The exclusion criteria of our study were as follows:
- Diagnosis of diabetes mellitus, defined as glycated hemoglobin ≥ 6.5% OR fasting plasma glucose ≥ 126 mg/dL OR glucose ≥ 200 mg/dL during an OGTT, OR random glucose ≥ 200 mg/dL in a patient with classic diabetic symptoms, like polyuria and/or polydipsia [7].
- Coded diagnosis of metabolic syndrome (obesity with waist circumference > 90th percentile and two risk factors among blood pressure ≥ 130/85 mmHg, HDL ≤ 40 mg/dL, triglycerides ≥ 150 mg/dL, or fasting glucose ≥ 100 mg/dL [4].
- Therapy with metformin and/or other antidiabetic drugs (excluding patients who started a specific therapy following the execution of the OGTT).
After the screening process, we retrospectively collected information from the medical records of 139 children and young adolescents; we divided patients into two groups based on the result of the OGTT.
Currently, the OGTT represents the main tool for assessing insulin peak, both in pediatric and adult populations. In the absence of a standardized guideline for interpreting insulin levels during OGTT, there is significant variability in the cut-off values, which may depend on the type of guidelines considered or clinicians’ experience. Currently, a defined cut-off value in the pediatric population has not yet been established. However, several possible cut-offs exist, including [8,9] the following:
- ✓ The sum of insulin measurements at different sampling times during the OGTT > or <2083.5 pmol/L (300 μU/mL).
- ✓ An insulin peak ≥ 1041.75 pmol/L.
- ✓ A blood insulin value ≥ 520.88 pmol/L (75 μU/mL) when sampled 120 min after glucose loading.
- ✓ An insulin peak above 100 uIU/mL.
In our center, in the absence of a commonly shared guideline, we considered a peak insulin level > 100 uIU/mL to be ‘pathological’. This cut-off is among those validated in the medical literature, and is not arbitrarily determined for the classification of OGTT results.
2.2. Cardiovascular Risk Indices
The cardiovascular risk indices calculated in our study were as follows:
- -
- Homeostasis model assessment of insulin resistance index (HOMA-IR) [10], calculated with the following formula: (Fasting plasma insulin × fasting plasma glucose)/22.5. This index combines basal blood glucose and insulin values to provide an indirect estimate of hyperinsulinism and increased insulin resistance. In general terms, HOMA-IR higher than 2.5 is considered pathological, although the cut-offs used may vary based on age, ethnicity, and gender.
- -
- Triglyceride glucose index (TyG) [11,12], calculated with the following formula: Ln [TG (mg/dL) × FPG (mg/dL)]/2; this index combines the value of triglycerides with glucose levels, incorporating two of the diagnostic criteria for the metabolic syndrome; it may be associated with increased cardiovascular risk when >4.5 [13].
- -
- Triglyceride to HDL ratio [14], a useful marker of cardiovascular risk applied also in overweight children; this index could be considered increased when >2 [15].
- -
- TyG-BMI [16], calculated with the following formula: TyG Index × BMI (kg/m2); this derives from the TyG index in association with patient’s BMI; however, there are no specific cut-offs available for its interpretation.
- -
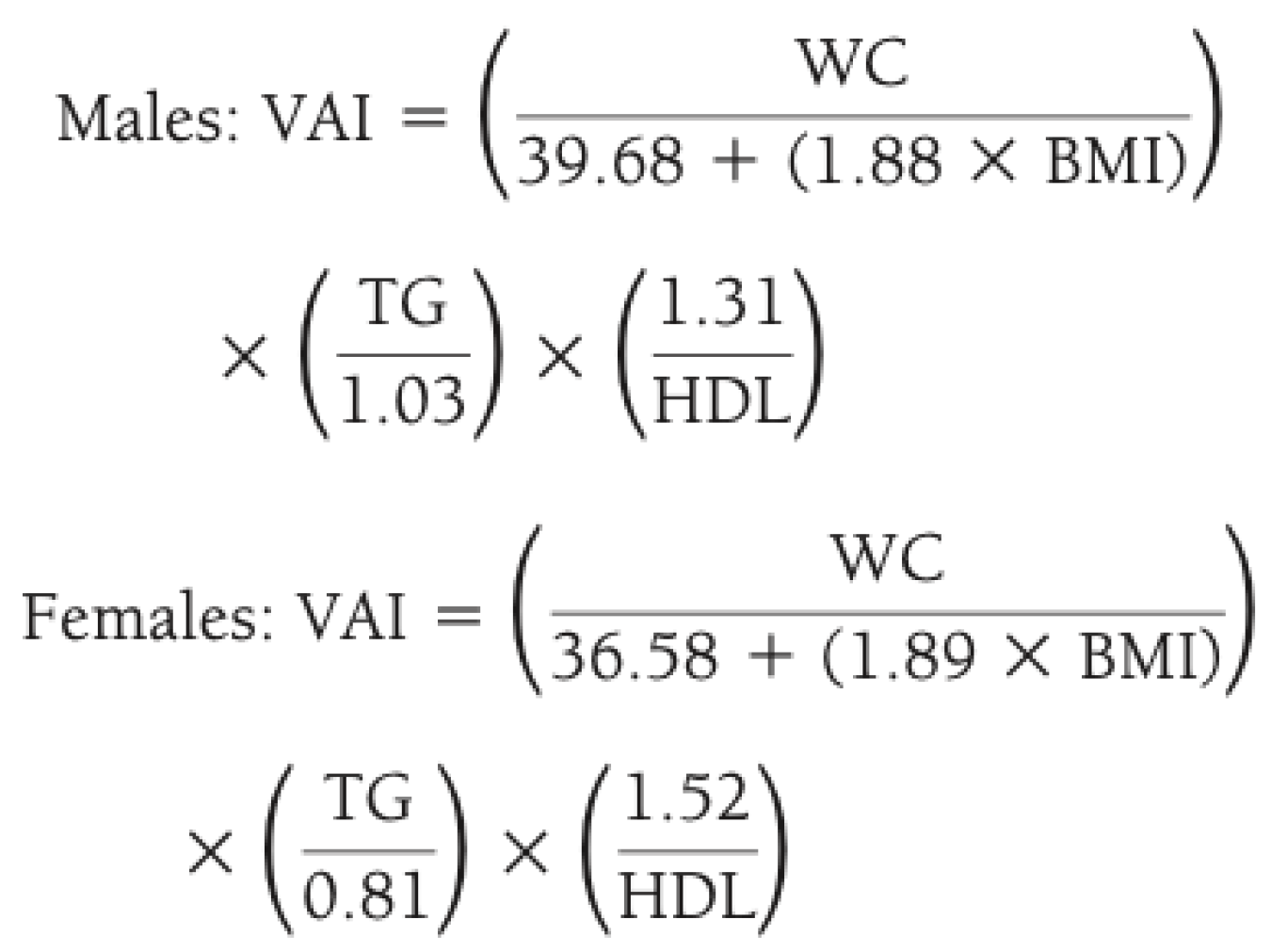
- Visceral adiposity index (VAI) [17], calculated as reported in Figure 1; it describes how all determinants of the metabolic syndrome increase their absolute value in the presence of a higher VAI (>2.5) [18].
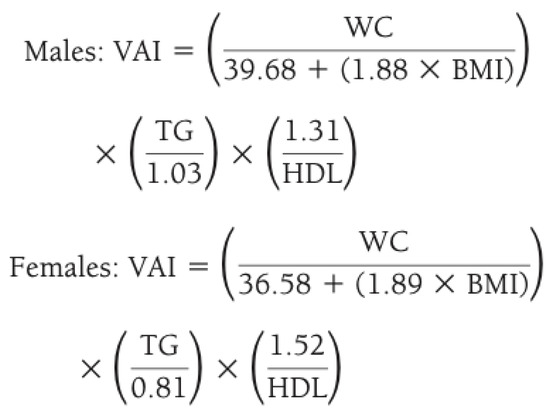 Figure 1. Method for calculating visceral adiposity index (VAI) by combining anthropometric and blood exams. Legend: WC waist circumference; BMI body mass index; TG triglycerides; HDL high density lipoprotein.
Figure 1. Method for calculating visceral adiposity index (VAI) by combining anthropometric and blood exams. Legend: WC waist circumference; BMI body mass index; TG triglycerides; HDL high density lipoprotein.
- -
- Lipid accumulation product index (LAP) [19], calculated with the following formula: (LAP = (WC (cm) − 65) × TG (mmol/L)) for males, and (LAP = (WC (cm) − 58) × TG (mmol/L)) for females; we usually consider ‘pathological’ an LAP > 30; in adults it could be considered ‘pathological’ if higher than 56.7 for men and higher than 30.4 for women [20].
- -
- Waist/hip ratio (WHR) [21], calculated with the following formula: waist circumference (cm)/hip circumference (cm); its increase is associated with higher cardiovascular risk in both men and women [22], and is generally considered ‘pathological’ when exceeding the 95th percentile for age and sex, as there is no unique and universally accepted cut-off value for all clinical contexts.
- -
- Waist/height ratio (WHtR) [23], calculated with the following formula: waist circumference (cm)/height (cm); usually, a value > 0.5 is associated with obesity [24].
- -
- Fatty liver index (FLI) [25], an algorithm used to estimate the presence of fatty liver disease and hepatic steatosis: it is calculated using the formula reported in Figure 2, that incorporates several parameters such as BMI, waist circumference, triglycerides, and gamma-glutamyl transferase (GGT) levels. In pediatric patients, an FLI exceeding 30 is considered pathological, as it is associated with hepatic steatosis and increased cardiovascular risk.
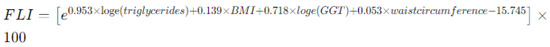 Figure 2. Method for calculating fatty liver index (FLI) by combining anthropometric and blood exams. Legend: BMI body mass index, GGT gamma-glutamyl transpeptidase.
Figure 2. Method for calculating fatty liver index (FLI) by combining anthropometric and blood exams. Legend: BMI body mass index, GGT gamma-glutamyl transpeptidase.
- -
- Hepatic steatosis index (HSI) [26], calculated using a formula based on BMI, waist circumference, and serum levels of AST (aspartate aminotransferase) and ALT (alanine aminotransferase), as reported in Figure 3. Similar to the FLI, an HSI > 30 is considered associated with hepatic steatosis and increased cardiovascular risk.
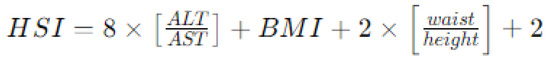 Figure 3. Method for calculating hepatic steatosis index (HSI) by combining anthropometric and blood exams. Legend: ALT aspartate aminotransferase, ALT alanine aminotransferase; BMI body mass index.
Figure 3. Method for calculating hepatic steatosis index (HSI) by combining anthropometric and blood exams. Legend: ALT aspartate aminotransferase, ALT alanine aminotransferase; BMI body mass index.
- -
- Alanine aminotransferase/aspartate aminotransferase (AST/ALT) ratio, primarily studied in rheumatologic diseases to predict the risk of poor response to pharmacological treatments and risk of coronary artery damage [27,28]; in this context, a ratio above 1 is considered elevated.
- -
- Atherogenic index of plasma (AIP) [29,30], used to assess the risk of cardiovascular diseases, based on lipid levels in the blood and calculated as follows: [AIP = log10 (triglyceride/HDL cholesterol)]. In our study, we considered a cut-off of 0.1 to identify patients with increased cardiovascular risk.
- -
- In addition to the aforementioned indices, we also assessed, for each patient, the association between hyperinsulinemia and presence of elevated blood pressure (above the 95th percentile) [31] and hepatic steatosis, evaluated by abdominal ultrasound.
2.3. Statistical Analysis
The statistical analysis was performed using IBM SPSS software (version 20). Continuous normally distributed variables were described as mean values ± standard deviation (SD), the other continuous data as median ± interquartile range (IQR). To describe dichotomous data, we used numerosity and percentages. Groups of continuous variables were compared with Student t-test (normally distributed data) and Mann–Whitney U Test (not normally distributed data). Dichotomous variables were compared with Chi Square test or Fisher exact test (if numerosity of one of the compared groups was less than 6). We performed a linear logistic regression with continuous variables that reached a p value less than 0.2 at the univariate analysis. We calculated an ROC curve for those variables that resulted independently associated with hyperinsulinemia at the linear regression and calculated the AUC to determine their accuracy in predicting hyperinsulinism (insulin peak above 100 uIU/mL). ROC curves were also used to choose the best cut-off values for all variables using the Youden index. We used the calculated cut-off to transform the continuous dichotomous variables. Finally, we performed a multiple logistic regression adjusted for sex and age to identify the variables independently associated with hyperinsulinism. A p < 0.05 was considered statistically significant.
2.4. Ethical Approval
Ethics committee approval was not obtained because the General Authorization to Process Personal Data for Scientific Research Purposes (Authorization No. 9/2014) states that retrospective archival studies using ID codes that prevent direct tracing of data to the subject do not require a formal ethics approval. However, all parents of recruited patients were informed about the purpose of this study and signed an informed written consent for authorizing the access to children’s medical records and processing their personal data. Of all patients considered, none refused to participate to our study.
3. Results
We enrolled 139 children (58 males, 81 females) with a mean age of 12.1 ± 2.9 years and a mean body weight of 65.21 ± 19.3 kg. Out of the 139 children analyzed, 95 (68.35%) showed a peak insulin level > 100 uIU/mL during the OGTT, while in 44 cases (31.65%) the suspected hyperinsulinemia was not confirmed. All children included in this study exhibited normal renal function, assessed through measurements of serum creatinine and calculation of the estimated glomerular filtration rate. The general characteristics of our cohort are summarized in Table 1.

Table 1.
Anthropometric and laboratory characteristics of patients with suspected hyperinsulinemia, confirmed or not confirmed following the OGTT.
All our patients were considered to have acquired hyperinsulinism, related to unhealthy dietary habits or unhealthy lifestyles. In none of the cases it was congenital, as signs and/or symptoms of neonatal hypoglycemia and other suggestive criteria for inherited conditions were absent [29]; therefore, genetic testing assays were not performed.
Patients with pathological OGTT were considered to be at increased cardiovascular risk; therefore, we calculated the various indices, previously described, assessing differences between patients with normal insulinemic response and those with insulinemia > 100 uIU/mL.
Comparing the two groups, we found statistically significant differences in weight, BMI, WC, HC, IGF-1, uric acid, VLDL, triglycerides, and basal insulin levels; p values are reported in Table 1.
After analyzing auxological and laboratory data, we calculated the previously described cardiovascular risk indices for each patient to assess differences between confirmed hyperinsulinemic and non-hyperinsulinemic patients. Comparing the two groups, we found statistically significant differences in HOMA-IR, TyG, TyG-BMI, VAI, LAP, FLI, HIS, and Triglycerides/HDL, while WHtR and WHR did not differ significantly. All p values are reported in Table 2.

Table 2.
Comparison of the main cardiovascular risk indices between the groups of patients with suspected hyperinsulinemia, confirmed or not, following the OGTT.
At the linear logistic regression, we found that IGF-1, HOMA-IR, and ALT/AST ratio were independently associated with a confirmed hyperinsulinism (Table 3).

Table 3.
Parameters associated with hyperinsulinemia based on the logistic linear regression analysis.
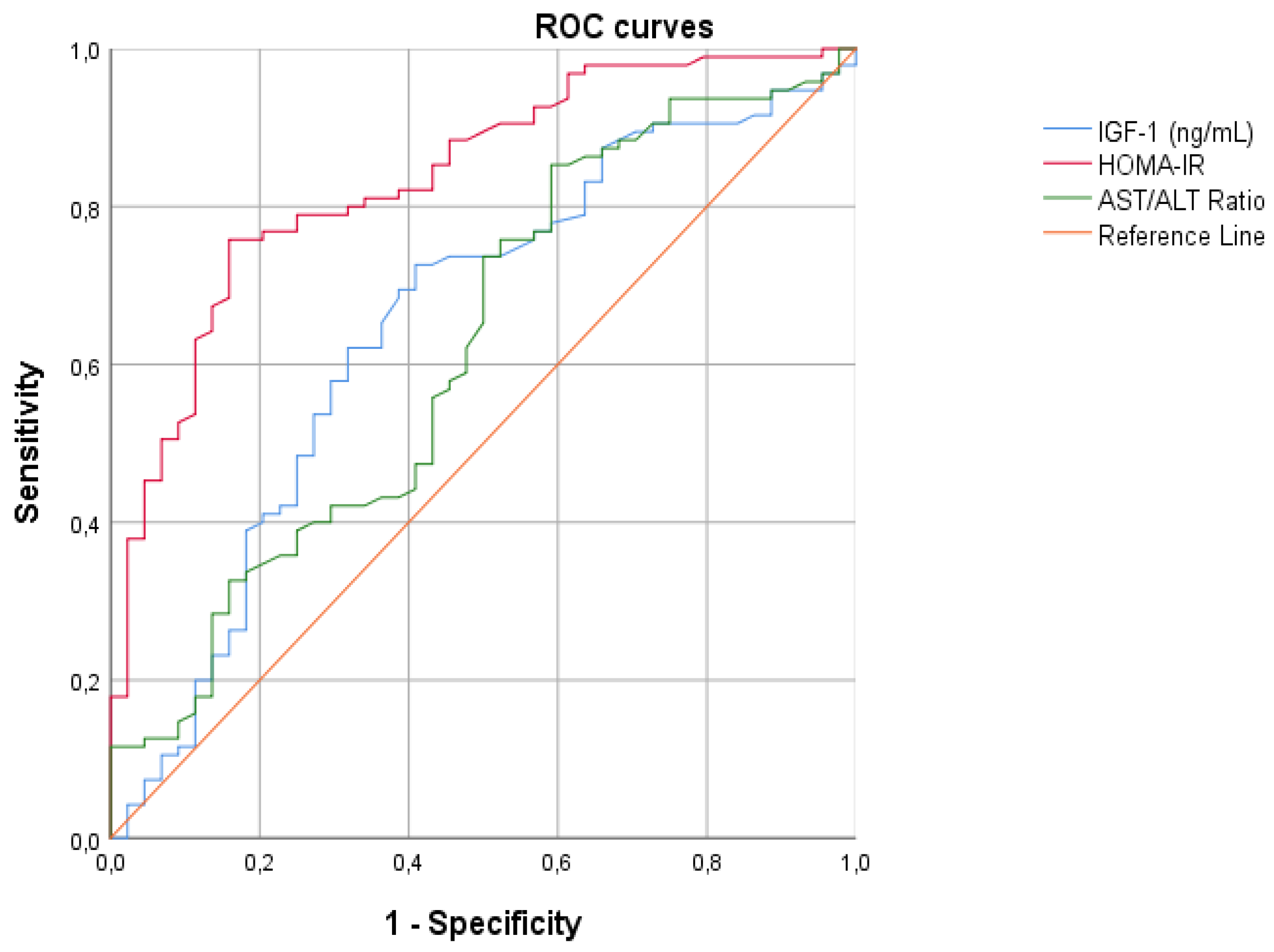
For all continuous variables correlated with hyperinsulinism, we created the ROC curves with the aim of finding the best cut-off points to differentiate patients with hyperinsulinemia from those without, and calculate the diagnostic accuracy using AUCs (Figure 4). From our analysis, HOMA-IR emerged as the best parameter for diagnosing hyperinsulinism (Table 4).
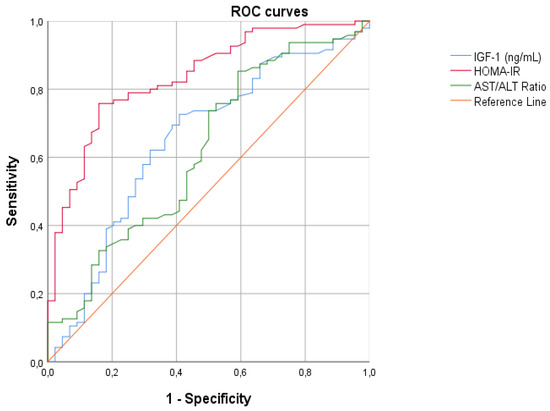
Figure 4.
ROC curves assessing the cardiovascular risk and laboratory parameters of patients. Legend: IGF-1 insulin growth factor-1; HOMA-IR homeostasis model assessment of insulin resistance index; AST/ALT ratio alanine aminotransferase/aspartate aminotransferase ratio.

Table 4.
Diagnostic accuracy (measured as AUC—area under the curve) for indirect indices and laboratory parameters of cardiovascular risk that resulted associated with hyperinsulinism at the linear logistic regression.
Thereafter, we calculated the optimal cut-off values of those parameters that were independently associated with hyperinsulinism at the linear logistic regression (Table 5).

Table 5.
Best cut-off values evaluated by means of the Youden index and multiple logistic regression analysis adjusted for age and sex to evaluate the association of laboratory parameters and indices of cardiovascular risk in our cohort of children with hyperinsulinism.
Using the identified cut-off, we transformed the variables from continuous to dichotomous, and performed a multinomial logistic regression, adjusting our data for sex and age. IGF-1 levels higher than 203 ng/mL and HOMA-IR values higher than 6.2 were respectively associated with a 9-times and 18-times higher risk of confirmed hyperinsulinism, compared to those with IGF levels less than 203 and HOMA-IR less than 6.2. The other investigated parameters were not significantly related to hyperinsulinism, and based on the results of our statistical analysis, they could not predict the presence of hyperinsulinemia in our cohort.
All analyzed children underwent a comprehensive endocrinological follow-up and are currently being monitored at our center; during the follow-up period, none of the patients experienced major cardiovascular events.
Given the results obtained and considering that it was not possible to match patients by sex and age due to sample size, we conducted subgroup analyses to assess whether differences in sex and age (prepubertal vs. pubertal patients, using 9 years as the age limit) could act as confounding factors for hyperinsulinemia.
When comparing sex in our populations in the univariate analysis, we observed no differences regarding the mean age. Furthermore, it appears that a smaller number of male subjects had IGF-1 values > 203: 27/58 (47%) vs. 60/81 (74%); p < 0.0001. A similar but non-significant trend was also observed for LAP > 15.9, although it did not reach a statistical significance (p = 0.08). No significant difference was found between sexes for the prevalence of hyperinsulinemia (p = 0.17).
Conducting a multivariate analysis including IGF-1 and LAP and correcting for age, we confirmed that IGF-1 values > 203 were more strongly associated with females, while LAP was not significantly associated with patients’ sex.
In the univariate analysis, it was found that patients over 9 years were more likely to have BMI < 25.72, WC > 82.5, IGF-1 > 203, HOMA-IR > 2.62, TyG-BMI > 131, LAP > 0.02, and WHR > 0.03. We also observed a trend, although not statistically significant, towards a higher likelihood of hyperinsulinemia (p = 0.06). We also performed a multivariate analysis correcting for sex and including all factors that were associated with age > 9 years in the univariate analysis with a significative p value: at the multivariate analysis, we confirmed that BMI, IGF-1, and WHR were the only factors associated with age > 9 years after correction for confounding factors.
Finally, we conducted further subgroup analyses to evaluate which indices were correlated with insulin resistance in females, in males, and in those aged more or less than 9 years. In female patients, at the multivariate analysis, correcting for age and including factors with a significative p value at the univariate analysis, we found that only HOMA-IR was associated with hyperinsulinemia. We repeated the same analysis in male patients, finding that only IGF-1 was associated with insulin resistance. In patients under 9 years, only HOMA-IR was statistically associated with insulin resistance, while in the subgroup over 9 years, only IGF-1 and HOMA-IR were effectively associated with hyperinsulinemia.
4. Discussion
The incidence of obesity in childhood is currently increasing, potentially leading to overt psychophysical issues during school age and several medical diseases in early adulthood [30,31,32,33,34]. In Italy, approximately 21.3% and 9.3% of school-aged children are classified as overweight and obese, respectively [34]. The Position Paper of the European Childhood Obesity Group and the European Academy of Pediatrics state that the main cause of overweight in pediatric age is the lack of a regular physical activity. Therefore, public health interventions are needed since school age to increase the level of physical activity and reduce a potential insulin resistance with hyperinsulinemia [35,36].
The increasing rate of obesity and the ever-growing healthcare expenditure to treat overweight-related complications make early diagnosis and appropriate prevention strategies imperative even during childhood. A recent consensus position statement of the Italian Society of Pediatric Endocrinology and diabetology, Italian Society of Pediatrics, and Italian Society of Pediatric Surgery has highlighted that a healthy lifestyle, a balanced diet, and a regular physical activity are milestones for managing pediatric patients with obesity, while pharmacological therapies should be introduced as second-line tools, and bariatric surgery reserved for those adolescents with severe obesity resistant to all other previous treatments [37]. Childhood obesity contributes to the development of early onset-cardiovascular diseases, though children can develop heart diseases due to other severe health issues, including Kawasaki disease or acute rheumatic fever, and if affected by peculiar lysosomal storage, disorders involving the heart [37,38,39,40,41,42]. The specific interaction of dietary macronutrients and the endocrine system might have a role in the etiology of obesity, and different genetic disorders display subverted processing of lipids following mutations in several genes involved in autoinflammation, as for children with mevalonate kinase deficiency [41]. Indeed, childhood obesity is related to a powerful array of cardiovascular risk factors, with increased triglycerides and increased lipoproteins being among the most relevant players, even if hypertriglyceridemia can be also a marker of systemic fatal complications in many pediatric diseases [40]. Hyperinsulinemia is an independent cardiovascular risk factor; therefore, identifying indices that can predict it in the pediatric population is crucial for the development of effective preventive strategies [1,3]; physical exercise training, particularly aerobic, should be capable of normalizing both auxological parameters related to overweight and obesity as well as improving insulin resistance in overweight or obese children and adolescents, though dietary interventions remain crucial [37].
During pediatric age, hyperinsulinemia can have a genetic basis, being caused by molecular alterations in insulin secretion or in insulin receptor response; these alterations can be classified as isolated hyperinsulinemia or as part of the clinical presentation of various genetic syndromes [43]. However, congenital hyperinsulinemia is a rare condition, and most of the elevated insulin peaks detected during OGTT might be explained by improper dietary habits and unhealthy lifestyles [8,34]; the bidirectional relationship between obesity and hyperinsulinemia is well established in the scientific literature. Obesity contributes to the development of insulin resistance, leading to compensatory hyperinsulinemia as the body attempts to maintain normal blood glucose levels [44]. Conversely, hyperinsulinemia promotes weight gain by stimulating lipogenesis and inhibiting lipolysis, thereby exacerbating the obesity phenotype [45]. This reciprocal interaction forms a vicious cycle, perpetuating both conditions. Different studies have elucidated this complex interplay, highlighting the pivotal role of hyperinsulinemia in the pathogenesis of obesity and vice versa [43,44,45,46].
The exact significance of hyperinsulinemia in pediatric patients is controversial, although it is a well-validated cardiovascular risk factor: in fact, several studies have highlighted that increased insulin secretion is not necessarily associated with weight gain or worsening BMI over time [47]. Moreover, elevated insulin levels have been shown to correlate with decreased cardiac diastolic function in the complete absence of any clinical symptoms [48].
In our study, we have applied various cardiovascular risk indices commonly used in adulthood using patients’ auxological parameters and their blood tests to assess their diagnostic accuracy in the pediatric age: our results have shown that these indices, with the exception of HOMA-IR, are not significantly associated with hyperinsulinemia in children and adolescents, and that they could not predict the risk of developing a metabolic syndrome or further cardiovascular comorbidities in childhood.
HOMA-IR has been proven to be an excellent marker of hyperinsulinemia in the pediatric age: the higher predictive value of HOMA-IR is associated with a close relationship between basal and stimulated insulin level after OGTT [49], as clearly shown by several studies [11]. Our statistical analysis has revealed that HOMA-IR is the only index that confirms its diagnostic accuracy in diagnosing hyperinsulinemia for both children and adolescents. Therefore, it could be applied to estimate the cardiovascular risk in this category of patients.
A recent study [50] analyzed 3203 Chinese children aged 6 to 18 years, determining the best predictive cut-offs for metabolic syndrome: the authors highlighted that the optimal HOMA-IR cut-off for diagnosis was 2.3 in the total participants, 1.7 in prepubertal children, and 2.6 in pubertal adolescents (>9 years); moreover, 44.3% of obese patients had values > 3. Our statistical analysis confirmed these results: indeed, in our population, the optimal cut-off was found to be 2.62, while the multivariate analysis showed that HOMA-IR > 6.2 was associated with risk of hyperinsulinemia approximately 18 times higher.
In addition, the importance of HOMA-IR lies in its ability to provide a quick and immediate estimate of insulin secretion and insulin resistance without resorting to investigative procedures like the OGTT [49], which are not always easily performed in the pediatric age [8]. This explains why this index is one of the most commonly used in clinical practice, widely employed even in the pediatric population.
Early diagnosis of hyperinsulinemia is crucial to enable early treatment with lifestyle modifications and improved dietary balance [35,36]. Indeed, while increased insulin levels are a well-established cardiovascular risk factor in adults, an elevation in insulin levels during pediatric age leads to worsened long-term outcomes. A recent study (The Pune Children’s Study) [51] examined the influence of glycemia, insulin, and HOMA-IR in a cohort of 8-year-old children, re-evaluated at the age of 21. The authors highlighted that values observed during pediatric age were statistically correlated with those measured in young adults. Furthermore, the authors found that higher levels of HOMA-IR were associated with a worse cardiovascular risk profile (assessed through measurement of blood pressure, plasma lipids, carotid intima-media thickness, and arterial pulse wave velocity). In fact, the authors showed that prepubertal glucose and insulin metabolism were associated with abnormal markers of atherosclerosis and early cardiovascular risk.
Less information is available about the application of other cardiovascular risk indices in the pediatric age, because they were only validated in the adult population and mostly applied for research purposes.
Regarding TyG, an analysis conducted on 367 children and adolescents showed that the cut-off of 7.96 had the best sensitivity and specificity (65% and 58%, respectively) for diagnosing insulin resistance, confirmed by HOMA-IR [52]. This cut-off was much higher than the one of 4.5 used for adults, which had higher rates of concordance. Our analysis did not confirm the triglyceride/HDL ratio as an index of hyperinsulinemia and insulin resistance. However, the study by de Giorgis et al. [14] highlighted that an alteration in this parameter could lead to changes in carotid intima-media thickness. Increasing our sample size may yield different results. Despite these considerations, the triglyceride/HDL ratio is a well-validated cardiovascular risk index [15] and should be assessed in patients at risk of developing metabolic syndrome, such as children with hyperinsulinemia [1,4]. VAI is a further parameter related to auxological and laboratory parameters, which can be considered an indirect index of dysfunction of the endocrine adipose tissue, identifying a higher risk of developing diabetes [53]. Amato et al. conducted a study on 1764 adult patients, calculating VAI and highlighting that a cut-off of 2.52 in subjects under 30 years had a sensitivity of 100% and specificity of 99.45% to identify adipose tissue dysfunction and cardiometabolic risk, while the optimal cut-off decreased with increasing age [54]. Vizzuso et al. have, however, demonstrated that the optimal cut-off for VAI in obese pediatric patients (with a mean age of 11 years) is 1.775, with differences between males (lower indices) and females [55]. Our statistical analysis has highlighted that the best cut-off for VAI was 1.66, confirming previous authors’ data.
LAP is another index that combines auxological parameters and laboratory data, estimating the accumulation of adipose tissue in the body, showing an excellent prediction of non-alcoholic fatty liver disease (NAFLD) in adults [56]. In childhood, there is limited evidence regarding this correlation, but the only published pediatric study analyzed 80 patients diagnosed with obesity, undergoing liver ultrasound screening for NAFLD; the authors demonstrated that LAP > 42.7 had a sensitivity of 53.7% and specificity of 84.6%, respectively, for diagnosing NAFLD [57]. Although our statistical analysis did not consider the correlation between LAP and steatosis, this index was not associated with hyperinsulinemia in our cohort; the best cut-off resulting from our multivariate analysis was 15.9, which should not allow a diagnosis of hepatic steatosis. In our cohort, both WHtR and WHR were also found inadequate to differentiate patients with or without hyperinsulinemia. In our multivariate analysis, WHR was not associated with insulin resistance. However, it is possible that our sample (139 children with suspected hyperinsulinemia) may not be sufficiently large for a proper validation of this index, as studies on numerous pediatric patients with comorbidities have shown that WHR is excellent to assess the cardiovascular risk if children have comorbidities [58], predicting the risk of developing a metabolic syndrome [24].
FLI and HSI are two commonly used clinical indices associated with metabolic syndrome and hepatic steatosis [25,26]. In a study conducted on 95 children aged between 5 and 15 years, all with BMI above the normal upper limits (>85th percentile corrected for sex and age), a high concordance between FLI and hepatic steatosis was found and confirmed by ultrasound in 36 children, with an AUROC of the FLI for predicting NAFLD of 0.692 (95% CI: 0.565–0.786); the cut-off of 30, analogous to that used in our study, showed sensitivity values of 58.3% and specificity of 69.4% [25]. Our multivariate analysis did not confirm the diagnostic accuracy of these two indices in assessing the risk of hyperinsulinemia; however, the correlation with hepatic steatosis is widely demonstrated in the medical literature. Therefore, although they are not associated with insulin dysregulation, both could be used clinically to study the cardiovascular risk in pediatric patients.
The measurement of transaminases is also included in the most commonly used screening hematochemical tests in clinical practice. The AST/ALT ratio has been proven to be an effective index in predicting the risk of coronary artery disease in patients with Kawasaki disease [27]. Additionally, studies conducted on adult patients have shown a higher correlation between this index and a diagnosis of hyperinsulinemia [28]. In our cohort of pediatric patients with hyperinsulinemia, we did not confirm this finding. This could be because hyperinsulinemia is not necessarily associated with hepatic steatosis [6], and it is possible that an increase in transaminases is only evident in more severe forms.
AIP is another marker currently undergoing validation in the pediatric population. Dag et al. [30] analyzed 136 adolescents (83 obese and 53 healthy controls) aged between 10 and 17 years, demonstrating that this index is higher in patients with hepatic steatosis and increased BMI compared to healthy controls. The same authors highlighted a significant statistical relationship between AIP and basal insulin, identifying this index as a potential marker for hyperinsulinemia. Our statistical analysis did not confirm this result, despite the sample size of our study; it is possible that by increasing the sample size, different results may be obtained, and future analyses are necessary to confirm the clinical relevance of this index.
The significant association between IGF-1 and hyperinsulinemia is already known, as they are closely related in intracellular signaling pathways: normal levels of IGF-1 contribute to the normal development and function of the cardiovascular system [59]. In our sample, an IGF-1 level higher than 203 was found to be associated with a 9-fold increased risk of hyperinsulinemia. Nevertheless, IGF-1 is not frequently measured in obese patients, and its use is largely referred to linear growth assessment and evaluation of growth hormone activity [60]. Despite this, a recent systematic review has analyzed the relationship among IGF-1, growth hormone, and obesity, highlighting that a modulation of these hormones could prevent the progression of metabolic syndrome and associated cardiovascular complications [61]. The development of pediatric cardiovascular risk indices might take into consideration this parameter combined with other anthropometric measurements.
Of course, our study has some limitations. Firstly, it is a retrospective single-center study, although our conclusions lay the groundwork for larger-scale prospective studies. The sample size is another limitation, so it is possible that the results of our statistical analysis may not be applicable to the general population. Despite these considerations, our study also has some strengths. We have contributed to defining possible cut-off values for common cardiovascular risk indices in a cohort of children and adolescents under 18 years. Currently, there are no well-defined normal ranges for these indices in the pediatric population. Furthermore, the OGTT is the gold standard for assessing hyperinsulinemia, not only in pediatric patients but also in adults. The implementation of specific cardiovascular risk indices for the pediatric population may, in the future, estimate the prevalence of hyperinsulinemia under 18 years, rendering unnecessary the execution of OGTT.
5. Conclusions
In our pediatric cohort of patients with suspected hyperinsulinism, the commonly used indirect indices of cardiovascular risk in the adult population, excluding HOMA-IR, were not accurate in confirming hyperinsulinemia. Comparing patients with and without hyperinsulinemia, we found statistically significant differences in weight, BMI, WC, HC, IGF-1, uric acid, VLDL, triglycerides, and basal insulin levels. The multivariate analysis highlighted that an IGF-1 level higher than 203 ng/mL and HOMA index higher than 6.2 are respectively associated with a 9-times and 18-times higher odds ratio for hyperinsulinism.
The results of our statistical analysis represent the starting point for future prospective studies to analyze the cardiovascular risk in pediatric patients displaying hyperinsulinemia and establish the optimal cut-offs of such indices.
Author Contributions
Conceptualization: C.C. and G.S.; methodology: G.S., D.R. and C.C.; formal analysis and investigation: M.C., L.C.P. and L.Q.; writing—original draft preparation: G.S.; writing—review and editing: L.C.P., L.Q. and L.S.; supervision: D.R., M.C. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics committee approval was not obtained because the General Authorization to Process Personal Data for Scientific Research Purposes (Authorization No. 9/2014) states that retrospective archival studies using ID codes that prevent direct tracing of data to the subject do not require a formal ethics approval.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- DeBoer, M.D. Assessing and managing the metabolic syndrome in children and adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch-Blüher, S.; Schwarz, P.; Klusmann, J.H. Childhood obesity: Increased risk for cardiometabolic disease and cancer in adulthood. Metabolism 2019, 92, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Han, J.C.; Lawlor, D.A.; Kimm, S.Y. Childhood obesity. Lancet 2010, 375, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Candelino, M.; Tagi, V.M.; Chiarelli, F. Cardiovascular risk in children: A burden for future generations. Ital. J. Pediatr. 2022, 48, 57. [Google Scholar] [CrossRef] [PubMed]
- Ighbariya, A.; Weiss, R. Insulin resistance, prediabetes, metabolic syndrome: What should every pediatrician know? J. Clin. Res. Pediatr. Endocrinol. 2017, 9 (Suppl. S2), 49–57. [Google Scholar] [CrossRef] [PubMed]
- Reckziegel, M.B.; Nepomuceno, P.; Machado, T.; Renner, J.D.P.; Pohl, H.H.; Nogueira-de-Almeida, C.A.; de Mello, E.D. The triglyceride-glucose index as an indicator of insulin resistance and cardiometabolic risk in Brazilian adolescents. Arch. Endocrinol. Metab. 2023, 67, 153–161. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.; Daar, S.; Tzoulis, P.; Di Maio, S.; Kattamis, C. Oral glucose tolerance test: How to maximize its diagnostic value in children and adolescents. Acta Biomed. 2022, 93, e2022318. [Google Scholar] [CrossRef] [PubMed]
- Sahin, N.M.; Kinik, S.T. Tekindal MAOGTT results in obese adolescents with normal HOMA-IR values. J. Pediatr. Endocrinol. Metab. 2013, 26, 285–291. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. S27), 7–19. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Song, P.; Xu, L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov. Ther. 2015, 9, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Sodero, G.; Pane, L.C.; Malavolta, E.; Rotunno, G.; Sessa, L.; Fraccascia, B.; Candelli, M.; Rigante, D.; Cipolla, C. Lipid Profile and Triglyceride-Glucose Index (TyG) Alterations in a Single-Center Cohort of Children Diagnosed with Central Precocious Puberty. Children 2024, 11, 639. [Google Scholar] [CrossRef]
- Ramdas Nayak, V.K.; Satheesh, P.; Shenoy, M.T.; Kalra, S. Triglyceride glucose (TyG) index: A surrogate biomarker of insulin resistance. J. Pak. Med. Assoc. 2022, 72, 986–988. [Google Scholar] [CrossRef]
- de Giorgis, T.; Marcovecchio, M.L.; Di Giovanni, I.; Giannini, C.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. Triglycerides-to-HDL ratio as a new marker of endothelial dysfunction in obese prepubertal children. Eur. J. Endocrinol. 2013, 170, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Valerio, G.; Grugni, G.; Licenziati, M.; Maffeis, C.; Manco, M.; del Giudice, E.M.; Pacifico, L.; Pellegrin, M.; Tomat, M.; et al. Comparison of non-HDL-cholesterol versus triglycerides-to-HDL-cholesterol ratio in relation to cardiometabolic risk factors and preclinical organ damage in overweight/obese children: The CARITALY study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Nilofer Sagana, M.K.; Arul Senghor, K.A.; Vinodhini, V.M.; Renuka, P. Irisin and triglyceride glucose index as markers of dyslipidemia in young adults. Indian. J. Clin. Biochem. 2024, 39, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, J.; Koo, S.H.; Kwon, G.C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS ONE 2019, 14, e0212963. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, Y.; Li, Y.; Wang, C.; Wang, Y.; Dong, M.; Xiao, J.; Lin, Z.; Lu, H.; Ji, X. Association between visceral adiposity index and heart failure: A cross-sectional study. Clin. Cardiol. 2023, 46, 310–319. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Seyedi, S.A.; Nabipoorashrafi, S.A.; Rabizadeh, S.; Sarzaeim, M.; Yadegar, A.; Mohammadi, F.; Bahri, R.A.; Pakravan, P.; Shafiekhani, P.; et al. Lipid accumulation product (LAP) index for the diagnosis of nonalcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Lipids Health Dis. 2023, 22, 41. [Google Scholar] [CrossRef]
- Ahn, N.; Baumeister, S.E.; Amann, U.; Rathmann, W.; Peters, A.; Huth, C.; Thorand, B.; Meisinger, C. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci. Rep. 2019, 9, 9693. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist: Hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- de Koning, L.; Merchant, A.T.; Pogue, J.; Anand, S.S. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: Meta-regression analysis of prospective studies. Eur. Heart J. 2007, 28, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Savva, S.C.; Lamnisos, D.; Kafatos, A.G. Predicting cardiometabolic risk: Waist-to-height ratio or BMI. A meta-analysis. Diabetes Metab. Syndr. Obes. 2013, 6, 403–419. [Google Scholar] [CrossRef] [PubMed]
- de Silva, M.H.A.D.; Hewawasam, R.P.; Kulatunge, C.R.; Chamika, R.M.A. The accuracy of fatty liver index for the screening of overweight and obese children for non-alcoholic fatty liver disease in resource limited settings. BMC Pediatr. 2022, 22, 511. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-H.; Hsieh, C.-S.; Lai, M.-W.; Chen, C.-C.; Chao, H.-C.; Yeh, H.-Y.; Lai, H.-H.; Tsui, P.-H. Detection of pediatric hepatic steatosis through ultrasound backscattering analysis. Eur. Radiol. 2021, 31, 3216–3225. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Ren, Y.; Shi, H.; Rong, X.; Zhang, X.; Shao, Y.; Wu, R.; Chu, M.; Qiu, H. Association between alanine aminotransferase/aspartate aminotransferase ratio (AST/ALT ratio) and coronary artery injury in children with Kawasaki disease. Cardiol. Res. Pract. 2020, 2020, 8743548. [Google Scholar] [CrossRef] [PubMed]
- Visaria, A.; Pai, S.; Cheung, M.; Ahlawat, S. Association between aspartate aminotransferase-to-alanine aminotransferase ratio and insulin resistance among US adults. Eur. J. Gastroenterol. Hepatol. 2022, 34, 316–323. [Google Scholar] [CrossRef]
- Nogay, N.H. Assessment of the correlation between the atherogenic index of plasma and cardiometabolic risk factors in children and adolescents: Might it be superior to the TG/HDL-C ratio? J. Pediatr. Endocrinol. Metab. 2017, 30, 947–955. [Google Scholar] [CrossRef]
- Dağ, H.; İncirkuş, F.; Dikker, O. Atherogenic index of plasma (AIP) and its association with fatty liver in obese adolescents. Children 2023, 10, 641. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; De Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, E.; De León, D.D. Bridging the gaps: Recent advances in diagnosis, care, and outcomes in congenital hyperinsulinism. Curr. Opin. Pediatr. 2023, 35, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.G. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J. Pediatr. 2016, 59, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Song, H.; Tian, Z.; Liu, Y. Prediction models for children/adolescents with obesity/overweight: A systematic review and meta-analysis. Prev. Med. 2024, 179, 107823. [Google Scholar] [CrossRef] [PubMed]
- Wyszynska, J.; Ring-Dimitriou, S.; Thivel, D.; Weghuber, D.; Hadjipanayis, A.; Grossman, Z.; Ross-Russell, R.; Deren, K.; Mazur, A. Physical activity in the prevention of childhood obesity: The position of the European Childhood Obesity Group and the European Academy of Pediatrics. Front. Pediatr. 2020, 8, 535705. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.; Cecchi, N.; Carbone, M.G.; Dinardo, M.; Gaudino, G.; Miraglia Del Giudice, E.; Umano, G.R. Pediatric obesity: Prevention is better than care. Ital. J. Pediatr. 2020, 46, 103. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Olivieri, F.; Valerio, G.; Verduci, E.; Licenziati, M.R.; Calcaterra, V.; Pelizzo, G.; Salerno, M.; Staiano, A.; Bernasconi, S.; et al. The treatment of obesity in children and adolescents: Consensus position statement of the Italian society of pediatric endocrinology and diabetology, Italian Society of Pediatrics and Italian Society of Pediatric Surgery. Ital. J. Pediatr. 2023, 49, 69. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, G.; Pardeo, M.; Rigante, D. Current recommendations for the pharmacological therapy in Kawasaki syndrome and management of its cardiovascular complications. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 301–308. [Google Scholar] [PubMed]
- Méndez Eirín, E.; Suárez Ouréns, Y.; Guerra Vázquez, J.L. Cardiac manifestations of rheumatic diseases. Med. Clin. 2021, 156, 615–621. [Google Scholar] [CrossRef]
- Rigante, D.; Segni, G. Cardiac structural involvement in mucopolysaccharidoses. Cardiology 2002, 98, 18–20. [Google Scholar] [CrossRef]
- Rigante, D.; Cantarini, L.; Imazio, M.; Lucherini, O.M.; Sacco, E.; Galeazzi, M.; Brizi, M.G.; Brucato, A. Autoinflammatory diseases and cardiovascular manifestations. Ann. Med. 2011, 43, 341–346. [Google Scholar] [CrossRef]
- Stabile, A.; Bertoni, B.; Ansuini, V.; La Torraca, I.; Sallì, A.; Rigante, D. The clinical spectrum and treatment options of macrophage activation syndrome in the pediatric age. Eur. Rev. Med. Pharmacol. Sci. 2006, 10, 53–59. [Google Scholar] [PubMed]
- Rosenfeld, E.; Ganguly, A.; De Leon, D.D. Congenital hyperinsulinism disorders: Genetic and clinical characteristics. Am. J. Med. Genet. C Semin. Med. Genet. 2019, 181, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef]
- Kazeminasab, F.; Sharafifard, F.; Miraghajani, M.; Behzadnejad, N.; Rosenkranz, S.K. The effects of exercise training on insulin resistance in children and adolescents with overweight or obesity: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1178376. [Google Scholar] [CrossRef] [PubMed]
- Halloun, R.; Galderisi, A.; Caprio, S.; Weiss, R. Lack of evidence for a causal role of hyperinsulinemia in the progression of obesity in children and adolescents: A longitudinal study. Diabetes Care 2022, 45, 1400–1407. [Google Scholar] [CrossRef]
- Burden, S.; Weedon, B.; Whaymand, L.; Rademaker, J.; Dawes, H.; Jones, A. The effect of overweight/obesity on diastolic function in children and adolescents: A meta-analysis. Clin. Obes. 2021, 11, e12476. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, C.; Lazzareschi, I.; Curatola, A.; Lasorella, C.; Pane, L.C.; Sessa, L.; Rotunno, G.; Rigante, D.; Sodero, G. Characteristics of children and adolescents with hyperinsulinemia undergoing oral glucose tolerance test: A single-center retrospective observational study. Diseases 2023, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, M.; Xu, L.; Wang, Y.; Cheng, H.; Zhao, X.; Mi, J. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. Diabetol. Metab. Syndr. 2013, 5, 71. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Katre, P.A.; Joshi, S.M.; Kumaran, K.; Bhat, D.S.; Lubree, H.G.; Memane, N.; Kinare, A.S.; Pandit, A.N.; Bhave, S.A.; et al. Higher glucose, insulin and insulin resistance (HOMA-IR) in childhood predict adverse cardiovascular risk in early adulthood: The Pune Children’s Study. Diabetologia 2015, 58, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Dikaiakou, E.; Vlachopapadopoulou, E.A.; Paschou, S.A.; Athanasouli, F.; Panagiotopoulos, Ι.; Kafetzi, M.; Fotinou, A.; Michalacos, S. Τriglycerides-glucose (TyG) index is a sensitive marker of insulin resistance in Greek children and adolescents. Endocrine 2020, 70, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, X.; Xue, H.; Wang, Y.; Shi, Z. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients 2019, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011, 10, 183. [Google Scholar] [CrossRef]
- Vizzuso, S.; Del Torto, A.; Dilillo, D.; Calcaterra, V.; Di Profio, E.; Leone, A.; Gilardini, L.; Bertoli, S.; Battezzati, A.; Zuccotti, G.V.; et al. Visceral adiposity index (VAI) in children and adolescents with obesity: No association with daily energy intake but promising tool to identify metabolic syndrome (MetS). Nutrients 2021, 13, 413. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Lu, S.; Xie, Q.; Peng, N.; Kuang, M.; Zou, Y. The usefulness of obesity and lipid-related indices to predict the presence of non-alcoholic fatty liver disease. Lipids Health Dis. 2021, 20, 134. [Google Scholar] [CrossRef]
- Özcabı, B.; Demirhan, S.; Akyol, M.; Öztürkmen Akay, H.; Güven, A. Lipid accumulation product is a predictor of nonalcoholic fatty liver disease in childhood obesity. Korean J. Pediatr. 2019, 62, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Sgambat, K.; Roem, J.; Mitsnefes, M.; Portale, A.A.; Furth, S.; Warady, B.; Moudgil, A. Waist-to-height ratio, body mass index, and cardiovascular risk profile in children with chronic kidney disease. Pediatr. Nephrol. 2018, 33, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Zafirovic, S.; Soskic, S.; Stanimirovic, J.; Trpkovic, A.; Jevremovic, D.; Isenovic, E.R. Effects of IGF-1 on the cardiovascular system. Curr. Pharm. Des. 2019, 25, 3715–3725. [Google Scholar] [CrossRef]
- Sodero, G.; Mariani, F.; Caprarelli, M.; Agazzi, C.; Quarta, L.; Benacquista, L.; Rigante, D.; Cipolla, C. Growth hormone responses during arginine and clonidine stimulation test: Correlations with patients’ auxological and metabolic parameters in a single centre study. Growth Horm. IGF Res. 2023, 68, 101522. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Radovick, S. Exploring the therapeutic potential of targeting GH and IGF-1 in the management of obesity: Insights from the interplay between these hormones and metabolism. Int. J. Mol. Sci. 2023, 24, 9556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).