Oocyte Maturation and miRNAs: Studying a Complicate Interaction to Reveal Possible Biomarkers for Female Infertility
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bendarska-Czerwinska, A.; Zmarzly, N.; Morawiec, E.; Panfil, A.; Brys, K.; Czarniecka, J.; Ostenda, A.; Dziobek, K.; Sagan, D.; Boron, D.; et al. Endocrine disorders and fertility and pregnancy: An update. Front. Endocrinol. 2022, 13, 970439. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.T.; Collins, J.; Egozcue, J.; Evers, L.H.; Gianaroli, L.; Leridon, H.; Sunde, A.; Templeton, A.; Van Steirteghem, A.; Cohen, J.; et al. Fertility and ageing. Hum. Reprod. Update 2005, 11, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar] [CrossRef] [PubMed]
- Vallee, M.; Gravel, C.; Palin, M.F.; Reghenas, H.; Stothard, P.; Wishart, D.S.; Sirard, M.A. Identification of novel and known oocyte-specific genes using complementary DNA subtraction and microarray analysis in three different species. Biol. Reprod. 2005, 73, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Dalbies-Tran, R.; Mermillod, P. Use of heterologous complementary DNA array screening to analyze bovine oocyte transcriptome and its evolution during in vitro maturation. Biol. Reprod. 2003, 68, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Braw-Tal, R. The initiation of follicle growth: The oocyte or the somatic cells? Mol. Cell. Endocrinol. 2002, 187, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Sabouni, R.; Cayton Vaught, K.C.; Owen, C.M.; Albertini, D.F.; Segars, J.H. Biomechanics and mechanical signaling in the ovary: A systematic review. J. Assist. Reprod. Genet. 2018, 35, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Dahl, K.D.; Czekala, N.M.; Hsueh, A.J. Estrogen-producing ovarian granulosa cells: Use of the granulosa cell aromatase bioassay (GAB) to monitor FSH levels in body fluids. Adv. Exp. Med. Biol. 1987, 219, 275–298. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Gonadotropin stimulation of the expansion of cumulus oophori isolated from mice: General conditions for expansion in vitro. J. Exp. Zool. 1979, 208, 111–120. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The role of miRNAs in regulating gene expression networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: MicroRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Glasner, M.E.; Yekta, S.; Burge, C.B.; Bartel, D.P. Vertebrate microRNA genes. Science 2003, 299, 1540. [Google Scholar] [CrossRef] [PubMed]
- Hutvagner, G.; McLachlan, J.; Pasquinelli, A.E.; Balint, E.; Tuschl, T.; Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001, 293, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Radmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Park, J.; Dang, T.L.; Choi, Y.G.; Kim, V.N. Microprocessor depends on hemin to recognize the apical loop of primary microRNA. Nucleic Acids Res. 2018, 46, 5726–5736. [Google Scholar] [CrossRef] [PubMed]
- Abd El Naby, W.S.; Hagos, T.H.; Hossain, M.M.; Salilew-Wondim, D.; Gad, A.Y.; Rings, F.; Cinar, M.U.; Tholen, E.; Looft, C.; Schellander, K.; et al. Expression analysis of regulatory microRNAs in bovine cumulus oocyte complex and preimplantation embryos. Zygote 2013, 21, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar] [PubMed]
- Dell’Aversana, C.; Cuomo, F.; Longobardi, S.; D‘Hooghe, T.; Caprio, F.; Franci, G.; Santonastaso, M.; Colacurci, N.; Barone, S.; Pisaturo, V.; et al. Age-related miRNome landscape of cumulus oophorus cells during controlled ovarian stimulation protocols in IVF cycles. Hum. Reprod. 2021, 36, 1310–1325. [Google Scholar] [CrossRef] [PubMed]
- Gonella-Diaza, A.M.; Lopes, E.; Ribeiro da Silva, K.; Perecin Nociti, R.; Mamede Andrade, G.; Atuesta-Bustos, J.E.; Coelho da Silveira, J.; Vieira Meirelles, F.; Binelli, M. Steroidal Regulation of Oviductal microRNAs Is Associated with microRNA-Processing in Beef Cows. Int. J. Mol. Sci. 2021, 22, 953. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Moya, J.M.; Vilella, F.; Simon, C. MicroRNA: Key gene expression regulators. Fertil. Steril. 2014, 101, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Soifer, H.S.; Rossi, J.J.; Saetrom, P. MicroRNAs in disease and potential therapeutic applications. Mol. Ther. 2007, 15, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Imbar, T.; Galliano, D.; Pellicer, A.; Laufer, N. Introduction: MicroRNAs in human reproduction: Small molecules with crucial regulatory roles. Fertil. Steril. 2014, 101, 1514–1515. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, W.J. Advance on Dicer gene and its role in female reproduction. Chin. J. Med. Genet. 2011, 28, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, H.; Sakurai, N.; Muto, N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: Role of cumulus cells. Biol. Reprod. 2000, 63, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Dompe, C.; Kulus, M.; Stefanska, K.; Kranc, W.; Chermula, B.; Bryl, R.; Pienkowski, W.; Nawrocki, M.J.; Petitte, J.N.; Stelmach, B.; et al. Human Granulosa Cells-Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- Jagarlamudi, K.; Rajkovic, A. Oogenesis: Transcriptional regulators and mouse models. Mol. Cell Endocrinol. 2012, 356, 31–39. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Veeramachaneni, D.N.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: A possible new form of cell communication within the ovarian follicle. Biol. Reprod. 2012, 86, 71. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Laukova, M.; Ovcharenko, D.; Brenaut, P.; Mlyncek, M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J. Cell. Physiol. 2010, 223, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.; Nunez, M.J.; Quinonero, A.; Martinez, S.; de la Orden, M.; Simon, C.; Pellicer, A.; Diaz-Garcia, C.; Dominguez, F. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil. Steril. 2015, 104, 1037–1046. [Google Scholar] [CrossRef]
- Santonocito, M.; Vento, M.; Guglielmino, M.R.; Battaglia, R.; Wahlgren, J.; Ragusa, M.; Barbagallo, D.; Borzi, P.; Rizzari, S.; Maugeri, M.; et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: Bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014, 102, 1751–1761.e1. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Lu, Y.; Li, L. MiRNA-451 is a potential biomarker for estrogenicity in mouse uterus. Front. Environ. Sci. Eng. 2014, 8, 99–105. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Peng, S.; Wu, L.; Lin, H.Y.; Wang, S.; Wang, H. Differentially expressed plasma microRNAs in premature ovarian failure patients and the potential regulatory function of mir-23a in granulosa cell apoptosis. Reproduction 2012, 144, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Assou, S.; Al-edani, T.; Haouzi, D.; Philippe, N.; Lecellier, C.H.; Piquemal, D.; Commes, T.; Ait-Ahmed, O.; Dechaud, H.; Hamamah, S. MicroRNAs: New candidates for the regulation of the human cumulus-oocyte complex. Hum. Reprod. 2013, 28, 3038–3049. [Google Scholar] [CrossRef] [PubMed]
- Andrei, D.; Nagy, R.A.; van Montfoort, A.; Tietge, U.; Terpstra, M.; Kok, K.; van den Berg, A.; Hoek, A.; Kluiver, J.; Donker, R. Differential miRNA Expression Profiles in Cumulus and Mural Granulosa Cells from Human Pre-ovulatory Follicles. Microrna 2019, 8, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, M.; Khodarahmi, P.; Tafvizi, F.; Bostanabad, S.Z. Evaluation of the potential of miR-21 as a diagnostic marker for oocyte maturity and embryo quality in women undergoing ICSI. Sci. Rep. 2023, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.P.; Ferreira, M.C.F.; Silveira, C.O.; Campos, J.R.; Borges, I.T.; Baeta, P.G.; Silva, F.H.S.; Reis, F.M.; Del Puerto, H.L. Clinical correlation of apoptosis in human granulosa cells-A review. Cell Biol. Int. 2018, 42, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Carletti, M.Z.; Fiedler, S.D.; Christenson, L.K. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol. Reprod. 2010, 83, 286–295. [Google Scholar] [CrossRef]
- Han, X.; Xue, R.; Yuan, H.J.; Wang, T.Y.; Lin, J.; Zhang, J.; Liang, B.; Tan, J.H. MicroRNA-21 plays a pivotal role in the oocyte-secreted factor-induced suppression of cumulus cell apoptosis. Biol. Reprod. 2017, 96, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, A.F.; Uliasz, T.; Peluso, J.J. MicroRNA-21 as a regulator of human cumulus cell viability and its potential influence on the developmental potential of the oocyte. Biol. Reprod. 2020, 103, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Gaia-Oltean, A.I.; Braicu, C.; Gulei, D.; Ciortea, R.; Mihu, D.; Roman, H.; Irimie, A.; Berindan-Neagoe, I. Ovarian endometriosis, a precursor of ovarian cancer: Histological aspects, gene expression and microRNA alterations (Review). Exp. Ther. Med. 2021, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lv, J.; Tang, R.; Feng, Y.; Zhao, Y.; Fei, X.; Chian, R.; Xie, Q. Association of exosomal microRNAs in human ovarian follicular fluid with oocyte quality. Biochem. Biophys. Res. Commun. 2021, 534, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Muti, P.; Donzelli, S.; Sacconi, A.; Hossain, A.; Ganci, F.; Frixa, T.; Sieri, S.; Krogh, V.; Berrino, F.; Biagioni, F.; et al. MiRNA-513a-5p inhibits progesterone receptor expression and constitutes a risk factor for breast cancer: The hOrmone and Diet in the ETiology of breast cancer prospective study. Carcinogenesis 2018, 39, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tong, L.; Wang, M.; Chang, X.; Wang, S.; Li, K.; Xiao, J. miR-505-3p is a repressor of the puberty onset in female mice. J. Endocrinol. 2018, 240, 279–392. [Google Scholar] [CrossRef] [PubMed]
- Barragan, M.; Pons, J.; Ferrer-Vaquer, A.; Cornet-Bartolome, D.; Schweitzer, A.; Hubbard, J.; Auer, H.; Rodolosse, A.; Vassena, R. The transcriptome of human oocytes is related to age and ovarian reserve. Mol. Hum. Reprod. 2017, 23, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Sang, Q.; Zhu, Y.; Fu, W.; Liu, M.; Xu, Y.; Shi, H.; Xu, Y.; Qu, R.; Chai, R.; et al. MiRNA-320 in the human follicular fluid is associated with embryo quality in vivo and affects mouse embryonic development in vitro. Sci. Rep. 2015, 5, 8689. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Rodosthenous, R.S.; Adir, M.; Mansour, A.; Racowsky, C.; Baccarelli, A.A.; Hauser, R. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: An exploratory study. J. Assist. Reprod. Genet. 2017, 34, 525–533. [Google Scholar] [CrossRef]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Hsieh, M.; Zamah, A.M.; Oh, J.S. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol. Cell. Endocrinol. 2012, 356, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Al-Edani, T.; Assou, S.; Ferrieres, A.; Bringer Deutsch, S.; Gala, A.; Lecellier, C.H.; Ait-Ahmed, O.; Hamamah, S. Female aging alters expression of human cumulus cells genes that are essential for oocyte quality. Biomed. Res. Int. 2014, 2014, 964614. [Google Scholar] [CrossRef]
- Barragan, M.; Cornet-Bartolome, D.; Molina, N.; Vassena, R. The expression levels of NOS2, HMOX1, and VEGFC in cumulus cells are markers of oocyte maturation and fertilization rate. Mol. Reprod. Dev. 2023, 90, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, L.; Fang, T.; Zhang, Q.; Wu, S.; Jiang, Y.; Sun, H.; Hu, Y. MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett. 2012, 586, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, W.; Yao, Y.; Du, X.; Zhou, J.; Ma, B.; Liu, H.; Li, Q.; Pan, Z. MiR-92a inhibits porcine ovarian granulosa cell apoptosis by targeting Smad7 gene. FEBS Lett. 2014, 588, 4497–4503. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.B.; Tesfaye, D.; Rings, F.; Hossien, M.; Hoelker, M.; Held, E.; Neuhoff, C.; Tholen, E.; Schellander, K.; Salilew-Wondim, D. MicroRNA-130b is involved in bovine granulosa and cumulus cells function, oocyte maturation and blastocyst formation. J. Ovarian Res. 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wu, W.; Zhou, X.; Liu, K.; Li, B.; Huang, X.; Zhang, Y.; Liu, H. Let-7g induces granulosa cell apoptosis by targeting MAP3K1 in the porcine ovary. Int. J. Biochem. Cell Biol. 2015, 68, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Mu, H.; Mei, Q.; Liu, Y.; Min, Z.; Zhang, L.; Su, P.; Xiang, W. Mir-484 contributes to diminished ovarian reserve by regulating granulosa cell function via YAP1-mediated mitochondrial function and apoptosis. Int. J. Biol. Sci. 2022, 18, 1008–1021. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod. Fertil. Dev. 1996, 8, 485–489. [Google Scholar] [CrossRef]
- Li, L.; Zheng, P.; Dean, J. Maternal control of early mouse development. Development 2010, 137, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Zheng, M.; Hayashi, M.; Lee, J.D.; Yoshino, O.; Lin, S.; Han, J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J. Clin. Investig. 2008, 118, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Kim, S.Y.; Carmell, M.A.; Murchison, E.P.; Alcorn, H.; Li, M.Z.; Mills, A.A.; Elledge, S.J.; Anderson, K.V.; Hannon, G.J. Dicer is essential for mouse development. Nat. Genet. 2003, 35, 215–217. [Google Scholar] [CrossRef]
- Yang, W.J.; Yang, D.D.; Na, S.; Sandusky, G.E.; Zhang, Q.; Zhao, G. Dicer is required for embryonic angiogenesis during mouse development. J. Biol. Chem. 2005, 280, 9330–9335. [Google Scholar] [CrossRef]
- Murchison, E.P.; Stein, P.; Xuan, Z.; Pan, H.; Zhang, M.Q.; Schultz, R.M.; Hannon, G.J. Critical roles for Dicer in the female germline. Genes Dev. 2007, 21, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Kaneda, M.; O‘Carroll, D.; Hajkova, P.; Barton, S.C.; Sun, Y.A.; Lee, C.; Tarakhovsky, A.; Lao, K.; Surani, M.A. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007, 21, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Tang, Y.; He, Z.; Rosenwaks, Z. Dicer is a key player in oocyte maturation. J. Assist. Reprod. Genet. 2010, 27, 571–580. [Google Scholar] [CrossRef] [PubMed]
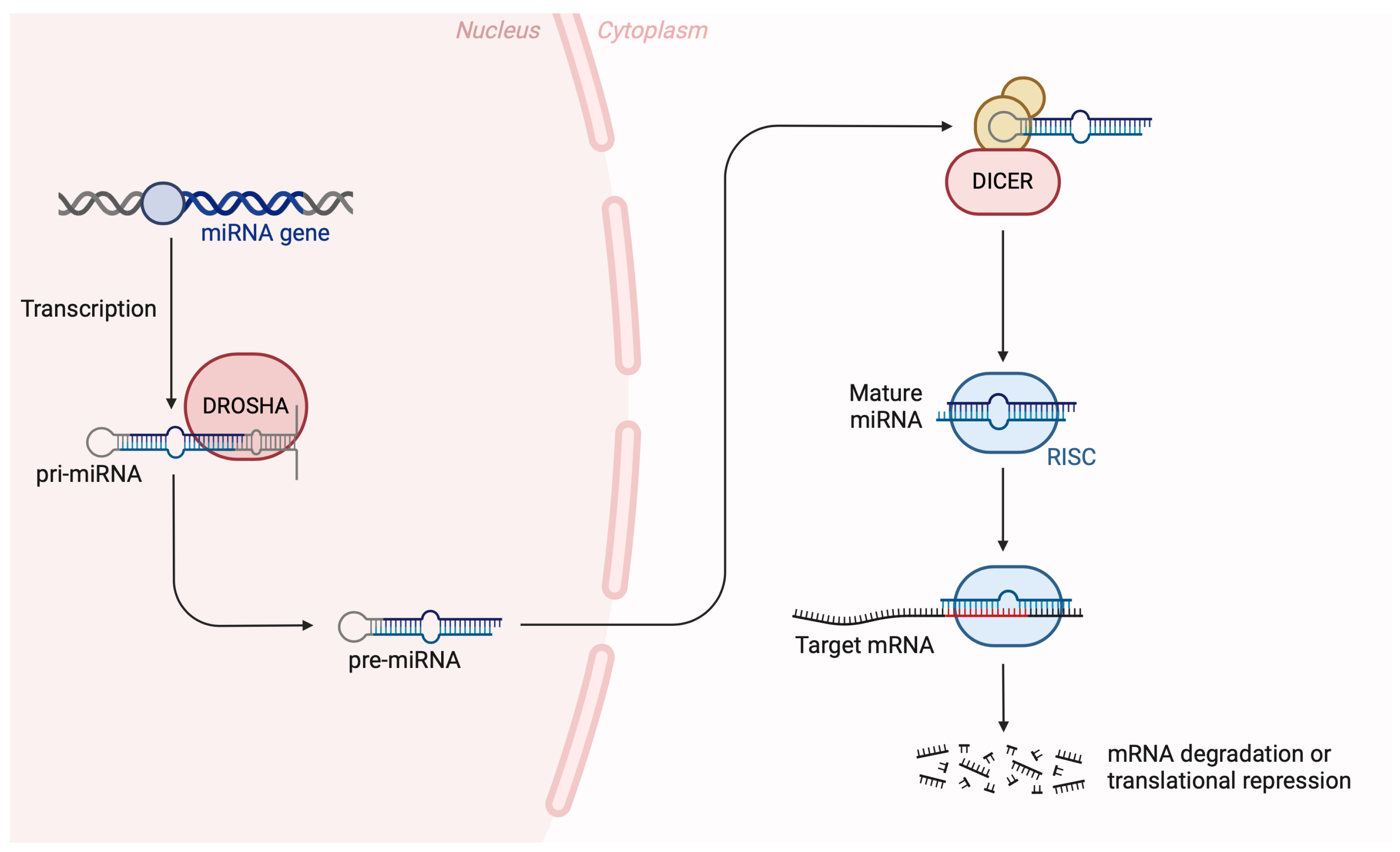


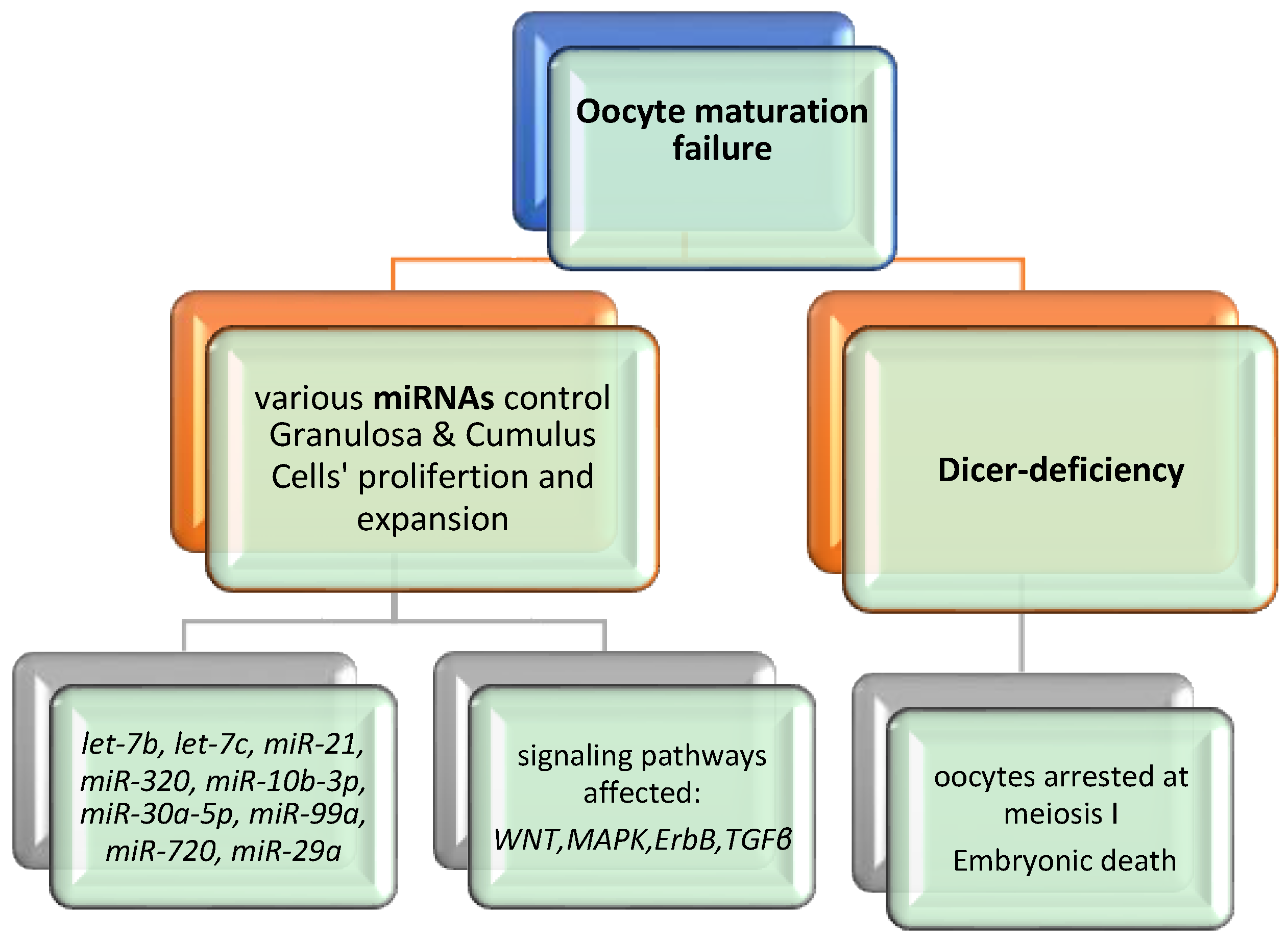
| miRNA | Cell Type | Regulation | Regulatory Role | References |
|---|---|---|---|---|
| let-7a-5p | GCs and CCs | up | Proliferation of GCs and CCs | [35] |
| let-7b | CCs | up | Steroidogenesis Apoptosis and proliferation of GCs | [34] |
| let-7c | CCs | up | Steroidogenesis Apoptosis and proliferation of GCs | [34] |
| let-7f-5p | GCs and CCs | up | Proliferation of GCs and CCs | [35] |
| miR-21 | CCs | up | Oocyte maturation Steroidogenesis Apoptosis and proliferation of GCs | [34,35,36,37,38,39,52,53,54] |
| miR-21 | CCs | down | Female infertility | [36] |
| miR-21-5p | GCs and CCs | up | Apoptosis of GCs and CCs | [35] |
| hsa-miR-320e | GCs | up | Proliferation of GCs affecting Oocyte degradation | [42] |
| miR-99a | GCs and CCs | up | Poor oocyte quality | [31,49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazou, E.; Potiris, A.; Mavrogianni, D.; Drakaki, E.; Vogiatzis, A.-A.; Sarli, V.; Vrantza, T.; Zikopoulos, A.; Louis, K.; Skentou, C.; et al. Oocyte Maturation and miRNAs: Studying a Complicate Interaction to Reveal Possible Biomarkers for Female Infertility. Diseases 2024, 12, 121. https://doi.org/10.3390/diseases12060121
Nazou E, Potiris A, Mavrogianni D, Drakaki E, Vogiatzis A-A, Sarli V, Vrantza T, Zikopoulos A, Louis K, Skentou C, et al. Oocyte Maturation and miRNAs: Studying a Complicate Interaction to Reveal Possible Biomarkers for Female Infertility. Diseases. 2024; 12(6):121. https://doi.org/10.3390/diseases12060121
Chicago/Turabian StyleNazou, Eleni, Anastasios Potiris, Despoina Mavrogianni, Eirini Drakaki, Aris-Anargyros Vogiatzis, Vaia Sarli, Tereza Vrantza, Athanasios Zikopoulos, Konstantinos Louis, Chara Skentou, and et al. 2024. "Oocyte Maturation and miRNAs: Studying a Complicate Interaction to Reveal Possible Biomarkers for Female Infertility" Diseases 12, no. 6: 121. https://doi.org/10.3390/diseases12060121






