Antimelanoma Effects of Alchemilla vulgaris: A Comprehensive In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Cells
2.2. Animals
2.3. Colorimetric Assays for Cellular viability (MTT and SRB)
2.4. Detection of Apoptosis, Activation of Caspases, and Autophagy
2.5. Measurement of Reactive Oxygen and Nitrogen Species (ROS/RNS) Generation
2.6. Detection of Cell Proliferation
2.7. Senescence Determination
2.8. Cell Cycle Analysis
2.9. PI Staining on Chamber Slides
2.10. Induction of Solid Melanoma and Experimental Treatment
2.11. Histopathology
2.12. Immunofluorescence PCNA Detection
2.13. Preparation of Single-Cell Suspensions
2.14. Flow Cytometric Analysis
2.15. Statistical Analysis
3. Results
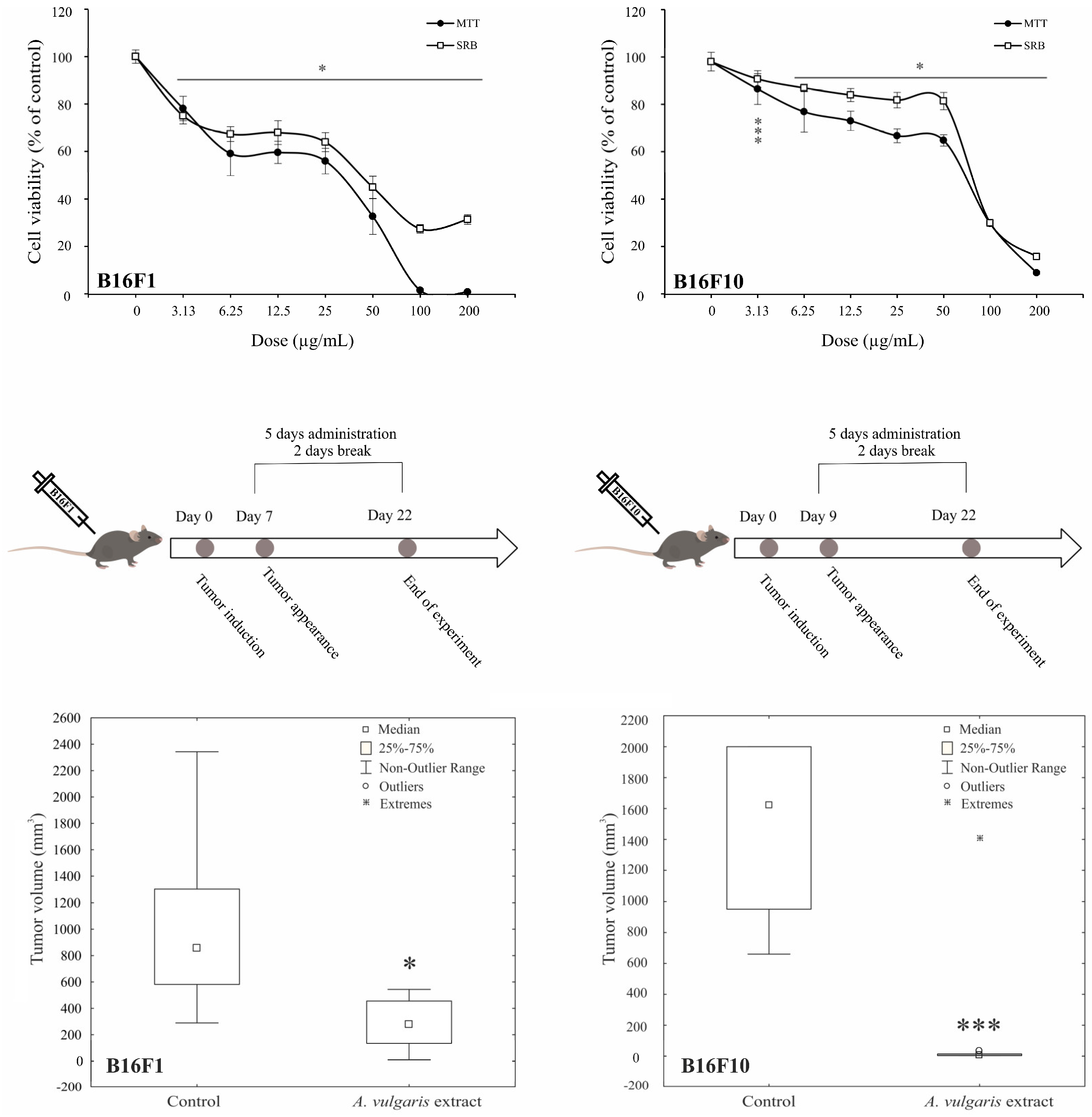
3.1. A. vulgaris Ethanolic Extract Downregulates Melanoma Cell Viability and Suppresses Tumor Cell Growth in Both, Low- and High-Grade Syngeneic Models of Primary Tumor
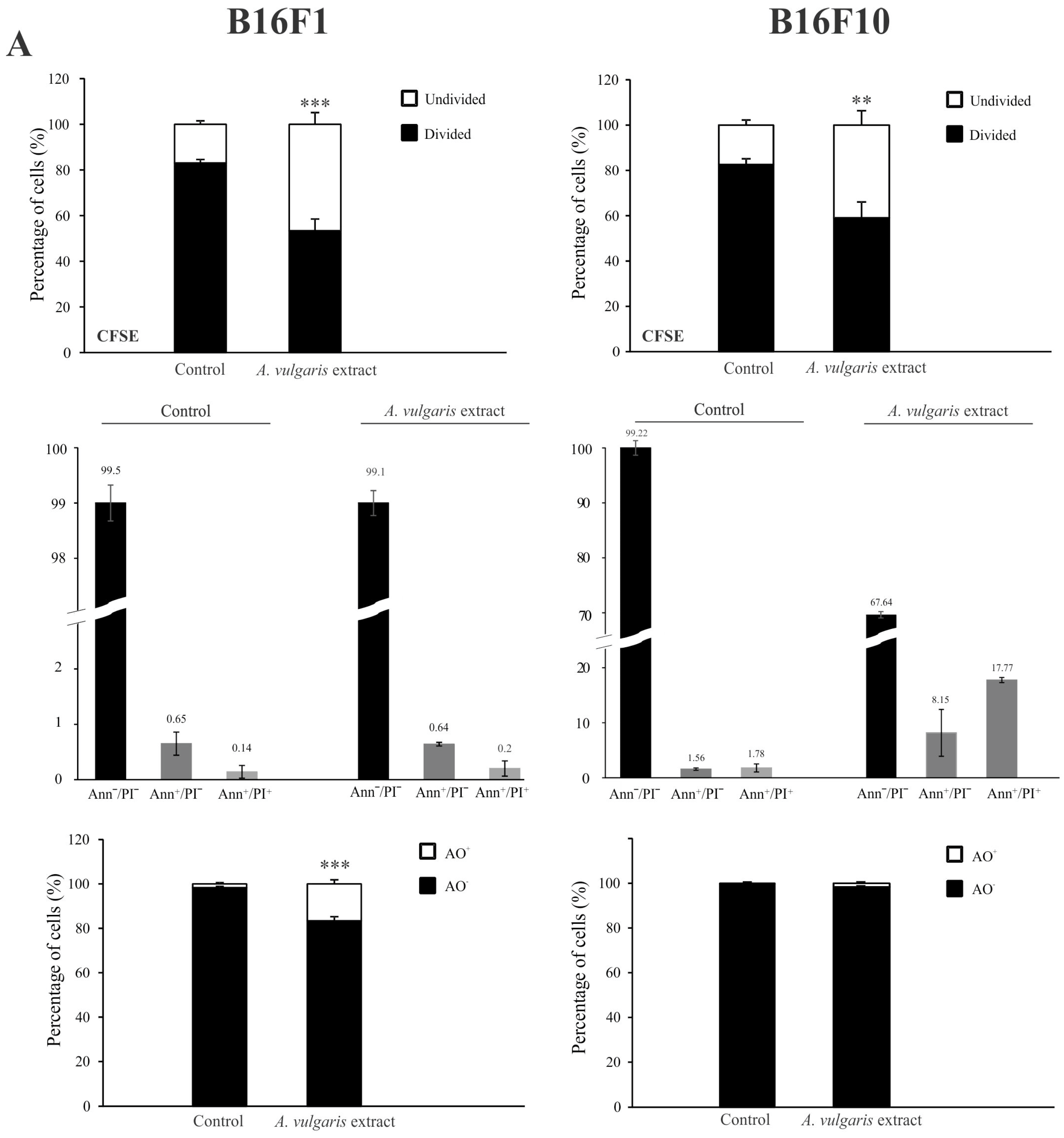
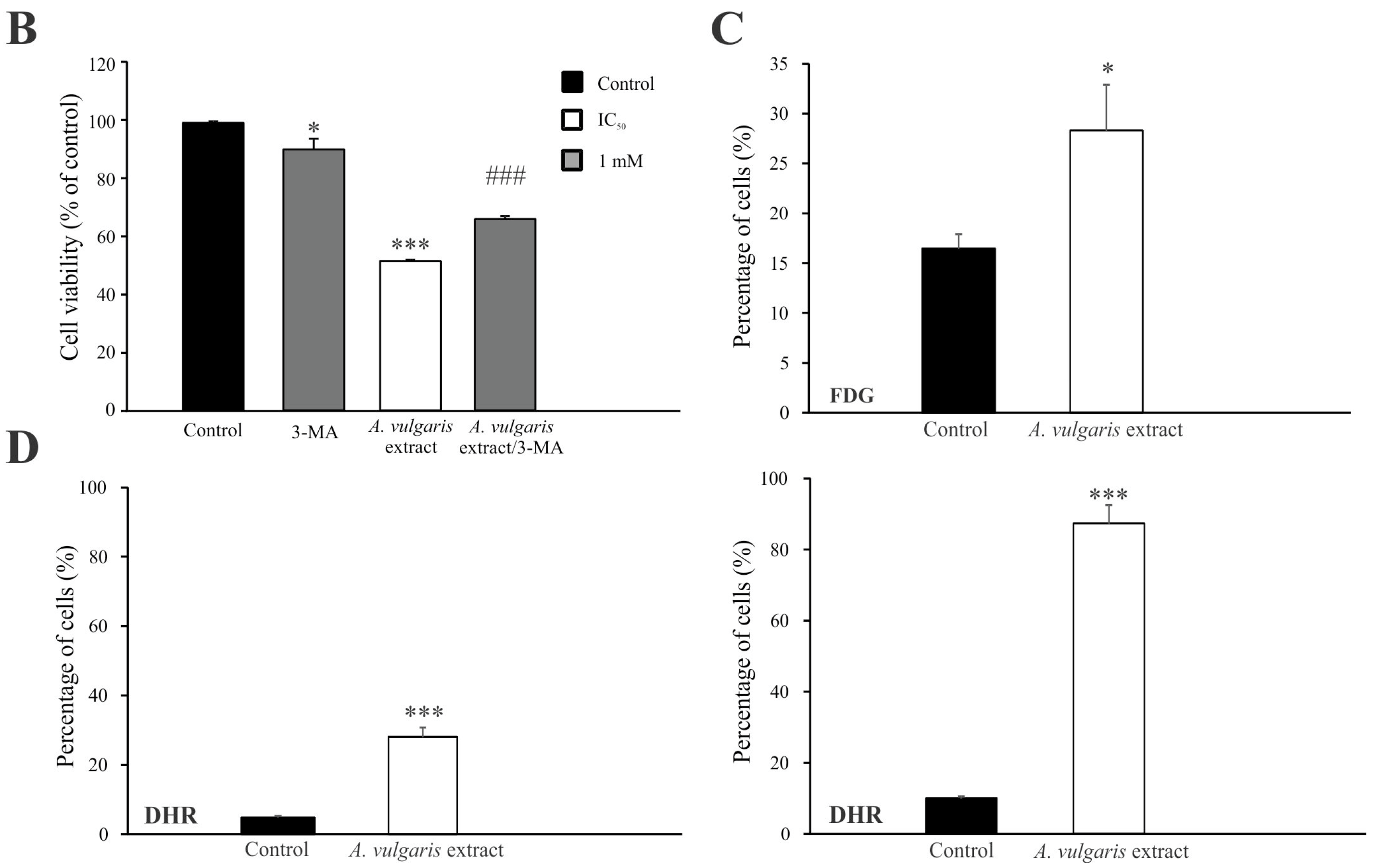
3.2. A. vulgaris Extract Manifests Dissimilar Mode of Action in B16 Melanoma Cell Lines of Different Grades In Vitro
3.3. A. vulgaris Extract Promoted Tumor Shrinkage in Both Low-End High Aggressive Syngeneic Models through Unexpectedly Different Routes in Comparison to Those Determined In Vitro
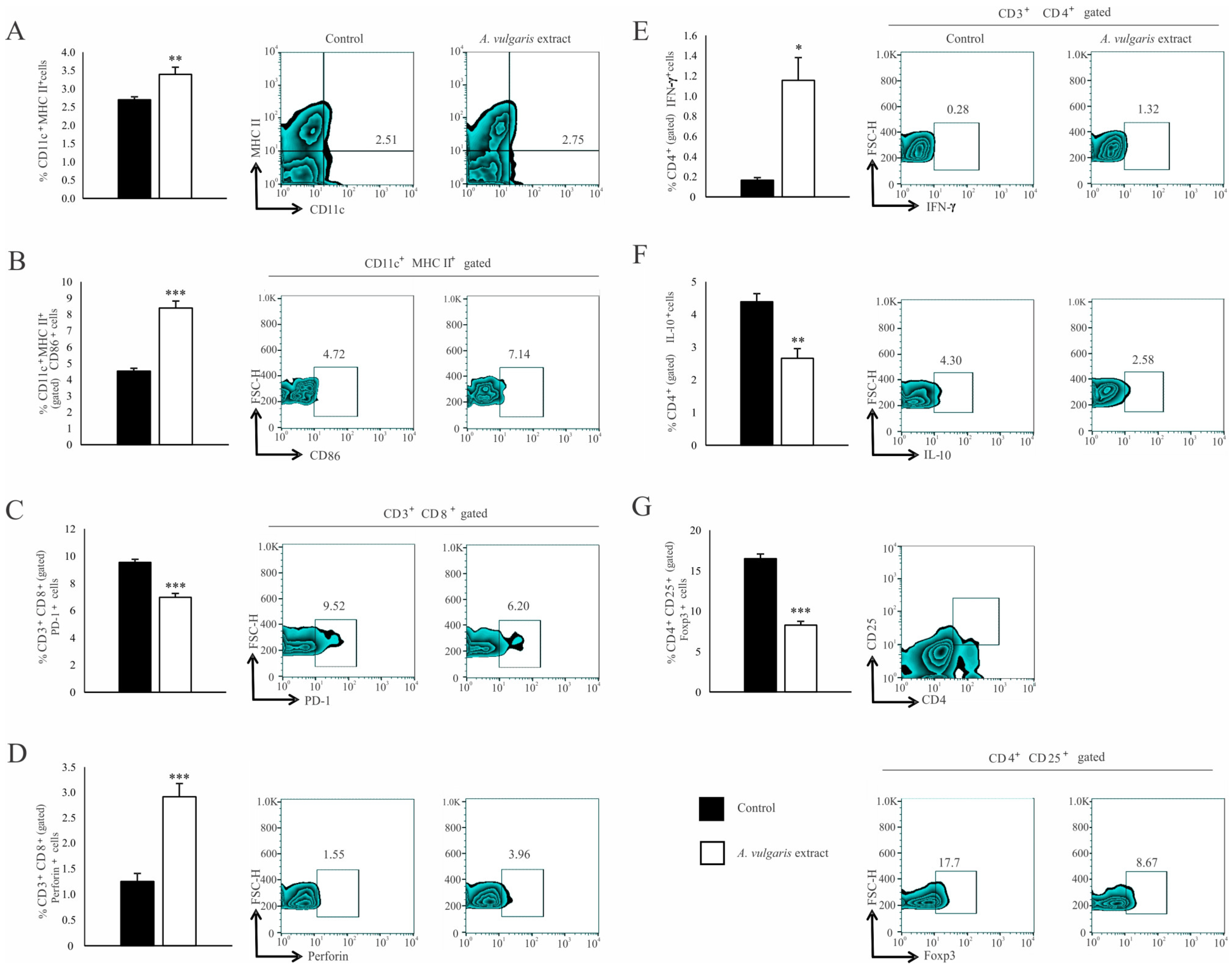
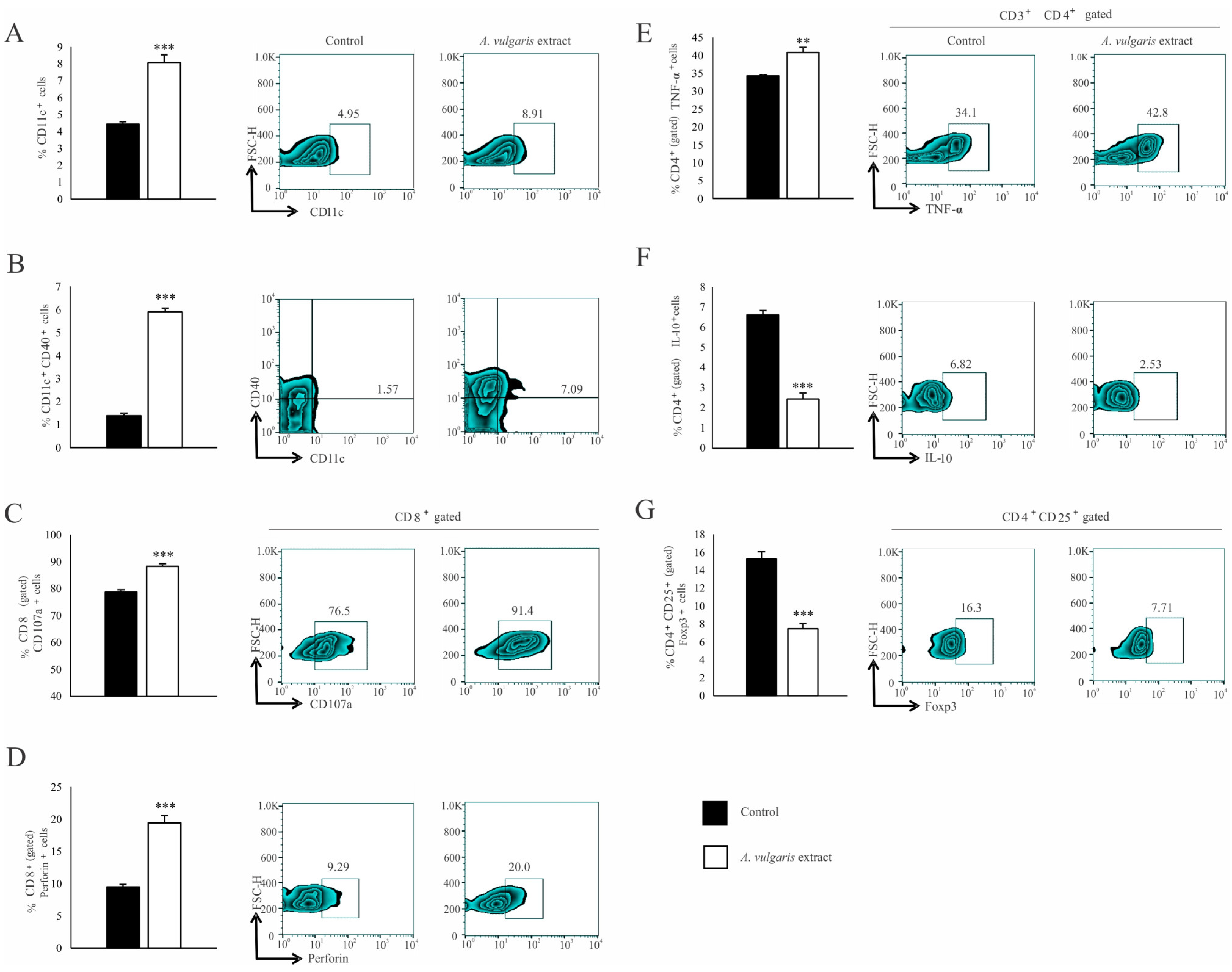
3.4. A. vulgaris Extract Enhances the Antitumor Immune Response in a Solid B16F1 Melanoma Model
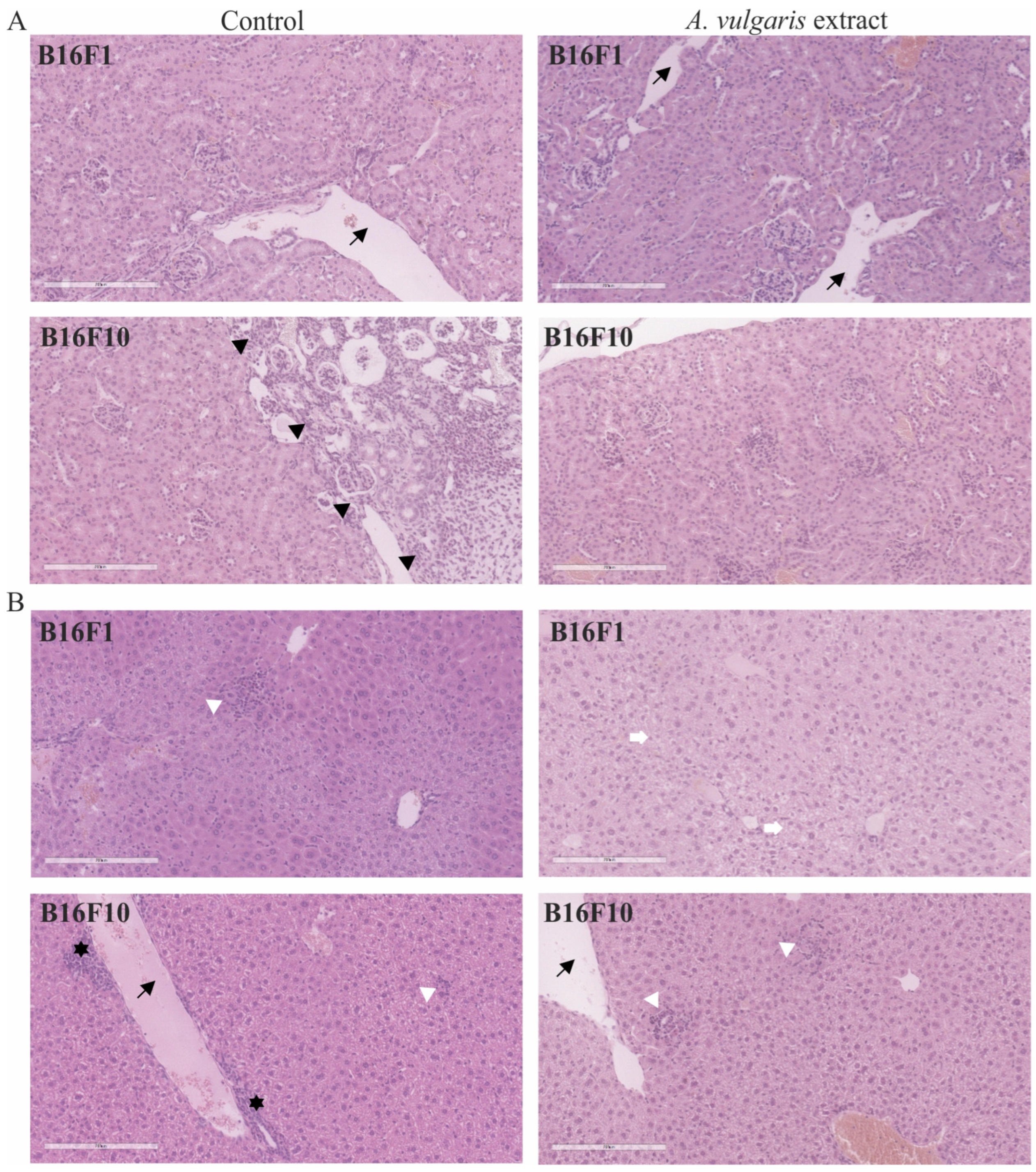
3.5. A. vulgaris Extract Realized a Strong Antitumor Effect in Both Melanoma Models without Remarkable Systemic Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin Melanocytes: Biology and Development. Adv. Dermatol. Allergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Bolick, N.L.; Geller, A.C. Epidemiology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 57–72. [Google Scholar] [CrossRef]
- Erdei, E.; Torres, S.M. A New Understanding in the Epidemiology of Melanoma. Expert Rev. Anticancer Ther. 2010, 10, 1811–1823. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ott, P.A.; Hodi, F.S.; Robert, C. CTLA-4 and PD-1/PD-L1 Blockade: New Immunotherapeutic Modalities with Durable Clinical Benefit in Melanoma Patients. Clin. Cancer Res. 2013, 19, 5300–5309. [Google Scholar] [CrossRef]
- Willsmore, Z.N.; Coumbe, B.G.T.; Crescioli, S.; Reci, S.; Gupta, A.; Harris, R.J.; Chenoweth, A.; Chauhan, J.; Bax, H.J.; McCraw, A.; et al. Combined Anti-PD-1 and Anti-CTLA-4 Checkpoint Blockade: Treatment of Melanoma and Immune Mechanisms of Action. Eur. J. Immunol. 2021, 51, 544–556. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Pop, T.D.; Diaconeasa, Z. Recent Advances in Phenolic Metabolites and Skin Cancer. Int. J. Mol. Sci. 2021, 22, 9707. [Google Scholar] [CrossRef]
- Gonçalves de Oliveira Júnior, R.; Adrielly Alves Ferraz, C.; Gama e Silva, M.; Martins de Lavor, É.; Araújo Rolim, L.; Tolentino de Lima, T.; Fleury, A.; Picot, L.; de Souza Siqueira Quintans, J.; José Quintans Júnior, L.; et al. Flavonoids: Promising Natural Products for Treatment of Skin Cancer (Melanoma). In Natural Products and Cancer Drug Discovery; InTech: London, UK, 2017; pp. 162–210. [Google Scholar]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Jaaks, P.; Coker, E.A.; Vis, D.J.; Edwards, O.; Carpenter, E.F.; Leto, S.M.; Dwane, L.; Sassi, F.; Lightfoot, H.; Barthorpe, S.; et al. Effective Drug Combinations in Breast, Colon and Pancreatic Cancer Cells. Nature 2022, 603, 166–173. [Google Scholar] [CrossRef]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’malley, B.W. Drug Combination in Cancer Treatment-From Cocktails to Conjugated Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Tomczyk, M. A Review of the Traditional Uses, Phytochemistry, Pharmacology, and Clinical Evidence for the Use of the Genus Alchemilla (Rosaceae). J. Ethnopharmacol. 2024, 320, 117439. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Jelača, S.; Zengin, G.; Mimica-Dukić, N.; Berežni, S.; Miljić, M.; Dajić Stevanović, Z. Alchemilla vulgaris Agg. (Lady’s Mantle) from Central Balkan: Antioxidant, Anticancer and Enzyme Inhibition Properties. RSC Adv. 2019, 9, 37474–37483. [Google Scholar] [CrossRef]
- Jelača, S.; Dajić-Stevanović, Z.; Vuković, N.; Kolašinac, S.; Trendafilova, A.; Nedialkov, P.; Stanković, M.; Tanić, N.; Tanić, N.T.; Acović, A.; et al. Beyond Traditional Use of Alchemilla vulgaris: Genoprotective and Antitumor Activity In Vitro. Molecules 2022, 27, 8113. [Google Scholar] [CrossRef]
- Ibrahim, O.H.M.; Abo-Elyousr, K.A.M.; Asiry, K.A.; Alhakamy, N.A.; Mousa, M.A.A. Phytochemical Characterization, Antimicrobial Activity and In Vitro Antiproliferative Potential of Alchemilla vulgaris Auct Root Extract against Prostate (PC-3), Breast (MCF-7) and Colorectal Adenocarcinoma (Caco-2) Cancer Cell Lines. Plants 2022, 11, 2140. [Google Scholar] [CrossRef]
- Potez, M.; Trappetti, V.; Bouchet, A.; Fernandez-Palomo, C.; Güç, E.; Kilarski, W.W.; Hlushchuk, R.; Laissue, J.; Djonov, V. Characterization of a B16-F10 Melanoma Model Locally Implanted into the Ear Pinnae of C57BL/6 Mice. PLoS ONE 2018, 13, e0206693. [Google Scholar] [CrossRef]
- Danciu, C.; Falamas, A.; Dehelean, C.; Soica, C.; Radeke, H.; Barbu-Tudoran, L.; Bojin, F.; Pînzaru, S.C.; Munteanu, M.F. A Characterization of Four B16 Murine Melanoma Cell Sublines Molecular Fingerprint and Proliferation Behavior. Cancer Cell Int. 2013, 13, 75. [Google Scholar] [CrossRef]
- Murugan, S.; Amaravadi, R.K. Methods for Studying Autophagy within the Tumor Microenvironment. Adv. Exp. Med. Biol. 2016, 899, 145–166. [Google Scholar] [CrossRef]
- Jovanovic, I.P.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/ST2 Axis Promotes Breast Cancer Growth and Metastases by Facilitating Intratumoral Accumulation of Immunosuppressive and Innate Lymphoid Cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef]
- Seglen, P.O.; Gordon, P.B. 3-Methyladenine: Specific Inhibitor of Autophagic/Lysosomal Protein Degradation in Isolated Rat Hepatocytes. Proc. Natl. Acad. Sci. USA 1982, 79, 1889–1892. [Google Scholar] [CrossRef]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-Acetylcysteine, Oral Glutathione (GSH) and a Novel Sublingual Form of GSH on Oxidative Stress Markers: A Comparative Crossover Study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef]
- Markovic, J.; Stojsic, J.; Zunic, S.; Ruzdijic, S.; Tanic, N. Genomic Instability in Patients with Non-Small Cell Lung Cancer Assessed by the Arbitrarily Primed Polymerase Chain Reaction. Cancer Investig. 2008, 26, 262–268. [Google Scholar] [CrossRef]
- Tanić, N.; Tanić, N.; Milašin, J.; Vukadinović, M.; Dimitrijević, B. Genomic Instability and Tumor-Specific DNA Alterations in Oral Leukoplakias. Eur. J. Oral Sci. 2009, 117, 231–237. [Google Scholar] [CrossRef]
- Gadiyar, V.; Lahey, K.C.; Calianese, D.; Devoe, C.; Mehta, D.; Bono, K.; Desind, S.; Davra, V.; Birge, R.B. Cell Death in the Tumor Microenvironment: Implications for Cancer Immunotherapy. Cells 2020, 9, 2207. [Google Scholar] [CrossRef]
- Liu, Z.G.; Jiao, D. Necroptosis, Tumor Necrosis and Tumorigenesis. Cell Stress 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Shu, L.; Su, Z.; Fuentes, F.; Lee, J.-H.; Kong, A.-N.T. Plants Against Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Majolo, F.; de Oliveira Becker Delwing, L.K.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal Plants and Bioactive Natural Compounds for Cancer Treatment: Important Advances for Drug Discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a Source of Anti-Cancer Agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy. Cancer Res. 2012, 72, 2473. [Google Scholar] [CrossRef]
- Moreno-Celis, U.; García-Gasca, T.; Mejía, C. Apoptosis-Induced Compensatory Proliferation in Cancer. In Metastasis; Exon Publications: Brisbane, Australia, 2022; pp. 149–162. [Google Scholar]
- Davis, A.J.; Tannock, I.F. Repopulation of Tumour Cells between Cycles of Chemotherapy: A Neglected Factor. Lancet Oncol. 2000, 1, 86–93. [Google Scholar] [CrossRef]
- Sajjad, H.; Imtiaz, S.; Noor, T.; Siddiqui, Y.H.; Sajjad, A.; Zia, M. Cancer Models in Preclinical Research: A Chronicle Review of Advancement in Effective Cancer Research. Anim. Models Exp. Med. 2021, 4, 87. [Google Scholar] [CrossRef]
- Olson, B.; Li, Y.; Lin, Y.; Liu, E.T.; Patnaik, A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018, 8, 1358. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The Apoptosis Paradox in Cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Engelhardt, J.J.; Boldajipour, B.; Beemiller, P.; Pandurangi, P.; Sorensen, C.; Werb, Z.; Egeblad, M.; Krummel, M.F. Marginating Dendritic Cells of the Tumor Microenvironment Cross-Present Tumor Antigens and Stably Engage Tumor-Specific T Cells. Cancer Cell 2012, 21, 402. [Google Scholar] [CrossRef]
- Gottfried, E.; Kunz-Schughart, L.A.; Ebner, S.; Mueller-Klieser, W.; Hoves, S.; Andreesen, R.; Mackensen, A.; Kreutz, M. Tumor-Derived Lactic Acid Modulates Dendritic Cell Activation and Antigen Expression. Blood 2006, 107, 2013–2021. [Google Scholar] [CrossRef]
- Townsend, S.E.; Allison, J.P. Tumor Rejection After Direct Costimulation of CD8+ T Cells by B7-Transfected Melanoma Cells. Science 1993, 259, 368–370. [Google Scholar] [CrossRef]
- Maeurer, M.J.; Gollin, S.M.; Martin, D.; Swaney, W.; Bryant, J.; Castelli, C.; Robbins, P.; Parmiani, G.; Storkus, W.J.; Lotze, M.T. Tumor Escape from Immune Recognition: Lethal Recurrent Melanoma in a Patient Associated with Downregulation of the Peptide Transporter Protein TAP-1 and Loss of Expression of the Immunodominant MART-1/Melan-A Antigen. J. Clin. Investig. 1996, 98, 1633–1641. [Google Scholar] [CrossRef]
- Seliger, B.; Maeurer, M.J.; Ferrone, S. TAP Off–Tumors On. Immunol. Today 1997, 18, 292–299. [Google Scholar] [CrossRef]
- Ossendorp, F.; Mengedé, E.; Camps, M.; Filius, R.; Melief, C.J.M. Specific T Helper Cell Requirement for Optimal Induction of Cytotoxic T Lymphocytes against Major Histocompatibility Complex Class II Negative Tumors. J. Exp. Med. 1998, 187, 693–702. [Google Scholar] [CrossRef]
- Wojtowicz-Praga, S. Reversal of Tumor-Induced Immunosuppression: A New Approach to Cancer Therapy. J. Immunother. 1997, 20, 165–177. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the Tumor Microenvironment in PD-L1/PD-1-Mediated Tumor Immune Escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Haabeth, O.A.W.; Tveita, A.A.; Fauskanger, M.; Schjesvold, F.; Lorvik, K.B.; Hofgaard, P.O.; Omholt, H.; Munthe, L.A.; Dembic, Z.; Corthay, A.; et al. How Do CD4+ T Cells Detect and Eliminate Tumor Cells That Either Lack or Express MHC Class II Molecules? Front. Immunol. 2014, 5, 174. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Li, T.; Wu, B.; Yang, T.; Zhang, L.; Jin, K. The Outstanding Antitumor Capacity of CD4+ T Helper Lymphocytes. Biochim. Biophys. Acta. Rev. Cancer 2020, 1874, 188439. [Google Scholar] [CrossRef]
- Braumüller, H.; Wieder, T.; Brenner, E.; Aßmann, S.; Hahn, M.; Alkhaled, M.; Schilbach, K.; Essmann, F.; Kneilling, M.; Griessinger, C.; et al. T-Helper-1-Cell Cytokines Drive Cancer into Senescence. Nature 2013, 494, 361–365. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Tsuji, T.; Luescher, I.F.; Shiku, H.; Mineno, J.; Okamoto, S.; Old, L.J.; Shrikant, P.; Gnjatic, S.; Odunsi, K. Direct Tumor Recognition by a Human CD4(+) T-Cell Subset Potently Mediates Tumor Growth Inhibition and Orchestrates Anti-Tumor Immune Responses. Sci. Rep. 2015, 5, 14896. [Google Scholar] [CrossRef]
- Chun, N.; Ang, R.L.; Chan, M.; Fairchild, R.L.; Baldwin, W.M.; Horwitz, J.K.; Gelles, J.D.; Chipuk, J.E.; Kelliher, M.A.; Pavlov, V.I.; et al. T Cell-Derived Tumor Necrosis Factor Induces Cytotoxicity by Activating RIPK1-Dependent Target Cell Death. JCI Insight 2021, 6, 148643. [Google Scholar] [CrossRef]
- Shklovskaya, E.; Terry, A.M.; Guy, T.V.; Buckley, A.; Bolton, H.A.; Zhu, E.; Holst, J.; De St Groth, B.F. Tumour-Specific CD4 T Cells Eradicate Melanoma via Indirect Recognition of Tumour-Derived Antigen. Immunol. Cell Biol. 2016, 94, 593–603. [Google Scholar] [CrossRef]
- Dang, N.; Waer, M.; Sprangers, B.; Lin, Y. Improved Anti-Tumour Adaptive Immunity Can Overcome the Melanoma Immunosuppressive Tumour Microenvironment. Cancers 2019, 11, 1694. [Google Scholar] [CrossRef]
- Facciabene, A.; Motz, G.T.; Coukos, G. T Regulatory Cells: Key Players in Tumor Immune Escape and Angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef]
- Jacobs, J.F.M.; Nierkens, S.; Figdor, C.G.; de Vries, I.J.M.; Adema, G.J. Regulatory T Cells in Melanoma: The Final Hurdle towards Effective Immunotherapy? Lancet Oncol. 2012, 13, 32–42. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Dong, W.; Fang, Y.; Lv, J.; Zhang, T.; Fiskesund, R.; Xie, J.; Liu, J.; Yin, X.; et al. Tumor-Repopulating Cells Induce PD-1 Expression in CD8+ T Cells by Transferring Kynurenine and AhR Activation. Cancer Cell 2018, 33, 480–494. [Google Scholar] [CrossRef]
- Chemnitz, J.M.; Parry, R.V.; Nichols, K.E.; June, C.H.; Riley, J.L. SHP-1 and SHP-2 Associate with Immunoreceptor Tyrosine-Based Switch Motif of Programmed Death 1 upon Primary Human T Cell Stimulation, but Only Receptor Ligation Prevents T Cell Activation. J. Immunol. 2004, 173, 945–954. [Google Scholar] [CrossRef]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a Expression and Cytotoxic Activity. Cell. Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef]
- Hoque, M.B.; Tanjila, M.J.; Hosen, M.I.; Hannan, M.A.; Haque, P.; Rahman, M.M.; Hasan, T. A Comprehensive Review of the Health Effects, Origins, Uses, and Safety of Tannins. Plant Soil 2024, 1–20. [Google Scholar] [CrossRef]
- Radović, J.; Suručić, R.; Niketić, M.; Kundaković-Vasović, T. Alchemilla viridiflora Rothm.: The Potent Natural Inhibitor of Angiotensin I-Converting Enzyme. Mol. Cell. Biochem. 2022, 477, 1893–1903. [Google Scholar] [CrossRef]
- Chen, P.Y.; Liao, Y.H.; Huang, W.T.; Lin, Y.C.; Hou, Y.T. Effects of Tannic Acid on Liver Function in a Small Hepatocyte–Based Detachable Microfluidic Platform. Biochem. Eng. J. 2023, 190, 108757. [Google Scholar] [CrossRef]
- Jelača, S.; Jovanovic, I.; Bovan, D.; Jovanovic, M.Z.; Jurisevic, M.M.; Dunđerović, D.; Dajic-Stevanovic, Z.; Arsenijevic, N.; Mijatović, S.; Maksimović-Ivanić, D. Dual Role of Alchemilla vulgaris L. Extract in Breast Cancer Regression: Reestablishment of Effective Immune Response. Pharmaceuticals 2024, 17, 286. [Google Scholar] [CrossRef]

| Cell Lines | Assay | IC50 [µg/mL] |
|---|---|---|
| B16F1 | MTT | 34.3 ± 4.2 1 |
| SRB | 43.2 ± 0.1 | |
| B16F10 | MTT | 72.2 ± 2.7 |
| SRB | 80.9 ± 1.0 | |
| 518A2 | MTT | 62.75 ± 6.5 |
| SRB | 88.55 ± 6.29 | |
| Fem-X | MTT | 78.4 ± 5.20 |
| SRB | 90.3 ± 8.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelača, S.; Jovanovic, I.; Bovan, D.; Pavlovic, S.; Gajovic, N.; Dunđerović, D.; Dajić-Stevanović, Z.; Acović, A.; Mijatović, S.; Maksimović-Ivanić, D. Antimelanoma Effects of Alchemilla vulgaris: A Comprehensive In Vitro and In Vivo Study. Diseases 2024, 12, 125. https://doi.org/10.3390/diseases12060125
Jelača S, Jovanovic I, Bovan D, Pavlovic S, Gajovic N, Dunđerović D, Dajić-Stevanović Z, Acović A, Mijatović S, Maksimović-Ivanić D. Antimelanoma Effects of Alchemilla vulgaris: A Comprehensive In Vitro and In Vivo Study. Diseases. 2024; 12(6):125. https://doi.org/10.3390/diseases12060125
Chicago/Turabian StyleJelača, Sanja, Ivan Jovanovic, Dijana Bovan, Sladjana Pavlovic, Nevena Gajovic, Duško Dunđerović, Zora Dajić-Stevanović, Aleksandar Acović, Sanja Mijatović, and Danijela Maksimović-Ivanić. 2024. "Antimelanoma Effects of Alchemilla vulgaris: A Comprehensive In Vitro and In Vivo Study" Diseases 12, no. 6: 125. https://doi.org/10.3390/diseases12060125
APA StyleJelača, S., Jovanovic, I., Bovan, D., Pavlovic, S., Gajovic, N., Dunđerović, D., Dajić-Stevanović, Z., Acović, A., Mijatović, S., & Maksimović-Ivanić, D. (2024). Antimelanoma Effects of Alchemilla vulgaris: A Comprehensive In Vitro and In Vivo Study. Diseases, 12(6), 125. https://doi.org/10.3390/diseases12060125






