SARS-CoV-2 Infection Positively Correlates with Hyperglycemia and Inflammatory Markers in COVID-19 Patients: A Clinical Research Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Ethical Considerations, Data Collection, and Case Enrollment
2.2. Sample Collection and Processing
2.3. Estimation of Serum Ferritin
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
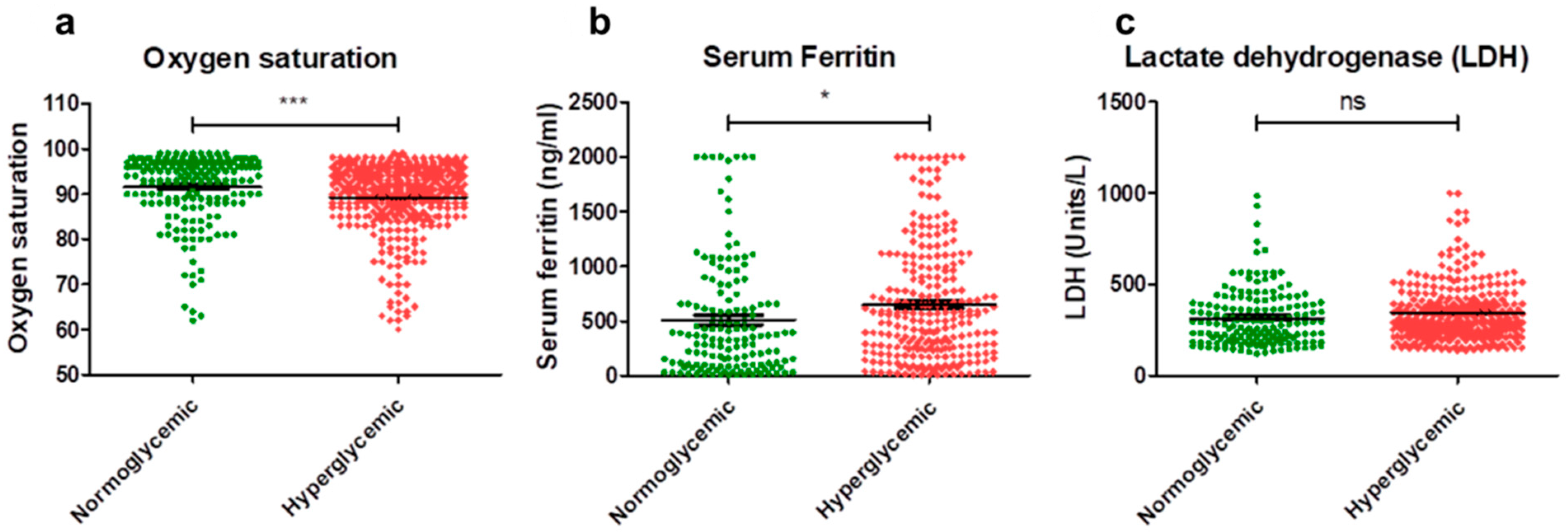
3.2. Inflammatory Parameters
3.3. Hematological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): An update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef]
- Care, D. Care in diabetesd2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.-Q.; Peng, H.-J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020, 9, 575. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Mehraeen, E.; Karimi, A.; Barzegary, A.; Vahedi, F.; Afsahi, A.M.; Dadras, O.; Moradmand-Badie, B.; Alinaghi, S.A.S.; Jahanfar, S. Predictors of mortality in patients with COVID-19—A systematic review. Eur. J. Integr. Med. 2020, 40, 101226. [Google Scholar] [CrossRef]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Chee, Y.J.; Ng, S.J.H.; Yeoh, E. Diabetic ketoacidosis precipitated by COVID-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 164, 108166. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, J.; Zuo, X.; Zhang, H.; Deng, A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes. Metab. 2020, 22, 1935–1941. [Google Scholar] [CrossRef]
- Ren, H.; Yang, Y.; Wang, F.; Yan, Y.; Shi, X.; Dong, K.; Yu, X.; Zhang, S. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc. Diabetol. 2020, 19, 58. [Google Scholar] [CrossRef]
- Barrett, C.E. Risk for newly diagnosed diabetes 30 days after SARS-CoV-2 infection among persons aged 18 years—United States, March 1, 2020–June 28, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 59–65. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.v.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, W.; Xu, Z.; Gu, J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res. Clin. Pract. 2020, 162, 108118. [Google Scholar] [CrossRef]
- Henry, B.M.; De Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 1021–1028. [Google Scholar] [CrossRef]
- Kumar, A.; Sepolia, S.; Shilpa, R.; Rezayani, G.; Kumari, S.; Gupta, S. Role of hematological and immunological parameters in COVID-19 patients. J. Pharm. Bioallied Sci. 2021, 13, 238–243. [Google Scholar]
- Usul, E.; Şan, İ.; Bekgöz, B.; Şahin, A. Role of hematological parameters in COVID-19 patients in the emergency room. Biomark. Med. 2020, 14, 1207–1215. [Google Scholar] [CrossRef]
- Elshazli, R.M.; Toraih, E.A.; Elgaml, A.; El-Mowafy, M.; El-Mesery, M.; Amin, M.N.; Hussein, M.H.; Killackey, M.T.; Fawzy, M.S.; Kandil, E. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS ONE 2020, 15, e0238160. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Is metformin ahead in the race as a repurposed host-directed therapy for patients with diabetes and COVID-19? Diabetes Res. Clin. Pract. 2020, 165, 108268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, X.; Wang, L.; Guo, J. Effects of hypertension, diabetes and coronary heart disease on COVID-19 diseases severity: A systematic review and meta-analysis. MedRxiv 2020. [Google Scholar] [CrossRef]
- Ji, D.; Zhang, D.; Xu, J.; Chen, Z.; Yang, T.; Zhao, P.; Chen, G.; Cheng, G.; Wang, Y.; Bi, J. Prediction for progression risk in patients with COVID-19 pneumonia: The CALL score. Clin. Infect. Dis. 2020, 71, 1393–1399. [Google Scholar] [CrossRef]
- Sahu, K.K.; Siddiqui, A.D. From Hematologistʼs desk: The effect of COVID-19 on the blood system. Am. J. Hematol. 2020, 95, E213. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kraemer, M.U.; Gutierrez, B.; Mekaru, S.; Sewalk, K.; Loskill, A.; Wang, L.; Cohn, E.; Hill, S.; Zarebski, A.; et al. Open access epidemiological data from the COVID-19 outbreak. Lancet Infect. Dis. 2020, 20, 534. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Bode, B.; Garrett, V.; Messler, J.; McFarland, R.; Crowe, J.; Booth, R.; Klonoff, D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020, 14, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef]
- Gianchandani, R.; Esfandiari, N.H.; Ang, L.; Iyengar, J.; Knotts, S.; Choksi, P.; Pop-Busui, R. Managing hyperglycemia in the COVID-19 inflammatory storm. Diabetes 2020, 69, 2048–2053. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.; Morieri, M.; Longato, E.; Avogaro, D.A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Investig. 2020, 43, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Feng, Y.; Yuan, M.; Yuan, S.; Fu, H.; Wu, B.; Sun, G.; Yang, G.; Zhang, X.; Wang, L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Lin, S.-S.; Ji, X.-J.; Guo, L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010, 47, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.; Campopiano, M.C.; Mantuano, M.; Mazoni, L.; Coppelli, A.; Del Prato, S. COVID-19 in people with diabetes: Understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020, 8, 782–792. [Google Scholar] [CrossRef]
- Jivanji, C.J.; Asrani, V.M.; Windsor, J.A.; Petrov, M.S. New-onset diabetes after acute and critical illness: A systematic review. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands; pp. 762–773.
- Li, H.; Tian, S.; Chen, T.; Cui, Z.; Shi, N.; Zhong, X.; Qiu, K.; Zhang, J.; Zeng, T.; Chen, L.; et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes. Metab. 2020, 22, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Laursen, J.C.; Jepsen, R.; Bruun-Rasmussen, N.E.; Frimodt-Møller, M.; Jørgensen, M.E.; Rossing, P.; Hansen, C.S. Blood oxygen saturation is lower in persons with pre-diabetes and screen-detected diabetes compared with non-diabetic individuals: A population-based study of the Lolland-Falster Health Study cohort. Front. Epidemiol. 2022, 2, 1022342. [Google Scholar] [CrossRef]
- Vold, M.L.; Aasebø, U.; Wilsgaard, T.; Melbye, H. Low oxygen saturation and mortality in an adult cohort: The Tromsø study. BMC Pulm. Med. 2015, 15, 9. [Google Scholar] [CrossRef]
- Sada, K.; Nishikawa, T.; Kukidome, D.; Yoshinaga, T.; Kajihara, N.; Sonoda, K.; Senokuchi, T.; Motoshima, H.; Matsumura, T.; Araki, E. Hyperglycemia induces cellular hypoxia through production of mitochondrial ROS followed by suppression of aquaporin-1. PLoS ONE 2016, 11, e0158619. [Google Scholar] [CrossRef]
- Vargas-Rodriguez, J.R.; Valdes Aguayo, J.J.; Garza-Veloz, I.; Martinez-Rendon, J.; del Refugio Rocha Pizaña, M.; Cabral-Pacheco, G.A.; Juarez-Alcala, V.; Martinez-Fierro, M.L. Sustained hyperglycemia and its relationship with the outcome of hospitalized patients with severe COVID-19: Potential role of ACE2 upregulation. J. Pers. Med. 2022, 12, 805. [Google Scholar] [CrossRef]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S.; et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef]
- Maheshwari, A.V.; Bhoi, B.K.; Sadariya, B.R.; Javia, H.N.; Gusani, J.K.; Sharma, H. Correlation between serum ferritin and glycaemic control in patients of type 2 diabetes mellitus: A case control study. Int. J. Res. Med. Sci. 2015, 3, 2327. [Google Scholar] [CrossRef][Green Version]
- Lu, J.; Wei, Z.; Jiang, H.; Cheng, L.; Chen, Q.; Chen, M.; Yan, J.; Sun, Z. Lactate dehydrogenase is associated with 28-day mortality in patients with sepsis: A retrospective observational study. J. Surg. Res. 2018, 228, 314–321. [Google Scholar] [CrossRef]
- Kang, H.E.; Park, D.W. Lactate as a biomarker for sepsis prognosis? Infect. Chemother. 2016, 48, 252. [Google Scholar] [CrossRef] [PubMed]
- Duman, A.; Akoz, A.; Kapci, M.; Ture, M.; Orun, S.; Karaman, K.; Turkdogan, K.A. Prognostic value of neglected biomarker in sepsis patients with the old and new criteria: Predictive role of lactate dehydrogenase. Am. J. Emerg. Med. 2016, 34, 2167–2171. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, B.; Liu, J.; Chen, Y.; Zhong, P. Risk factors associated with long-term hospitalization in patients with COVID-19: A single-centered, retrospective study. Front. Med. 2020, 7, 553604. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, W.; Ye, B.; Chen, C.; Huang, R.; Wu, F.; Wei, Q.; Zhang, W.; Hu, J. Changes of hematological and immunological parameters in COVID-19 patients. Int. J. Hematol. 2020, 112, 553–559. [Google Scholar] [CrossRef]
- Saurabh, A.; Dey, B.; Raphael, V.; Barman, B.; Dev, P.; Tiewsoh, I.; Lyngdoh, B.S.; Dutta, K. Evaluation of hematological parameters in predicting intensive care unit admission in COVID-19 patients. SN Compr. Clin. Med. 2022, 4, 39. [Google Scholar] [CrossRef]
- Matin, S.; Safarzadeh, E.; Rezaei, N.; Negaresh, M.; Salehzadeh, H.; Matin, S.; Sharifiazar, A.H.; Abazari, M.; Dadkhah, M. Hematological parameters as diagnostic factors: Correlation with severity of COVID-19. Acta Bio Medica Atenei Parm. 2022, 93, e2022061. [Google Scholar]
- Liao, D.; Zhou, F.; Luo, L.; Xu, M.; Wang, H.; Xia, J.; Gao, Y.; Cai, L.; Wang, Z.; Yin, P.; et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: A retrospective cohort study. Lancet Haematol. 2020, 7, e671–e678. [Google Scholar] [CrossRef]
- Anurag, A.; Jha, P.K.; Kumar, A. Differential white blood cell count in the COVID-19: A cross-sectional study of 148 patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 2099–2102. [Google Scholar] [CrossRef]
- Ayalew, G.; Mulugeta, B.; Haimanot, Y.; Adane, T.; Bayleyegn, B.; Abere, A. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio can predict the severity in COVID-19 patients from Ethiopia: A retrospective study. Int. J. Gen. Med. 2022, 15, 7701. [Google Scholar] [CrossRef]
- Gujar, R.K.; Meena, A.; Chouhan, S.S.; Likhar, K. Hematological profiles of COVID-19 patients at the Ratlam district, Madhya Pradesh State, India. Bioinformation 2021, 17, 686. [Google Scholar]
- Gunardi, W.D. Corona Virus Disease 2019 dan Perkembangannya; PT. Scifintech Andrew Wijaya: South Jakarta, Indonesia, 2022. [Google Scholar]
- Zahorec, R.; Hulin, I.; Zahorec, P. Rationale Use of Neutrophil-to-lymphocyte ratio for early diagnosis and stratification of COVID-19. Bratisl. Med. J./Bratisl. Lek. Listy 2020, 121, 466–470. [Google Scholar] [CrossRef]
- Ramesh, J.; Reddy, S.S.; Rajesh, M.; Varghese, J. Evaluation of simple and cost-effective immuno-haematological markers to predict outcome in hospitalized severe COVID-19 patients, with a focus on diabetes mellitus-A retrospective study in Andhra Pradesh, India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 739–745. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.-S.; et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef]
- Cheng, Y.; Yue, L.; Wang, Z.; Zhang, J.; Xiang, G. Hyperglycemia associated with lymphopenia and disease severity of COVID-19 in type 2 diabetes mellitus. J. Diabetes Its Complicat. 2021, 35, 107809. [Google Scholar] [CrossRef]
- Wu, D.; Gao, S. Analysis of the lymphocyte count in type 2 diabetic patients with coronavirus disease (COVID-19): A retrospective study in a centralized treatment center. Diabetes Res. Clin. Pract. 2020, 166, 108340. [Google Scholar] [CrossRef]
- Goel, V.; Raizada, A.; Aggarwal, A.; Madhu, S.; Kar, R.; Agrawal, A.; Mahla, V.; Goel, A. Long-Term Persistence of COVID-Induced Hyperglycemia: A Cohort Study. Am. J. Trop. Med. Hyg. 2024, 110, 512. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, B.; Wang, S.; Chen, X.; Wen, Z. Effect of hyperglycemia on the immune function of COVID-19 patients with type 2 diabetes mellitus: A retrospective study. PeerJ 2022, 10, e14570. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes/Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Nieuwdorp, M.; van Haeften, T.W.; Gouverneur, M.C.; Mooij, H.L.; van Lieshout, M.H.; Levi, M.; Meijers, J.C.; Holleman, F.; Hoekstra, J.B.; Vink, H.; et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006, 55, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The end of the beginning. Cell Metab. 2021, 33, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Al-Mulla, F.; Thanaraj, T.A.; Kavalakatt, S.; Ali, H.; Abdul Ghani, M.; Abubaker, J. Impact of diabetes in patients diagnosed with COVID-19. Front. Immunol. 2020, 11, 576818. [Google Scholar] [CrossRef]
- Zahedi, M.; Kordrostami, S.; Kalantarhormozi, M.; Bagheri, M. A Review of Hyperglycemia in COVID-19. Cureus 2023, 15, e37487. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.P.; Bellomo, R.; Cousins, C.E.; Annink, C.E.; Sundararajan, K.; Reddi, B.A.; Raj, J.P.; Chapman, M.J.; Horowitz, M.; Deane, A.M. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014, 40, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.K.; Hao, W.; Brooks-Worrell, B.M.; Palmer, J.P. Low-dose interleukin 1 and tumor necrosis factor individually stimulate insulin release but in combination cause suppression. Eur. J. Endocrinol. 1994, 130, 208–214. [Google Scholar] [CrossRef]
- Vedantam, D.; Poman, D.S.; Motwani, L.; Asif, N.; Patel, A.; Anne, K.K. Stress-induced hyperglycemia: Consequences and management. Cureus 2022, 14, e26714. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | Total | Normoglycemic (RBS < 140 mg/dL) | Hyperglycemic (RBS > 140 mg/dL) |
|---|---|---|---|
| Total number of subjects | n = 550 | n = 202 | n = 348 |
| Gender | |||
| Male | 354 (64.36) | 127 (62.87) | 227 (65.22) |
| Female | 196 (35.63) | 75 (37.12) | 121 (34.77) |
| Age distribution | |||
| <30 | 57 (10.36) | 28 (13.86) | 29 (8.33) |
| 30–60 | 368 (66.90) | 136 (67.32) | 232 (66.66) |
| >60 | 125 (22.72) | 38 (18.81) | 87 (35.08) |
| Vaccination status | |||
| Unvaccinated | 442 (80.36) | 159 (78.71) | 283 (81.32) |
| Vaccinated | 108 (19.63) | 43 (21.28) | 65 (18.67) |
| Single Dose vaccination | 75 (69.44) | 31 (72.09) | 44 (67.69) |
| Double Dose vaccination | 33 (30.55) | 12 (27.90) | 21 (32.30) |
| Clinical features | |||
| Fever | 379 (68.90) | 131 (64.85) | 248 (71.26) |
| Cough | 417 (75.81) | 149 (73.76) | 268 (77.01) |
| Breathlessness | 249 (45.27) | 83 (41.08) | 166 (47.70) |
| Preexisting diabetes | 158 (28.72) | 32 (15.84) | 126 (36.20) |
| New onset hyperglycemia | 225 (40.90) | None | 225 (64.65) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikkahonnaiah, P.; Dallavalasa, S.; Tulimilli, S.V.; Dubey, M.; Byrappa, S.H.; Amachawadi, R.G.; Madhunapantula, S.V.; Veeranna, R.P. SARS-CoV-2 Infection Positively Correlates with Hyperglycemia and Inflammatory Markers in COVID-19 Patients: A Clinical Research Study. Diseases 2024, 12, 143. https://doi.org/10.3390/diseases12070143
Chikkahonnaiah P, Dallavalasa S, Tulimilli SV, Dubey M, Byrappa SH, Amachawadi RG, Madhunapantula SV, Veeranna RP. SARS-CoV-2 Infection Positively Correlates with Hyperglycemia and Inflammatory Markers in COVID-19 Patients: A Clinical Research Study. Diseases. 2024; 12(7):143. https://doi.org/10.3390/diseases12070143
Chicago/Turabian StyleChikkahonnaiah, Prashanth, Siva Dallavalasa, SubbaRao V. Tulimilli, Muskan Dubey, Shashidhar H. Byrappa, Raghavendra G. Amachawadi, SubbaRao V. Madhunapantula, and Ravindra P. Veeranna. 2024. "SARS-CoV-2 Infection Positively Correlates with Hyperglycemia and Inflammatory Markers in COVID-19 Patients: A Clinical Research Study" Diseases 12, no. 7: 143. https://doi.org/10.3390/diseases12070143
APA StyleChikkahonnaiah, P., Dallavalasa, S., Tulimilli, S. V., Dubey, M., Byrappa, S. H., Amachawadi, R. G., Madhunapantula, S. V., & Veeranna, R. P. (2024). SARS-CoV-2 Infection Positively Correlates with Hyperglycemia and Inflammatory Markers in COVID-19 Patients: A Clinical Research Study. Diseases, 12(7), 143. https://doi.org/10.3390/diseases12070143









