Acute Effect of Simultaneous Exercise and Cognitive Tasks on Cognitive Functions in Elderly Individuals with Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
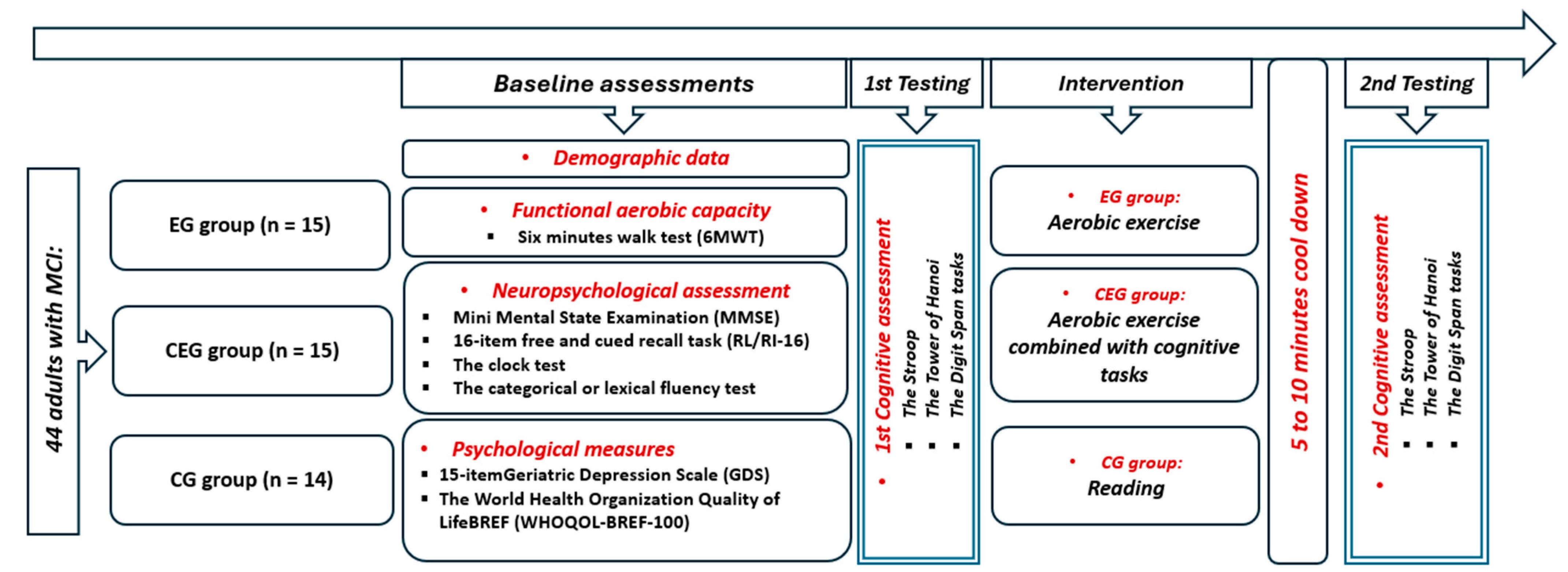
2.1. Study Population
2.2. Procedure
2.3. Measurements
2.3.1. Neuropsychological Measures
2.3.2. Psychological Measures
2.3.3. Cognitive Function Measures
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Effects on Cognitive Performance: A Comparative Analysis Based on Pre–Post Values
3.2.1. Stroop Test
3.2.2. Hanoi Time
3.2.3. Hanoi Time
3.2.4. Digit Span Forward
3.2.5. Digit Span Backward
3.3. Effects on Cognitive Performances: A Comparative Analysis Based on the Percentage of Change from Pre- to Postsession
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The Global Prevalence of Dementia: A Systematic Review and Metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; David Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Blough, J.; Ryu, S.; Kang, M. Experimental effects of exercise on memory function among mild cognitive impairment: Systematic review and meta-analysis. Phys. Sportsmed. 2019, 47, 21–26. [Google Scholar] [CrossRef]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Harveson, A.T.; Hannon, J.C.; Brusseau, T.A.; Podlog, L.; Papadopoulos, C.; Durrant, L.H.; Hall, M.S.; Kang, K.D. Acute Effects of 30 Minutes Resistance and Aerobic Exercise on Cognition in a High School Sample. Res. Q. Exerc. Sport 2016, 87, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hötting, K.; Schickert, N.; Kaiser, J.; Röder, B.; Schmidt-Kassow, M. The Effects of Acute Physical Exercise on Memory, Peripheral BDNF, and Cortisol in Young Adults. Neural Plast. 2016, 2016, 6860573. [Google Scholar] [CrossRef]
- Hwang, J.; Brothers, R.M.; Castelli, D.M.; Glowacki, E.M.; Chen, Y.T.; Salinas, M.M.; Kim, J.; Jung, Y.; Calvert, H.G. Acute High-Intensity Exercise-Induced Cognitive Enhancement and Brain-Derived Neurotrophic Factor in Young, Healthy Adults. Neurosci. Lett. 2016, 630, 247–253. [Google Scholar] [CrossRef]
- McSween, M.-P.; Coombes, J.S.; MacKay, C.P.; Rodriguez, A.D.; Erickson, K.I.; Copland, D.A.; McMahon, K.L. The Immediate Effects of Acute Aerobic Exercise on Cognition in Healthy Older Adults: A Systematic Review. Sports Med. 2019, 49, 67–82. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute Effects of Moderate Aerobic Exercise on Specific Aspects of Executive Function in Different Age and Fitness Groups: A Meta-Analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
- Chang, Y.K.; Alderman, B.L.; Chu, C.H.; Wang, C.C.; Song, T.F.; Chen, F.T. Acute Exercise Has a General Facilitative Effect on Cognitive Function: A Combined ERP Temporal Dynamics and BDNF Study. Psychophysiology 2017, 54, 289–300. [Google Scholar] [CrossRef]
- Ji, Z.; Feng, T.; Mei, L.; Li, A.; Zhang, C. Influence of Acute Combined Physical and Cognitive Exercise on Cognitive Function: An NIRS Study. PeerJ 2019, 7, e7418. [Google Scholar] [CrossRef]
- Pellegrini-Laplagne, M.; Dupuy, O.; Sosner, P.; Bosquet, L. Acute Effect of a Simultaneous Exercise and Cognitive Task on Executive Functions and Prefrontal Cortex Oxygenation in Healthy Older Adults. Brain Sci. 2022, 12, 455. [Google Scholar] [CrossRef]
- Devenney, K.E.; Guinan, E.M.; Kelly, A.M.; Mota, B.C.; Walsh, C.; Rikkert, M.O.; Schneider, S.; Lawlor, B. Acute high-intensity aerobic exercise affects brain-derived neurotrophic factor in mild cognitive impairment: A randomised controlled study. BMJ Open Sport Exerc. Med. 2019, 5, e000499. [Google Scholar] [CrossRef]
- Ben Ayed, I.; Castor-Guyonvarch, N.; Amimour, S.; Naija, S.; Aouichaoui, C.; Ben Omor, S.; Tabka, Z.; El Massioui, F. Acute Exercise and Cognitive Function in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82, 749–760. [Google Scholar] [CrossRef]
- Lo, Y.-H.; Ai, J.-Y.; Kao, S.-C.; Chen, F.-T.; Chang, Y.-K. Effects of acute fitness exercise on executive function in patients with Alzheimer’s disease and mild cognitive impairment: A review of the literature. Chin. Sports Q. 2023, 37, 25–39. [Google Scholar]
- Chang, Y.-K.; Etnier, J.L.; Li, R.-H.; Ren, F.-F.; Ai, J.Y.; Chu, C.-H. Acute Exercise Effect on Neurocognitive Function Among Cognitively Normal Late-Middle-Aged Adults With/Without Genetic Risk of AD: The Moderating Role of Exercise Volume and APOE Genotype. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad179. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, S.; Lang, M.; He, R.; Li, J. The more the better? A metaanalysis on efects of combined cognitive and physical intervention on cognition in healthy older adults. Ageing Res. Rev. 2016, 31, 67–79. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Dong, C.; Minkov, R.; Bahar-Fuchs, A.; Ellis, K.A.; Lautenschlager, N.T.; Mellow, M.L.; Wade, A.T.; Smith, A.E.; Finke, C.; et al. Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 2021, 66, 101232. [Google Scholar] [CrossRef]
- Damirchi, A.; Hosseini, F.; Babaei, P. Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women with MCI: A Small-Scale Study. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 20–29. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Sullivan, M.J.; Thompson, P.J.; Fallen, E.L.; Pugsley, S.O.; Taylor, D.W.; Berman, L.B. The 6-minute walk: A new measure of exercise capacity in patients with chronic heart failure. Can. Med. Assoc. J. 1985, 132, 919–992. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Omar Hammouda, O.; Trabelsi, K.; Chiboub, J.; Turki, M.; AbdelKarim, O.; El Abed, K.; Ben Ali, M.; Hoekelmann, A.; et al. Acute and delayed responses of C-reactive protein, malondialdehyde and antioxidant markers after resistance training session in elite weightlifters: Effect of time of day. Chronobiol. Int. 2015, 32, 1211–1222. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Souissi, N. Effect of Time-of-Day on Biochemical Markers in Response to Physical Exercise. J. Strength Cond. Res. 2017, 31, 272–282. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Shulman, K.I. Clock-drawing: Is it the ideal cognitive screening test? Int. J. Geriatr. Psychiatry 2000, 15, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.G.; White, D.A. Using verbal fluency to detect very mild dementia of the Alzheimer type. Arch. Clin. Neuropsychol. 2006, 21, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.J.; Woods, C.M.; Storandt, M. Model stability of the 15-item Geriatric Depression Scale across cognitive impairment and severe depression. Psychol. Aging 2007, 22, 372–379. [Google Scholar] [CrossRef]
- Lucas-Carrasco, R.; Skevington, S.M.; Gómez-Benito, J.; Rejas, J.; March, J. Using the WHOQOL-BREF in persons with dementia: A validation study. Alzheimer Dis. Assoc. Disord. 2011, 25, 345–351. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Golden, C.J. A group version the Stroop color and word test. J. Person. Assess. 1975, 39, 386–388. [Google Scholar] [CrossRef]
- Godefroy, O.; Martinaud, O.; Verny, M.; Mosca, C.; Lenoir, H.; Bretault, E.; Roussel, M. The dysexecutive syndrome of Alzheimer’s disease: The GREFEX study. J. Alzheimers Dis. 2014, 42, 1203–1208. [Google Scholar] [CrossRef]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Holdnack, J. Reliability and validity of the Delis-Kaplan Executive Function System: An update. J. Int. Neuropsychol. Soc. 2004, 10, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Troyer, A.K.; Leach, L.; Strauss, E. Aging and Response Inhibition: Normative Data for the Victoria Stroop Test. Aging Neuropsychol. Cogn. 2007, 13, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, J.; Van der Linden, M. Effect of age on forward and backward digit spans. Aging Neuropsychol. Cogn. 1997, 4, 140–149. [Google Scholar] [CrossRef]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Douglass, S. Tower of Hanoi: Evidence for the cost of goal retrieval. J. Exp. Psychol. Learn. Mem. Cogn. 2001, 27, 1331–1346. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. A scale of magnitudes for effect statistics. New View Stat. 2002, 502, 411. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; 2020, 4.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Singmann, H.; Bolker, B.; Westfall, J.; Aust, F.; Ben-Shachar, M.S. afex: Analysis of Factorial Experiments; R Package Version 0.16-1; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests, version 0.7; R Package: Vienna, Austria, 2021. [Google Scholar]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. nparLD: An R software package for the nonparametric analysis of longitudinal data in factorial experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Takenaka, S.; Suga, T.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Isaka, T.; Hashimoto, T. Impact of Exercise Intensity and Duration on Postexercise Executive Function. Med. Sci. Sports Exerc. 2017, 49, 774–784. [Google Scholar] [CrossRef]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Callow, D.D.; Smith, J.C. Brain activation during executive control after acute exercise in older adults. Int. J. Psychophysiol. 2019, 146, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Wenig, T.B.; Narayana-Kumanan, K.; Cole, R.C.; Wharff, C.; Reist, L.; Dubose, L.; Sigurdsson, G.; Mills, J.A.; Long, J.D.; et al. Acute Exercise Effects Predict Training Change in Cognition and Connectivity. Med. Sci. Sports Exerc. 2020, 52, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K.; Sng, E.; Ashpole, N. The effects of exercise on memory function among young to middle aged adults: Systematic review and recommendations for future research. Am. J. Health Promot. 2018, 32, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Marin Bosch, B.; Bringard, A.; Logrieco, M.G.; Lauer, E.; Imobersteg, N.; Thomas, A.; Ferretti, G.; Schwartz, S.; Igloi, K. Effect of acute physical exercise on motor sequence memory. Sci. Rep. 2020, 10, 15322. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Blough, J.; Crawford, L.; Ryu, S.; Zou, L.; Li, H. The temporal effects of acute exercise on episodic memory function: Systematic review with meta-analysis. Brain Sci. 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Festa, F.; Medori, S.; Macrì, M. Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups. Biomedicines 2023, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yin, H.; Wang, S.; Shang, B.; Meng, X.; Yan, M.; Li, G.; Chu, J.; Chen, L. The effect of combined cognitive intervention and physical exercise on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Li, P.W.C.; Yu, D.S.F.; Rose, S.Y.; Lin, R.S.Y. Combined exercise and cognitive interventions for adults with mild cognitive impairment and dementia: A systematic review and network meta-analysis. Int. J. Nurs. Stud. 2023, 147, 104592. [Google Scholar] [CrossRef]
- Ben Ayed, I.; Aouichaoui, C.; Ammar, A.; Naija, S.; Tabka, O.; Jahrami, H.; Trabelsi, K.; Trabelsi, Y.; El Massioui, N.; El Massioui, F. Mid-Term and Long-Lasting Psycho-Cognitive Benefits of Bidomain Training Intervention in Elderly Individuals with Mild Cognitive Impairment. Eur. J. Investig. Health Psychol. Educ. 2024, 14, 284–298. [Google Scholar]
- Nath, K.; Ferguson, I.; Puleio, A.; Wall, K.; Stark, J.; Clark, S.; Story, C.; Cohen, B.; Anderson-Hanley, C. Brain Health Indicators Following Acute Neuro-Exergaming: Biomarker and Cognition in Mild Cognitive Impairment (MCI) after Pedal-n-Play (iPACES). Brain Sci. 2024, 13, 844. [Google Scholar] [CrossRef]
- Paillard, T.; Blain, H.; Bernar, P.L. The impact of exercise on Alzheimer’s disease progression. Expert Rev. Neurother. 2024, 24, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Xia, R.; Zhou, W.; Tao, J.; Chen, L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sport. Med. 2016, 50, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Wang, X.-X.; Chen, L.; Liu, Y.; Li, Y.-R. A systematic review and network meta-analysis comparing various non-pharmacological treatments for older people with mild cognitive impairment. Asian J. Psychiatr. 2023, 86, 103635. [Google Scholar] [CrossRef]


| Exercise Group (EG, n = 15) | Combined Group (ECG, n = 15) | Control Group (CG, n = 14) | p Value | ES | |
|---|---|---|---|---|---|
| Age (years) | 67.93 ± 5.18 | 67.13 ± 3,04 | 69.24 ± 4.84 | 0.444 | 0 * |
| Age of the onset of the disease (years) | 63.26 ± 4.75 | 62.00 ± 3.84 | 64.57 ± 4.44 | 0.1 | 0.064 * |
| Sexe (M/F) | 5/10 | 5/10 | 2/12 | ||
| Neuropsychological measures | |||||
| MMSE | 26.20 ± 0.56 | 26.13 ± 0.35 | 26.07 ± 0.26 | 0.65 | 0 * |
| RL/RI 16 | 13.13 ± 1.18 | 13.00 ± 1.25 | 13.14 ± 1.16 | 0.937 | 0.003 |
| Letter fluency | 10.53 ± 0.74 | 10.80 ± 0.77 | 10.71 ± 0.72 | 0.561 | 0 * |
| Category fluency | 13.86 ± 0.99 | 13.33 ± 0.97 | 13.07 ± 0.82 | 0.106 | 0.061 * |
| Clock test | 6.00 ± 0.53 | 5.66 ± 0.48 | 5.57 ± 0.64 | 0.101 | 0.063 * |
| Psychological measures | |||||
| GDS 15 | 5.93 ± 0.45 | 5.20 ± 0.41 | 6.21 ± 0.57 | 0.001 | 0.451 * |
| WHOQOL-BREF-100D1 | 21.00 ± 1.36 | 21.73 ± 1.83 | 21.71 ± 1.43 | 0.354 | 0.049 |
| WHOQOL-BREF-100D2 | 20.86 ± 1.30 | 20.33 ± 1.54 | 19.35 ± 1.78 | 0.039 | 0.147 |
| WHOQOL-BREF-100D3 | 9.06 ± 1.38 | 9.67 ± 1.40 | 9.64 ± 0.74 | 0.393 | 0 * |
| WHOQOL-BREF-100D4 | 27.00 ± 1.64 | 26.93 ± 2.37 | 26.21 ± 2.11 | 0.535 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ayed, I.; Ammar, A.; Boujelbane, M.A.; Salem, A.; Naija, S.; Amor, S.B.; Trabelsi, K.; Jahrami, H.; Chtourou, H.; Trabelsi, Y.; et al. Acute Effect of Simultaneous Exercise and Cognitive Tasks on Cognitive Functions in Elderly Individuals with Mild Cognitive Impairment. Diseases 2024, 12, 148. https://doi.org/10.3390/diseases12070148
Ben Ayed I, Ammar A, Boujelbane MA, Salem A, Naija S, Amor SB, Trabelsi K, Jahrami H, Chtourou H, Trabelsi Y, et al. Acute Effect of Simultaneous Exercise and Cognitive Tasks on Cognitive Functions in Elderly Individuals with Mild Cognitive Impairment. Diseases. 2024; 12(7):148. https://doi.org/10.3390/diseases12070148
Chicago/Turabian StyleBen Ayed, Ines, Achraf Ammar, Mohamed Ali Boujelbane, Atef Salem, Salma Naija, Sana Ben Amor, Khaled Trabelsi, Haitham Jahrami, Hamdi Chtourou, Yassine Trabelsi, and et al. 2024. "Acute Effect of Simultaneous Exercise and Cognitive Tasks on Cognitive Functions in Elderly Individuals with Mild Cognitive Impairment" Diseases 12, no. 7: 148. https://doi.org/10.3390/diseases12070148
APA StyleBen Ayed, I., Ammar, A., Boujelbane, M. A., Salem, A., Naija, S., Amor, S. B., Trabelsi, K., Jahrami, H., Chtourou, H., Trabelsi, Y., & El Massioui, F. (2024). Acute Effect of Simultaneous Exercise and Cognitive Tasks on Cognitive Functions in Elderly Individuals with Mild Cognitive Impairment. Diseases, 12(7), 148. https://doi.org/10.3390/diseases12070148









