Unveiling the Immunostimulatory Potential of Rhus Toxicodendron in Immunocompromised Balb/C Mice Induced with Cyclophosphamide
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of a Stock Solution of the Drug
2.3. Experimental Animals
2.4. Experimental Design
2.4.1. Sample Size
2.4.2. Cyclophosphamide-Induced Immunosuppression Model
2.5. Calculation of Immune Organ Indices
2.6. Phagocytic Index Assay
2.7. Determination of Cytokines in Serum and Spleen by ELISA
2.8. Gene Expression Analysis
2.9. Histopathological Analysis (H&E Staining) of the Spleen
2.10. Statistical Analysis
3. Results
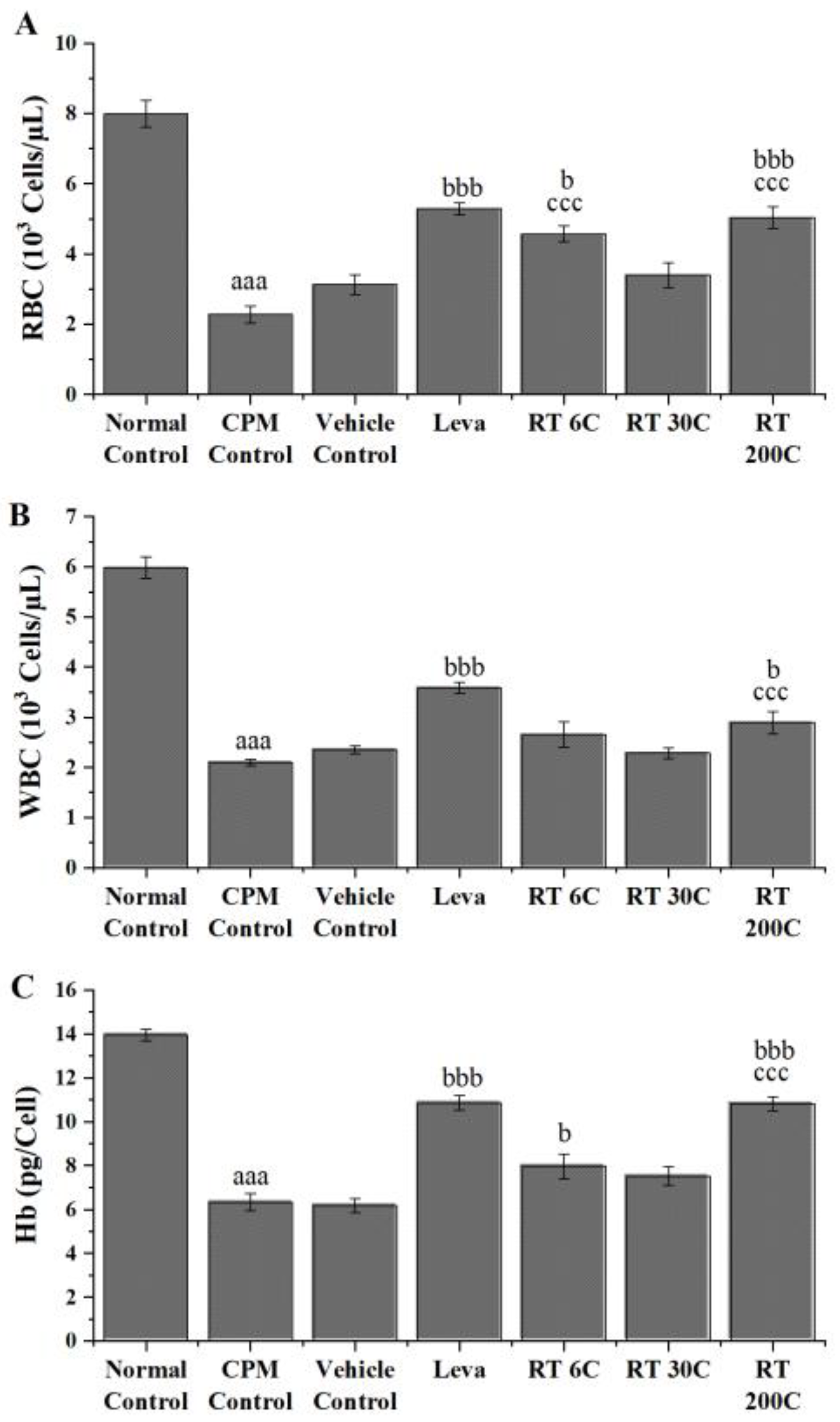
3.1. Effect of RT on RBC, WBC, and Hb in CPM-Treated Mice
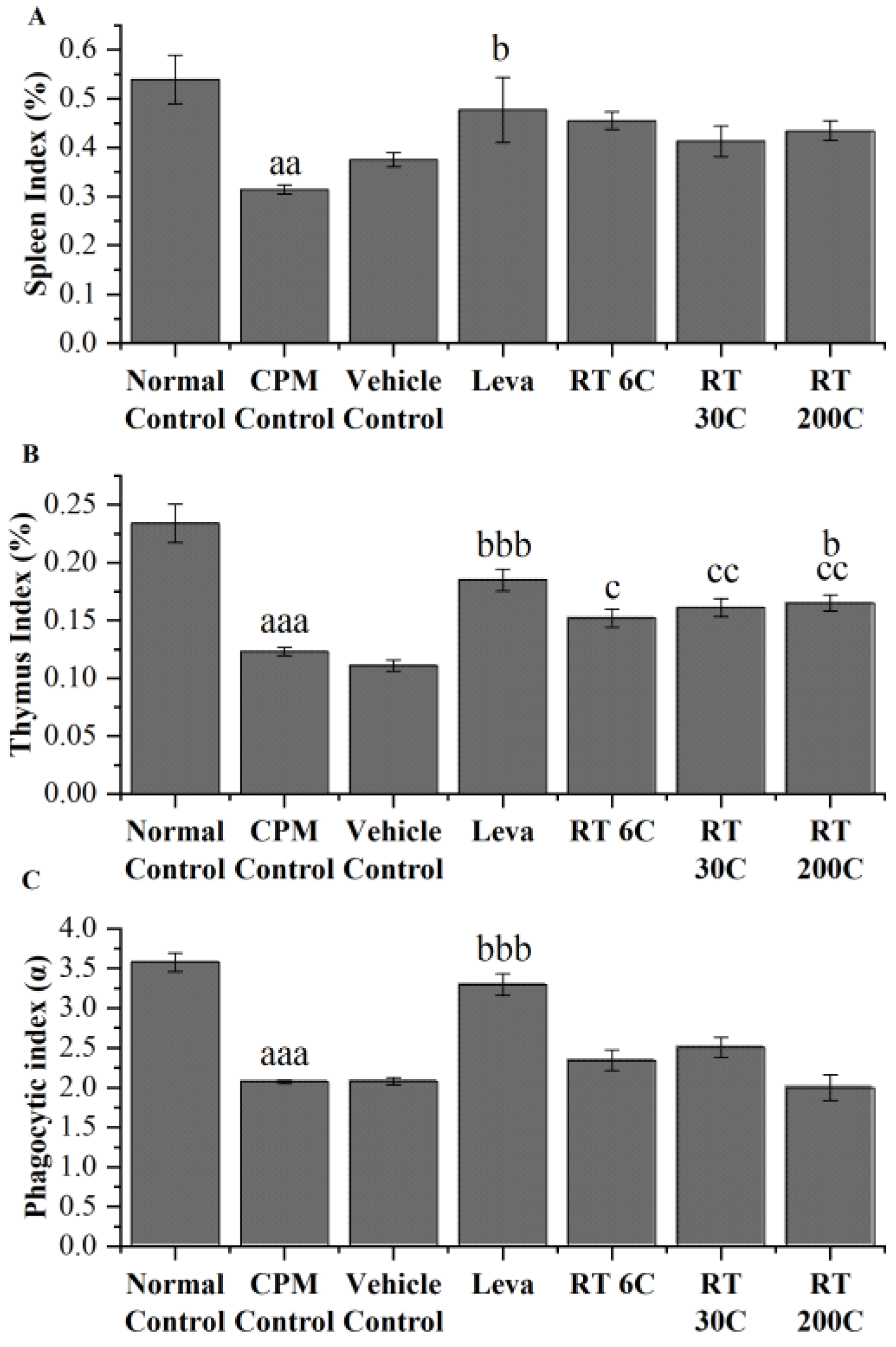
3.2. Effect of RT on Thymus and Spleen Indices in the CPM-Treated Group
3.3. Effect of RT on the Phagocytic Index in CPM-Treated Mice
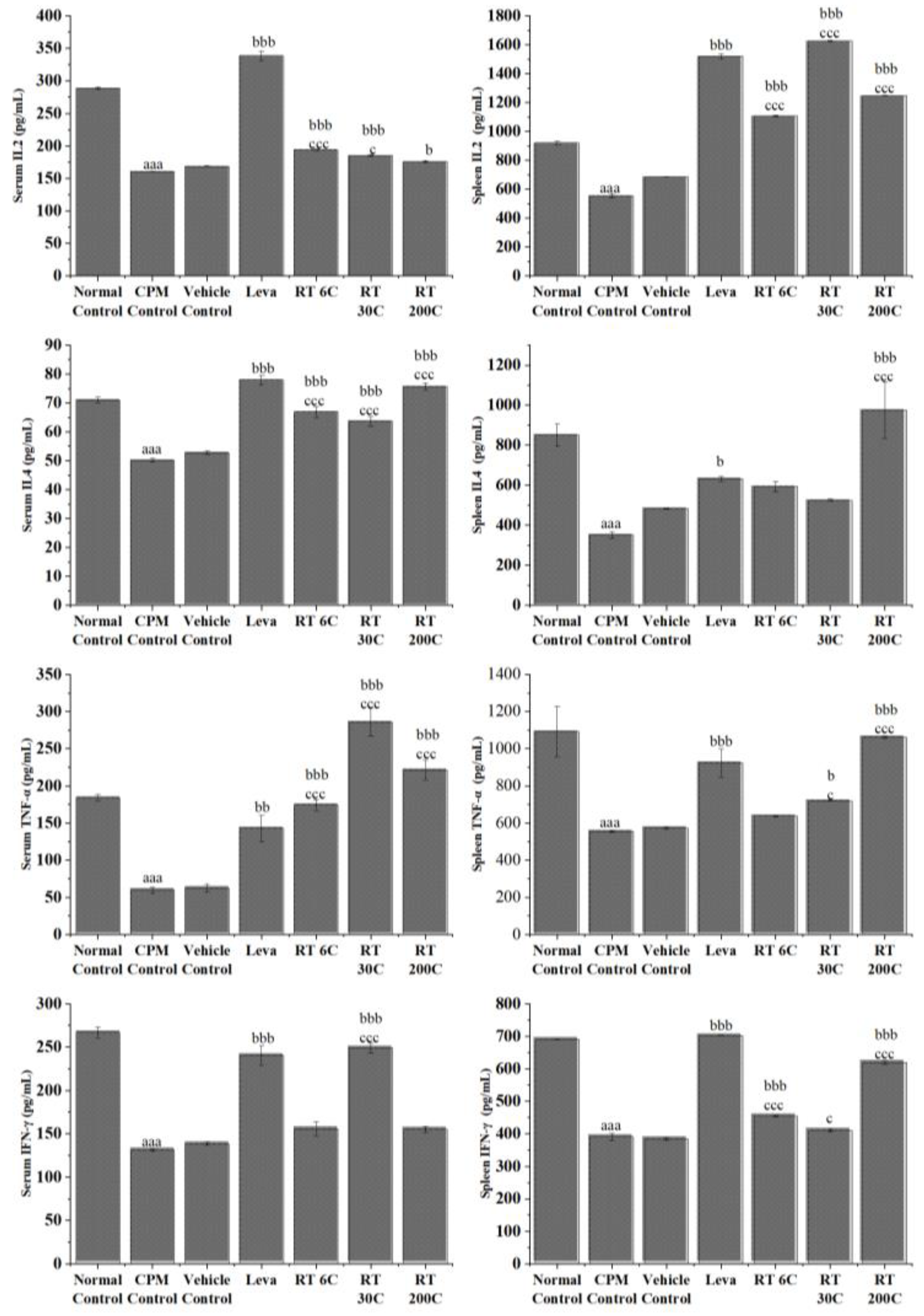
3.4. Effect of RT on Cytokine Levels in the Serum and Spleen of CPM-Treated Mice
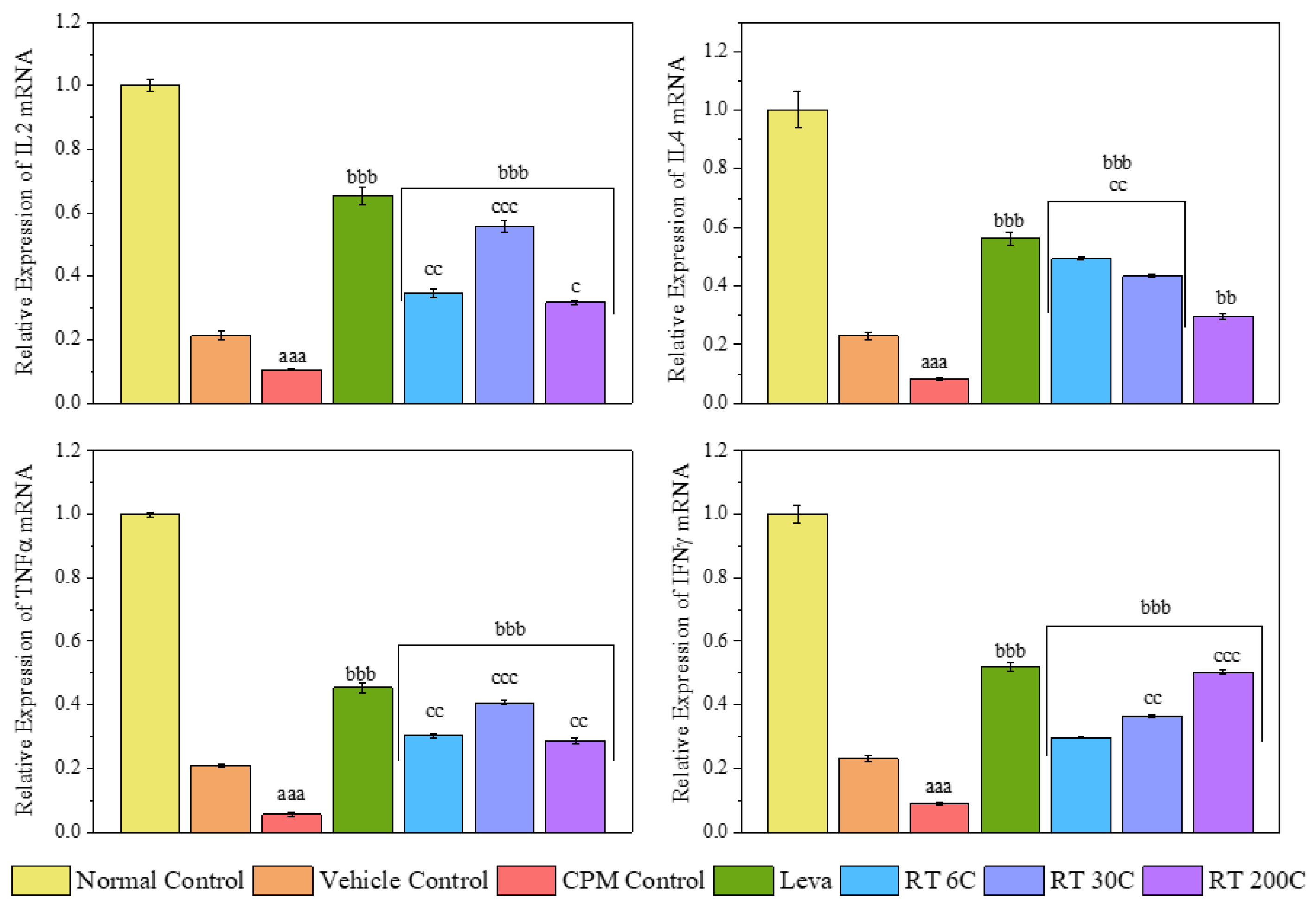
3.5. Effect of RT on the mRNA Expression Levels of Cytokines (IL2, IL4, TNF-α, and IFN-γ) in the Spleens of CPM-Treated Mice
3.6. Effect of RT on Spleen Histology in CPM-Treated Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loh, L.; Wang, Z.; Sant, S.; Koutsakos, M.; Jegaskanda, S.; Corbett, A.J.; Liu, L.; Fairlie, D.P.; Crowe, J.; Rossjohn, J. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18–dependent activation. Proc. Natl. Acad. Sci. USA 2016, 113, 10133–10138. [Google Scholar] [CrossRef]
- Ferlazzo, G.; Tsang, M.L.; Moretta, L.; Melioli, G.; Steinman, R.M.; Munz, C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 2002, 195, 343–351. [Google Scholar] [CrossRef]
- Shruthi, S.; Vijayalaxmi, K.; Shenoy, K.B. Immunomodulatory effects of gallic acid against cyclophosphamide-and cisplatin-induced immunosuppression in Swiss albino mice. Indian J. Pharm. Sci. 2018, 80, 150–160. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Zhou, X.; Yang, M.; Ma, X.; Liu, X. Norcantharidin alleviates cyclophosphamide-induced immunosuppression via circBCL2L1/miR-30c-3-3p/TRAF6 axis. Qual. Assur. Saf. Crops Foods 2022, 14, 94–102. [Google Scholar] [CrossRef]
- Winkelstein, A. Mechanisms of immunosuppression: Effects of cyclophosphamide on cellular immunity. Blood 1973, 41, 273–284. [Google Scholar] [CrossRef]
- Hussain, K.; Iqbal, Z.; Zahid Abbas, R.; Kasib Khan, M.; Kashif Saleemi, M. Immunomodulatory activity of Glycyrrhiza glabra extract against mixed Eimeria infection in chickens. Int. J. Agric. Biol. 2017, 19, 928. [Google Scholar]
- Dos Santos, A.; Perazzo, F.; Cardoso, L.; Carvalho, J. In vivo study of the anti-inflammatory effect of Rhus toxicodendron. Homeopathy 2007, 96, 95–101. [Google Scholar] [CrossRef]
- Goswami, A.; Saka, V.P.; GV, N.K.; Biswas, B. A Comprehensive Review on Phytochemistry and Pharmacology of Homoeopathic Medicine Rhus Toxicodendron. Homœopathic Links 2022, 35, 271–275. [Google Scholar] [CrossRef]
- Pfaff, F. On the active principle of Rhus toxicodendron and Rhus venenata. J. Exp. Med. 1897, 2, 181. [Google Scholar] [CrossRef]
- Patil, C.; Salunkhe, P.; Gaushal, M.; Gadekar, A.; Agrawal, A.; Surana, S. Immunomodulatory activity of Toxicodendron pubescens in experimental models. Homeopathy 2009, 98, 154–159. [Google Scholar] [CrossRef]
- Xia, Z.; Miyakoshi, T.; Yoshida, T. Lipoxygenase-catalyzed polymerization of phenolic lipids suggests a new mechanism for allergic contact dermatitis induced by urushiol and its analogs. Biochem. Biophys. Res. Commun. 2004, 315, 704–709. [Google Scholar] [CrossRef]
- Vickers, A.; Zollman, C. ABC of Complementary Medicine; netLibrary: Boulder, CO, USA, 2001. [Google Scholar]
- Patil, C.R.; Rambhade, A.D.; Jadhav, R.B.; Patil, K.R.; Dubey, V.K.; Sonara, B.M.; Toshniwal, S.S. Modulation of arthritis in rats by Toxicodendron pubescens and its homeopathic dilutions. Homeopathy 2011, 100, 131–137. [Google Scholar] [CrossRef]
- Patil, C.R.; Gadekar, A.R.; Patel, P.N.; Rambhade, A.; Surana, S.J.; Gaushal, M.H. Dual effect of Toxicodendron pubescens on Carrageenan induced paw edema in rats. Homeopathy 2009, 98, 89–91. [Google Scholar] [CrossRef]
- Alam, M.F.; Ajeibi, A.O.; Safhi, M.H.; Alabdly, A.J.A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Jali, A.M.; Alqahtani, S.; Nomier, Y.; et al. Therapeutic Potential of Capsaicin against Cyclophosphamide-Induced Liver Damage. J. Clin. Med. 2023, 12, 911. [Google Scholar] [CrossRef]
- Yadav, V.; Krishnan, A.; Zahiruddin, S.; Ahmad, S.; Vohora, D. Amelioration of Cyclophosphamide-Induced DNA Damage, Oxidative Stress, and Hepato- and Neurotoxicity by Piper Longum Extract in Rats: The Role of ΓH2AX and 8-OHdG. Front. Pharmacol. 2023, 14, 1147823. [Google Scholar] [CrossRef]
- Sato-Okabayashi, Y.; Isoda, K.; Heissig, B.; Kadoguchi, T.; Akita, K.; Kitamura, K.; Shimada, K.; Hattori, K.; Daida, H. Low-dose oral cyclophosphamide therapy reduces atherosclerosis progression by decreasing inflammatory cells in a murine model of atherosclerosis. IJC Hear. Vasc. 2020, 28, 100529. [Google Scholar] [CrossRef]
- Quan, X.; Chen, H.; Liang, S.; Yang, C.; Yao, C.; Xu, Y.; Liu, H.; An, N. Revisited Cyclophosphamide in the Treatment of Lupus Nephritis. Biomed Res. Int. 2022, 2022, 8345737. [Google Scholar] [CrossRef]
- Ministry of Health & Family Welfare(India). Homoeopathic Pharmacopoeia of India; Government of India: New Delhi, India, 1990; Volume 1, pp. 1–234.
- Goswami, A.; Kumar, G.V.N.; Saka, V.P.; Gupta, P.; Verma, D. Safety Assessment of Ultra High Dilutions of Camphora Officinarum: A Preclinical Investigation. Altern. Ther. Health Med. 2024, 30, AT10469. [Google Scholar]
- Lal, R.; Sharma, M.; Behera, S.; Regar, R.K.; Tripathi, D.; Kumar, G.N.; Singh, S.; Verma, D.; Gupta, P.; Kaushik, S. Safety Evaluation of Arsenicum Album in Acute and Sub-Acute Toxicity Studies in Rats. Toxicol. Int. 2023, 30, 233–247. [Google Scholar] [CrossRef]
- Saka, V.P.; Kumar, G.N.; Goswami, A.; Verma, D.; Gupta, P. Camphora Augments Humoral Mediated Immunity and Decreases Delayed type Hypersensitivity in BALB/c Mice. J. Nat. Remedies 2023, 23, 157–167. [Google Scholar] [CrossRef]
- Yan, H.; Lu, J.; Wang, J.; Chen, L.; Wang, Y.; Li, L.; Miao, L.; Zhang, H. Prevention of cyclophosphamide-induced immunosuppression in mice with traditional Chinese medicine Xuanfei Baidu decoction. Front. Pharmacol. 2021, 12, 730567. [Google Scholar] [CrossRef]
- Cheng, W.; Li, J.; You, T.; Hu, C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linne. J. Ethnopharmacol. 2005, 101, 334–337. [Google Scholar] [CrossRef]
- Raj, S.; Gothandam, K. Immunomodulatory activity of methanolic extract of Amorphophallus commutatus var. wayanadensis under normal and cyclophosphamide induced immunosuppressive conditions in mice models. Food Chem. Toxicol. 2015, 81, 151–159. [Google Scholar] [CrossRef]
- Han, L.; Meng, M.; Guo, M.; Cheng, D.; Shi, L.; Wang, X.; Wang, C. Immunomodulatory Activity of a Water-Soluble Polysaccharide Obtained from Highland Barley on Immunosuppressive Mice Models. Food Funct. 2019, 10, 304–314. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kim, Y.-S.; Lee, D.-R.; Choi, B.-K.; Kwon, K.-B.; Bae, G.-S. Echinacea purpurea alleviates cyclophosphamide-induced immunosuppression in mice. Appl. Sci. 2021, 12, 105. [Google Scholar] [CrossRef]
- House of Commons Science and Technology Committee. Evidence Check 2: Homeopathy. 2010. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://publications.parliament.uk/pa/cm200910/cmselect/cmsctech/45/45.pdf&ved=2ahUKEwjt5azRhryHAxX7sVYBHe5IBE4QFnoECBkQAQ&usg=AOvVaw3ZMuHyefSTMUsVoOYb79I5 (accessed on 18 June 2024).
- Vithoulkas, G. Serious mistakes in meta-analysis of homeopathic research. J. Med. Life 2017, 10, 47. [Google Scholar]
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS ONE 2018, 13, e0204152. [Google Scholar] [CrossRef]
- Zhang, W.-N.; Gong, L.-L.; Liu, Y.; Zhou, Z.-B.; Wan, C.-X.; Xu, J.-J.; Wu, Q.-X.; Chen, L.; Lu, Y.-M.; Chen, Y. Immunoenhancement effect of crude polysaccharides of Helvella leucopus on cyclophosphamide-induced immunosuppressive mice. J. Funct. Foods 2020, 69, 103942. [Google Scholar] [CrossRef]
- Yingjian, L.; Junming, H.; Min, C.; Chenyue, L.; Dachao, Z.; Yuanhua, H.; Zhi, L. A health food high-peptide meal alleviates immunosuppression induced by hydrocortisone and cyclophosphamide in mice. Food Funct. 2013, 4, 1352–1359. [Google Scholar] [CrossRef]
- Barrea, L.; Di Somma, C.; Muscogiuri, G.; Tarantino, G.; Tenore, G.C.; Orio, F.; Colao, A.; Savastano, S. Nutrition, inflammation and liver-spleen axis. Crit. Rev. Food Sci. Nutr. 2018, 58, 3141–3158. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Qu, H.; Li, X.; Feng, B. Holothurian Wall Hydrolysate Ameliorates Cyclophosphamide-Induced Immunocompromised Mice via Regulating Immune Response and Improving Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 12583. [Google Scholar] [CrossRef] [PubMed]
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 2009, 106, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.-X.; Chen, H.-X.; Zhang, J.; Zhang, X.-D.; Liang, Y.-X. Protective effect of chitooligosaccharides against cyclophosphamide-induced immunosuppression in mice. Int. J. Biol. Macromol. 2013, 62, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Fan, Y.; Wang, S.; Si, L.; Li, B. Immunomodulatory activity of Alaska pollock hydrolysates obtained by glutamic acid biosensor–Artificial neural network and the identification of its active central fragment. J. Funct. Foods 2016, 24, 37–47. [Google Scholar] [CrossRef]
- Woo, S.M.; Choi, W.R.; Jang, D.; Yi, C.S.; Kim, H.L.; Kim, K.H.; Kim, J.T.; Choi, W.H.; Jang, S.H.; Kim, M.J. Immune enhancement effect of an herb complex extract through the activation of natural killer cells and the regulation of cytokine levels in a cyclophosphamide-induced immunosuppression rat model. Asian Pac. J. Trop. Med. 2018, 11, 653–658. [Google Scholar]
- Kumar, V.P.; Venkatesh, Y.P. Alleviation of cyclophosphamide-induced immunosuppression in Wistar rats by onion lectin (Allium cepa agglutinin). J. Ethnopharmacol. 2016, 186, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hong, H.; Zhang, C.; Wang, K.; Zhang, B.; Han, Q.-A.; Liu, H.; Luo, Y. Immunomodulatory effects of collagen hydrolysates from yak (Bos grunniens) bone on cyclophosphamide-induced immunosuppression in BALB/c mice. J. Funct. Foods 2019, 60, 103420. [Google Scholar] [CrossRef]
- Dirchwolf, M.; Podhorzer, A.; Marino, M.; Shulman, C.; Cartier, M.; Zunino, M.; Paz, S.; Muñoz, A.; Bocassi, A.; Gimenez, J. Immune dysfunction in cirrhosis: Distinct cytokines phenotypes according to cirrhosis severity. Cytokine 2016, 77, 14–25. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Meng, Y.; Li, B.; Jin, D.; Zhan, M.; Lu, J.; Huo, G. Immunomodulatory activity of Lactobacillus plantarum KLDS1. 0318 in cyclophosphamide-treated mice. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Saha, S.K.; Roy, S.; Khuda-Bukhsh, A.R. Ultra-Highly Diluted Plant Extracts of Hydrastis Canadensis and Marsdenia Condurango Induce Epigenetic Modifications and Alter Gene Expression Profiles in HeLa Cells in Vitro. J. Integr. Med. 2015, 13, 400–411. [Google Scholar] [CrossRef]
- Mohan, T.; Periandavan, K.; Nayak, D. Evaluation of Hypolipidemic Activity of Homeopathic Drug Allium Sativum 6C Potency on Different Grades of Dyslipidemia in Wistar Albino Rat Models. Phytomed. Plus 2022, 2, 100354. [Google Scholar] [CrossRef]
- Joshi, J.; Bandral, C.; Manchanda, R.K.; Khurana, A.; Nayak, D.; Kaur, S. Evidence for Reversal of Immunosuppression by Homeopathic Medicine to a Predominant Th1-Type Immune Response in BALB/c Mice Infected with Leishmania Donovani. Homeopathy 2022, 111, 031–041. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, S.; Maurya, V.K.; Nayak, D.; Kaushik, S.; Manchanda, R.K.; Gadugu, S. Antiviral and Anti-Inflammatory Activity of Novel Belladonna Formulation against Japanese Encephalitis Virus via Inhibition of P65 Nuclear Translocation and TNF-α Mediated NF-KB Signaling. In Biotechnology and Genetic Engineering Reviews; Taylor & Francis Group: Abingdon, UK, 2023; pp. 1–23. [Google Scholar]
- Sarkar, A.; Roy, A.; Maity, M.; Nayak, D.; Das, S. Ultradiluted Eupatorium Perfoliatum Alleviates DENV-Induced Fibrosis By Regulation of TGFβ1, MMP-9 and Interferons. Future Virol. 2023, 18, 767–782. [Google Scholar] [CrossRef]
- Mohan, T.; Rajkumar, A.; Panchalingam, G.; Nayak, D.; Raghunathan, M.; Periandavan, K. Homeopathic Preparation of Allium Sativum Abrogates OxLDL Mediated Atherogenic Events in Macrophages: An in Vitro and in Silico Approach. J. Ayurveda Integr. Med. 2024, 15, 100850. [Google Scholar] [CrossRef]
- Rath, S.; Jema, J.P.; Kesavan, K.; Mallick, S.; Pradhan, J.; Chainy, G.B.N.; Nayak, D.; Kaushik, S.; Dandapat, J. Arsenic Album 30C Exhibits Crystalline Nano Structure of Arsenic Trioxide and Modulates Innate Immune Markers in Murine Macrophage Cell Lines. Sci. Rep. 2024, 14, 745. [Google Scholar] [CrossRef]
- Ullman, D. Exploring Possible Mechanisms of Hormesis and Homeopathy in the Light of Nanopharmacology and Ultra-High Dilutions. Dose-Response 2021, 19, 155932582110229. [Google Scholar] [CrossRef]

| Gene | Sequence 5′-3′ | Size bp |
|---|---|---|
| IL-2 | F-GCAGCTGTTGATGGACCTAC | 20 |
| R-TCCACCACAGTTGCTGACTC | 20 | |
| IL-4 | F-TCGGCATTTTGAACGAGGTC | 20 |
| R-GAAAAGCCCGAAAGAGTCTC | 20 | |
| TNF-α | F-ATGAGCACAGAAAGCATGATC | 21 |
| R-TACAGGCTTGTCACTGGAATT | 21 | |
| IFN-γ | F-TGAGCAGAGCTCTTGTGGTC | 20 |
| R-CGTTCCTCCTTGTGGCCTAA | 20 | |
| GAPDH | F-5′GTGGAGTCTACTGGTGTCTTC′3 | 21 |
| R-5′GTGCAGGAGGCATTGCTTACA3′ | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saka, V.P.; G. V., N.K.; Sanapalli, B.K.R.; Goswami, A.; Roy, A.; Agrawal, A.; Gupta, P.; Verma, D.; Kaushik, S. Unveiling the Immunostimulatory Potential of Rhus Toxicodendron in Immunocompromised Balb/C Mice Induced with Cyclophosphamide. Diseases 2024, 12, 178. https://doi.org/10.3390/diseases12080178
Saka VP, G. V. NK, Sanapalli BKR, Goswami A, Roy A, Agrawal A, Gupta P, Verma D, Kaushik S. Unveiling the Immunostimulatory Potential of Rhus Toxicodendron in Immunocompromised Balb/C Mice Induced with Cyclophosphamide. Diseases. 2024; 12(8):178. https://doi.org/10.3390/diseases12080178
Chicago/Turabian StyleSaka, Vara Prasad, Narasimha Kumar G. V., Bharat Kumar Reddy Sanapalli, Abanti Goswami, Anirban Roy, Anurag Agrawal, Pankaj Gupta, Digvijay Verma, and Subhash Kaushik. 2024. "Unveiling the Immunostimulatory Potential of Rhus Toxicodendron in Immunocompromised Balb/C Mice Induced with Cyclophosphamide" Diseases 12, no. 8: 178. https://doi.org/10.3390/diseases12080178
APA StyleSaka, V. P., G. V., N. K., Sanapalli, B. K. R., Goswami, A., Roy, A., Agrawal, A., Gupta, P., Verma, D., & Kaushik, S. (2024). Unveiling the Immunostimulatory Potential of Rhus Toxicodendron in Immunocompromised Balb/C Mice Induced with Cyclophosphamide. Diseases, 12(8), 178. https://doi.org/10.3390/diseases12080178







